Abstract
The protein tyrosine kinase Syk plays a central role in Fcγ receptor–mediated phagocytosis in the adaptive immune system. We show here that Syk also plays an essential role in complement-mediated phagocytosis in innate immunity. Macrophage-like differentiated HL60 cells and C3bi-opsonized zymosan comprised the pathogen-phagocyte system. C3bi-opsonized zymosan particles promptly attached to the cells and were subsequently engulfed via complement receptor 3. During this process, Syk became tyrosine phosphorylated and accumulated around the nascent phagosomes. The transfer of Syk-siRNA or dominant-negative Syk (DN-Syk) into HL60 cells resulted in impaired phagocytosis. Quenching assays using fluorescent zymosan revealed that most of the attached zymosan particles were located inside parental HL60 cells, whereas few were ingested by the mutant cells. These data indicated that Syk is required for the engulfment of C3bi-opsonized zymosan. During C3bi-zymosan–induced phagocytosis, actin accumulation occurred around phagosomes and was followed by depolymerization, and further RhoA was activated together with tyrosine phosphorylation of Vav. These responses including the actin remodeling were suppressed in Syk-siRNA– or DN-Syk–expressing cells. Our results demonstrated that Syk plays an indispensable role in complement-mediated phagocytosis by regulating both actin dynamics and the RhoA activation pathway and that these functions of Syk lead to phagosome formation and pathogen engulfment.
Introduction
Phagocytosis is a central event in the innate immune responses that are triggered by the association between ligands on the surface of pathogens and receptors on the membrane of phagocytes. Phagocytes then engulf and eliminate the pathogens. Among the phagocytic receptor types expressed on mammalian neutrophils and macrophages, Fcγ receptors and complement receptor 3 (CR3) have been characterized in detail. The clustering of these receptors by their ligands bound to pathogens is followed by the activation of signaling pathways that trigger dynamic rearrangements of the cytoskeleton, which lead to the formation of phagosomes and their fusion to the lysosome.1,2 Some signaling molecules such as Rho-GTPases, ADP-ribosylation factor 6 (ARF6), and PLCγ regulate actin polymerization during phagosome formation.3-5
The complement system comprises many distinct plasma proteins that react with one another to opsonize pathogens and induce a series of inflammatory responses that help to fight infection.6,7 The most important action in this system is the acceleration of the uptake and destruction of pathogens by phagocytes. This complement-mediated phagocytosis begins with the specific recognition of bound complement components by complement receptors (CRs). In these combinations, C3bi binding to CR3 is a highly effective signal because this interaction is sufficient to stimulate phagocytosis.
The protein tyrosine kinase Syk is expressed in a wide range of hematopoietic and nonhematopoietic cells and it plays a key role in immunoreceptor (B-cell receptor and Fc receptors) signaling including Fcγ receptor–mediated phagocytosis.8-14 On the other hand, the role of Syk in nonadaptive immune mechanisms is not well understood and the roles of Syk in innate immunity have received considerable focus.15 Besides these roles in immunoreceptor function, Syk is also activated upon ligation with cell-surface integrins,16 such as integrin αIIbβ3 in platelets17,18 or β2 integrin in neutrophils19-21 or monocytic cell lines.22,23
Among complement receptors, CR3 (integrin αMβ2) and CR4 (integrin αXβ2) are the main phagocytic receptors and both types belong to the integrin receptor family. Considering the particular role of Syk in integrin signaling, Syk should play a critical role in innate immunity through complement-mediated phagocytosis.
The present study examines the role of Syk in complement-mediated phagocytosis using macrophage-like differentiated HL60 cells and C3bi-opsonized zymosan. Our results indicated that Syk plays a crucial role in the process from phagosome formation to engulfment by controlling the accumulation and disassembly of polymerized actin and Rho-GTPase activation.
Materials and methods
Reagents and antibodies
Zymosan A and RPMI 1640 medium were purchased from Sigma (St Louis, MO). Anti-Syk polyclonal antibody (polyAb; N-19), anti-Syk monoclonal antibody (monoAb; 4D10), anti-PLCγ2 polyAb, and anti-Vav polyAb (C-14) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-C3bi monoAb was obtained from Quidel (Santa Clara, CA). Antiphosphotyrosine (4G10) monoAb was from Upstate Biotechnology (Lake Placid, NY). Hygromycin B was from Wako Pure Chemical Industries (Osaka, Japan). Penicillin-Streptomycin Mixed Solution was from Nacalai Tesque (Kyoto, Japan). Antihuman integrin αM (CD11b) for immunoblotting was from R&D Systems (Minneapolis, MN). Antihuman CD11b for flow cytometry was from DAKO (Glostrup, Denmark). Antihuman integrin αM (CD11b) activating monoAb VIM12 F(ab)2 was from Orbigen (San Diego, CA).24 A C3-convertase inhibitor, compstatin, is a 13–amino acid cyclic peptide that binds to and inhibits cleavage of C3.25,26 The acetylated form of compstatin (Ac-ICVVQDWGHHRCT-NH2) and the control peptide (IAVVQDWGHHRAT-NH2) were purchased from Peptide Institute (Osaka, Japan) and Sigma-Aldrich (Tokyo, Japan), respectively.
Cells and cell culture
The human promyelocytic cell line HL60 was maintained in RPMI 1640 medium supplemented with 8% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, and 100 μg/mL streptomycin in 5% carbon dioxide (CO2) humidified air at 37°C. The cells were induced to undergo differentiation to macrophages by seeding them on dishes at a concentration of 2 × 106/100-mm dish in the presence of 10–7 M vitamin D3 and 10 ng/mL 12-O-tetradecanoylphorbol-13-acetate (TPA) and incubating for 3 days. Cell differentiation was confirmed morphologically by evaluating CR3 with flow cytometry.
Separation of human primary monocytes
Human monocytes were collected using the magnetic cell sorting (MACS) system and microbeads conjugated with monoclonal mouse anti–human CD14 antibodies purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Briefly, peripheral blood mononuclear cells of healthy blood donors were isolated by density gradient centrifugation using Ficoll-Paque (Pharmacia Biotech AB, Uppsala, Sweden). CD14-expressing cells were positively separated by MACS according to the protocol provided by the manufacturer. The purity of the CD14+ cell fraction was consistently over 90%.
Plasmid and transfection
Human syk cDNA, provided by Muller et al,27 was modified as previously described.28 The polymerase chain reaction (PCR) product of the Flag-tagged dominant-negative (DN) form of human syk gene expressing only the first 261 amino acids of Syk protein was subcloned into pcDNA3.1/Hygro (Invitrogen, Carlsbad, CA).
To inhibit syk gene expression, a vector for short interfering RNA (siRNA) expression, the pSilencer 2.1-U6 hygro (Ambion, Austin, TX), was used. Synthetic oligonucleotides for the sense and antisense target sequences of the human Syk-coding sequences including bp 1192 to 1210 and control sequences with stem-loop sequence to form the small hairpin RNA (a pair of oligonucleotides for annealing is 5′-GATCCACCGTGGCTGTGAAAATACTTCAAGAGAGTATTTTCACAGCCACGGTTTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAAACCGTGGCTGTGAAAATACTCTCTTGAAGTATTTTCACAGCCACGGTG-3′) were annealed and ligated into BamHI- and HindIII-cleaved backbone of pSilencer 2.1-U6 according to the manufacturer's instructions. Control sequences with stem-loop sequence to form the small hairpin RNA (a pair of oligonucleotides for annealing is 5′-GATCCGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAACG-3′) were annealed in the same way. Plasmids were transfected into HL60 cells by electroporation, and positive clones were selected with 1 mg/mL hygromycin B.
To support the knockdown effects of Syk-siRNA, we constructed a Flag-rescue–Syk expression vector containing 4 silent mismatches in the knockdown oligonucleotide sequence, 5′-ACGGTCGCAGTCAAAATAC-3′, corresponding to nucleotides 1192 to 1210 (mutation points are indicated by the underscores) by two-step overlap extension PCR. To amplify 2 fragments between 839 and 867, sense primer (5′-ATCCTGCGACTTGGTCAGCGGGTGGAATA-3′) or 1512-1540 antisense primer (5′-CGAAATCACTGATCTTGGCGTAATGTTGG-3′) and the specific mutation primer of the appropriate sense or antisense strand were used. The sense strand sequences of the mutation primers were 1186-1223 sense primer (5′-GTGAAAACGGTCGCTGTGAAAATACTGAAAAACGAGGC-3′) for 1194C>G and 1197G>C and additionally 1192-1229 (5′-ACGGTCGCAGTCAAAATACTGAAAAACGAGGCCAATGA-3′) for 1200T>A and 1203G>C. Two fragments were combined by the PCR with the 839-867 sense primer and 1512-1540 antisense primer and digested with ScaI. The ScaI fragment was inserted between the ScaI sites of Syk. Mutations were verified by sequencing both strands (ABI PRISM Cycle Sequencing FS Ready Reaction Kit; Applied Biosystems, Foster City, CA). The Flag-rescue–Syk was subcloned in the expression vector pcDNA4/TO (Invitrogen).
Flag-rescue–Syk was introduced into Syk-siRNA/HL cells by electroporation and positive clones were selected with 0.2 mg/mL zeocin (Invitrogen) for pcDNA4/TO. The expression of Flag-tagged DN-Syk or Flagrescue–Syk was confirmed by immunoblotting analysis with the anti-Flag epitope antibody M2 (Sigma) and anti-Syk polyAb. The effects of siRNA on expression of Syk were also confirmed by immunoblotting analysis with anti-Syk polyAb.
Phagocytosis assay
To opsonize zymosan with C3bi, complement activation cascade in serum was used. Zymosan A was incubated in 50% human serum at 37°C for 30 minutes in the presence or absence of compstatin or control peptide and then washed with PBS twice at 4°C. Binding of C3bi to zymosan was confirmed by flow cytometry with anti-C3bi antibody. For comparison, zymosan was also treated with PBS at 37°C or 50% human serum at 4°C. In some cases protein A coupled to agarose (Pierce, Rockford, IL) was used to remove IgG from human serum. Serum IgG concentration was measured by nephelometry using the IMMAGE Immunochemistry System (Beckman Coulter, Fullerton, CA). As a result of repeated treatment with protein A, IgG concentration in the treated serum was decreased from 12.5 mg/mL to the undetectable level.
C3bi-opsonized or nonopsonized zymosan was added to macrophage-like differentiated HL60 cells (cell–zymosan particle ratio, 1:10) and incubated for indicated times at 37°C in a 5% CO2 humidified atmosphere. In some cases, phagocytosis was recorded by a time-lapse microscope (Olympus IX71 microscope, Olympus, Tokyo, Japan; and CoolSNAPcf camera, Roper Scientific, Tucson, AZ) every 15 seconds for indicated times and analyzed by an image analyzing software, Mac SCOPE (Mitani, Fukui, Japan). In the case of quantitative analysis of phagocytosis assay, Texas Red zymosan (Sigma) was used similarly as described for zymosan A and analyzed by flow cytometry (FACSCalibur; Becton Dickinson, San Jose, CA). In some experiments, cells were treated with serum-opsonized or nonopsonized Texas Red zymosan for 10 minutes at 0°C or 37°C, washed with PBS, further incubated for 10 minutes at 37°C, and analyzed by flow cytometry.
To make a distinction whether zymosan particles exist inside or outside the cells, AlexaFluor 488 zymosan (Invitrogen) was opsonized and phagocytosis assay was performed, then the cells were analyzed by fluorescence microscopy before and after treatment of 0.2% trypan blue in PBS.
Analysis for actin accumulation around phagosomes
Phagocytosis assay was started, and then at 10 minutes after incubation with C3bi-zymosan, cells were washed with medium 2 times to remove unbound C3bi-zymosan and then reincubated for indicated times at 37°C in a 5% CO2 humidified atmosphere. Cells were fixed and then incubated with AlexaFluor 594–labeled phalloidin (Invitrogen) and observed with a confocal laser-scanning microscope (LSM 510 META; Carl Zeiss, Oberkochen, Germany).
Immunoprecipitation and immunoblotting
Cells were lysed with lysis buffer (0.05% SDS; 0.5% sodium deoxycholate; 50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 5 mM EDTA; 2 mM phenylmethylsulfonyl fluoride [PMSF]; 1 mM Na3VO4; 20 μg/mL aprotinin). Cellular debris was sonicated and removed by centrifugation. In some cases, cell lysate was immunoprecipitated with anti-Syk antibody or anti-Vav antibody at 4°C for 60 minutes and then incubated with protein A or G–Sepharose beads for 60 minutes, washed 3 times, and subjected to immunoblotting. For detection of tyrosine phosphorylation proteins, agarose-conjugated antiphosphotyrosine monoAb (clone 4G10) was directly used. Cell lysates or immunoprecipitated samples were separated in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA). The membrane was blocked with 5% skim milk in T-TBS (25 mM Tris-HCl, pH 8.0; 150 mM NaCl; 0.1% Tween 20) for 60 minutes at room temperature and then incubated with the appropriate antibodies. The membrane was washed 3 times with T-TBS and incubated with horseradish peroxidase–conjugated goat antirabbit or goat antimouse antibodies for 30 minutes, and specific proteins were detected using an enhanced chemiluminescence immunoblotting system.
Preparation of GST-rhotekin RBD proteins
For the preparation of GST-rhotekin Rho binding domain (RBD) proteins, RBD cDNA corresponding to the 7-89 amino acid residues of mouse rhotekin29 that were produced by PCR was incorporated into pGEX4T-3 and subsequently introduced into Escherichia coli DH5α. Expression of the proteins was induced with Isopropyl β-d-thiogalactopyranoside (IPTG; Nacalai Tesque), and the proteins were purified with GST-Sepharose beads.
GST pull-down assay for activated RhoA
GST pull-down assay was performed as previously described.30 Briefly, 2 × 106 cells were lysed in lysis buffer (50 mM Tris-HCl, pH 7.2; 1% Triton X-100; 100 mM NaCl; 10 mM MgCl2; and 2 mM PMSF). After centrifugation (15 000g, 15 minutes, 4°C), lysates were incubated with GST-rhotekin RBD immobilized on glutathione-Sepharose 4B beads (Amersham Biosciences, Uppsala, Sweden) for 60 minutes at 4°C. After washing, the proteins on the beads were run on SDS-PAGE. GTP-bound RhoA was detected by immunoblotting with anti-RhoA polyclonal antibody (Santa Cruz Biotechnology).
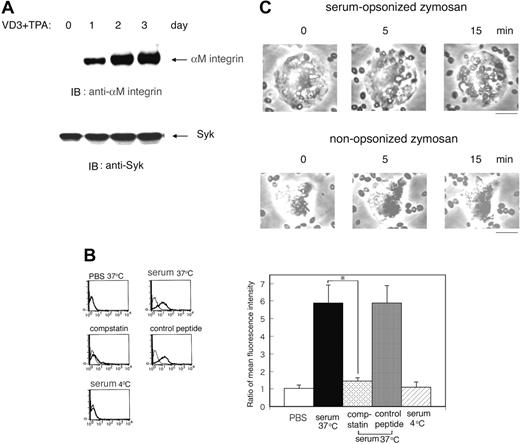
Complement-mediated phagocytosis using macrophage-like differentiated HL60 cells and serum-treated zymosan. (A) Expression of the CR3/integrin αM in macrophage-like differentiated HL60 cells. HL60 cells were treated with 10–7 M vitamin D3 (VD3) and 10 ng/mL TPA for indicated days and the expression of the CR3/integrin αM and Syk was examined by immunoblotting (IB) analysis with the corresponding antibodies. The blot is a representative of 3 independent experiments. (B) Binding of C3bi to zymosan. To opsonize zymosan with C3bi, zymosan was incubated in 50% human serum at 37°C for 30 minutes in the presence or absence of compstatin or control peptide and then washed with PBS twice at 4°C. For comparison, zymosan was also treated with PBS at 37°C for 30 minutes or in 50% human serum at 4°C. Binding of C3bi to zymosan was confirmed by flow cytometry with anti-C3bi antibody (thick line) or with control mouse IgG (thin line). The representative flow cytometric patterns (left) and the ratio of mean fluorescence intensity (anti-C3bi antibody/control IgG) with SD of triplicate experiments at the indicated conditions (right) are presented. The statistically significant difference was assessed by the Student t test; *P < .05. (C) Phagocytosis of zymosan particles by HL60 cells. Serum-opsonized (top panel) or nonopsonized zymosan (bottom panel) was added to macrophage-like differentiated Day3-HL60 cells (cell-zymosan ratio, 1:10) and incubated at 37°C. Phagocytosis was recorded by a time-lapse microscope every 15 seconds and analyzed by MacSCOPE image analyzing software. An LCPlan 20 ×/0.40 numeric aperture (NA) objective was used to visualize the images. The bar indicates 10 μm.
Complement-mediated phagocytosis using macrophage-like differentiated HL60 cells and serum-treated zymosan. (A) Expression of the CR3/integrin αM in macrophage-like differentiated HL60 cells. HL60 cells were treated with 10–7 M vitamin D3 (VD3) and 10 ng/mL TPA for indicated days and the expression of the CR3/integrin αM and Syk was examined by immunoblotting (IB) analysis with the corresponding antibodies. The blot is a representative of 3 independent experiments. (B) Binding of C3bi to zymosan. To opsonize zymosan with C3bi, zymosan was incubated in 50% human serum at 37°C for 30 minutes in the presence or absence of compstatin or control peptide and then washed with PBS twice at 4°C. For comparison, zymosan was also treated with PBS at 37°C for 30 minutes or in 50% human serum at 4°C. Binding of C3bi to zymosan was confirmed by flow cytometry with anti-C3bi antibody (thick line) or with control mouse IgG (thin line). The representative flow cytometric patterns (left) and the ratio of mean fluorescence intensity (anti-C3bi antibody/control IgG) with SD of triplicate experiments at the indicated conditions (right) are presented. The statistically significant difference was assessed by the Student t test; *P < .05. (C) Phagocytosis of zymosan particles by HL60 cells. Serum-opsonized (top panel) or nonopsonized zymosan (bottom panel) was added to macrophage-like differentiated Day3-HL60 cells (cell-zymosan ratio, 1:10) and incubated at 37°C. Phagocytosis was recorded by a time-lapse microscope every 15 seconds and analyzed by MacSCOPE image analyzing software. An LCPlan 20 ×/0.40 numeric aperture (NA) objective was used to visualize the images. The bar indicates 10 μm.
Statistical analysis
In some experiments, statistical significance was determined by the Student t test.
Results
Syk is tyrosine phosphorylated during complement-mediated phagocytosis in macrophage-like differentiated HL60 cells
We investigated the role of Syk in innate immunity, especially in complement-mediated phagocytosis, using macrophage-like differentiated HL60 cells incubated with vitamin D3 and TPA for 3 days as described.31 Three days thereafter, the cells became morphologically macrophage-like and the cell surface expression of CR3 (αMβ2 integrin/CD11bCD18) gradually increased (Figure 1A, top panel), whereas the amount of Syk remained unchanged (Figure 1A, bottom panel).
We studied the mechanism of phagocytosis mediated by complement activation using C3bi-opsonized zymosan, the membrane particles of Saccharomyces cerevisiae, in human serum at 37°C or 4°C. Activation of the complement pathway was then examined by flow cytometry using anti-C3bi antibody. C3bi bound to zymosan at 37°C but not at 4°C. In contrast, in the presence of a C3-convertase inhibitor, compstatin,25,26 binding of C3bi to zymosan was significantly suppressed but not in the presence of a control peptide (Figure 1B). Using complement-opsonized zymosan, we assayed phagocytosis on macrophage-like HL60 cells after incubation with vitamin D3 and TPA for 3 days (Day3-HL60). Microscopy showed that C3bi-opsonized, but not nonopsonized, zymosan particles promptly attached to and were absorbed by the cells (Figure 1C). Zymosan incubated with serum in the presence of compstatin or incubated at 4°C did not become attached (data not shown). In addition, to confirm that our methods are also adequate for primary phagocytes, we isolated human monocytes from the peripheral blood and performed phagocytosis assay. Primary monocytes also adsorbed C3bi-opsonized but not nonopsonized zymosan particles (data not shown).
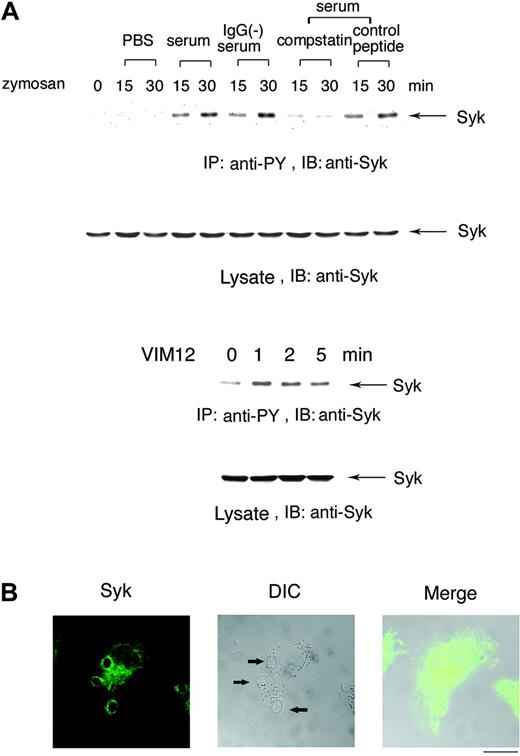
To determine whether Syk is involved in complement-mediated phagocytosis, Day3-HL60 cells were incubated with zymosan pretreated with PBS, human serum, IgG-removed serum, serum in the presence of compstatin, or a control peptide. Figure 2A (top panel) shows that Syk was tyrosine phosphorylated after incubation with zymosan pretreated with both serum and IgG-removed serum (C3bi-zymosan) but not with zymosan pretreated with either PBS or with serum in the presence of compstatin. Further, cross-linking with CR3-activating F(ab)2 antibody VIM12 caused tyrosine phosphorylation of Syk (Figure 2A, top panel). At 5 minutes after the onset of phagocytosis, Syk obviously accumulated in the region of forming phagosomes (Figure 2B).
Syk is tyrosine phosphorylated in the process of complement-mediated phagocytosis. (A) Tyrosine phosphorylation of Syk by the treatment with serum-opsonized zymosan. Macrophage-like differentiated Day3-HL60 cells were incubated with zymosan particles that were treated with PBS, serum, IgG-removed serum, serum containing compstatin (10 μM), or the control peptide to compstatin (10 μM) for 15 or 30 minutes (top). Macrophage-like differentiated Day3-HL60 cells were stimulated with CR3-activating F(ab)2 antibody VIM12 (2 μg/mL; bottom). Cell lysates were immunoprecipitated (IP) with antiphosphotyrosine (anti-PY) monoAb and immunoblotting analysis was performed with anti-Syk polyAb. As to the whole cell lysates, immunoblotting analysis was also done with anti-Syk polyAb. (B) Accumulation of Syk around the phagocytosed zymosan. At 5 minutes after the onset of phagocytosis, Day3-HL60 cells were fixed and stained with anti-Syk polyAb and AlexaFluor 488–conjugated secondary antibody. The arrows indicate the phagocytosed zymosan (the bar indicates 10 μm). A 63 ×/1.4 NA oil objective was used to visualize the images. DIC indicates differential interference contrast.
Syk is tyrosine phosphorylated in the process of complement-mediated phagocytosis. (A) Tyrosine phosphorylation of Syk by the treatment with serum-opsonized zymosan. Macrophage-like differentiated Day3-HL60 cells were incubated with zymosan particles that were treated with PBS, serum, IgG-removed serum, serum containing compstatin (10 μM), or the control peptide to compstatin (10 μM) for 15 or 30 minutes (top). Macrophage-like differentiated Day3-HL60 cells were stimulated with CR3-activating F(ab)2 antibody VIM12 (2 μg/mL; bottom). Cell lysates were immunoprecipitated (IP) with antiphosphotyrosine (anti-PY) monoAb and immunoblotting analysis was performed with anti-Syk polyAb. As to the whole cell lysates, immunoblotting analysis was also done with anti-Syk polyAb. (B) Accumulation of Syk around the phagocytosed zymosan. At 5 minutes after the onset of phagocytosis, Day3-HL60 cells were fixed and stained with anti-Syk polyAb and AlexaFluor 488–conjugated secondary antibody. The arrows indicate the phagocytosed zymosan (the bar indicates 10 μm). A 63 ×/1.4 NA oil objective was used to visualize the images. DIC indicates differential interference contrast.
Dominant-negative Syk and Syk-siRNA do not affect the complement receptor 3 expression
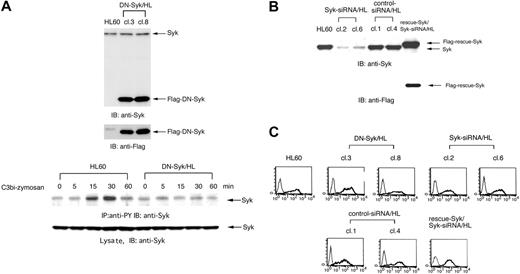
To further investigate the effect of Syk on the mechanism of complement-mediated phagocytosis, DN-Syk (a truncated form of human Syk that contains only tandem SH2 domains with no catalytic domain that interrupts endogenous active Syk kinase) or Syk-siRNA was introduced into HL60 cells, and cell clones stably expressing DN-Syk (DN-Syk/HL) and cell clones stably expressing Syk-siRNA (Syk-siRNA/HL) were isolated. Further, to support the effects of Syk-siRNA, Flag-rescue–Syk was transferred into Syk-siRNA/HL clone 6 (cl6) cells and stable mutant was isolated. The expression was confirmed by immunoblotting analyses (Figure 3A-B). In DN-Syk/HL, tyrosine phosphorylation of Syk induced by C3bi-zymosan was suppressed (Figure 3A, bottom panel). Introduction of Syk-siRNA significantly reduced the expression of endogenous Syk (clone 2, 85%; clone 6, 80% suppression) but transfer of Flag-rescue–Syk showed the restoration of Syk expression (Figure 3B). Flow cytometry showed that the level of CR3 surface expression in these cell clones was identical to that of parental Day3-HL60 cells (Figure 3C).
Transfer of either DN-Syk or Syk-siRNA resulted in the inhibition of phagocytosis
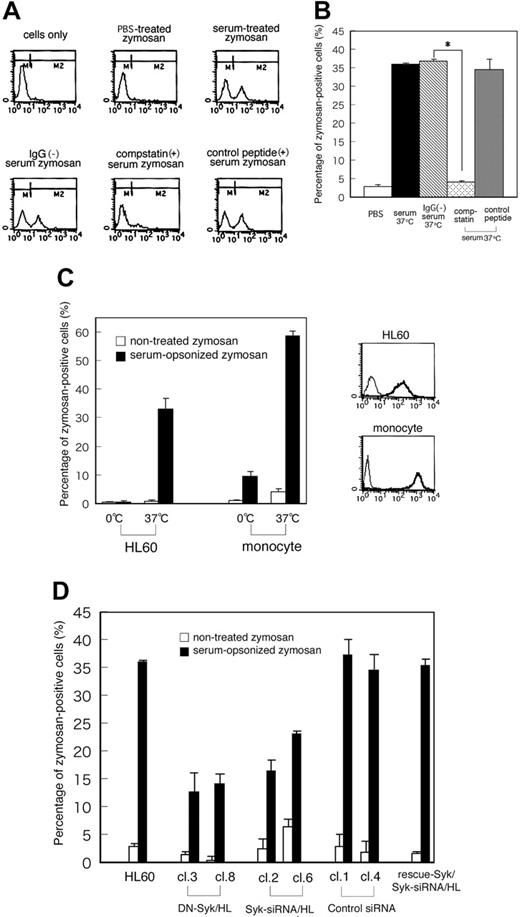
To determine whether Syk is required for complement-mediated phagocytosis, we analyzed phagocytic activity using fluorescence-labeled zymosan. Day3-HL60 cells were incubated with zymosan particles that were pretreated with PBS, serum, IgG-removed serum, serum containing compstatin (10 μM), or the control peptide to compstatin (10 μM) for 30 minutes at 37°C and then analyzed by flow cytometry to detect phagocytosis of fluorescent zymosan. Figure 4A shows the representative patterns of flow cytometry and Figure 4B shows the result of quantitative analysis. The fluorescence intensity was increased and shifted to the M2 region in the cells incubated with C3bi-zymosan but not in those with PBS-treated zymosan. Removal of IgG from the serum revealed the similar phagocytosis of C3bi-zymosan, whereas this phagocytosis was clearly inhibited in the presence of compstatin but not in the presence of a control peptide. These results indicated that C3bi-CR3 binding in this system was specific. Next, phagocytic activity was analyzed in primary monocytes and macrophage-like differentiated HL60 cells under the 2 binding conditions of C3bi-zymosan to CR3: at 0°C or 37°C before incubation at 37°C. In both cells the percentage of C3bi-zymosan–positive cells increased depending upon C3bi binding at 37°C (Figure 4C). The effects of DN-Syk and Syk-siRNA on phagocytic activity were analyzed using C3bi-opsonized zymosan. About 30% of parental Day3-HL60 cells were zymosan positive but both the transfer of DN-Syk and the transfer of Syk-siRNA reduced this ratio to 10% to 20%. Transfer of control siRNA did not affect the phagocytosis, and transfer of Flag-rescue–Syk restored the phagocytosis (Figure 4D). These data showed that Syk plays a significant role in the process of C3bi-CR3–mediated phagocytosis.
Syk is essential for pathogen engulfment rather than for attachment via complement receptor
To determine whether fluorescent zymosan particles are intracellularly or extracellularly located, the cells were stained with trypan blue, which quenches the fluorescence of extracellular zymosan.32 After the phagocytosis assay with C3bi-opsonized fluorescent zymosan, the cells were analyzed before and after trypan blue staining by fluorescence microscopy and fluorescent zymosan particles were counted.
Neither DN-Syk nor Syk-siRNA affects the expression of the complement receptor CR3. DN-Syk was transferred into HL60 cells and stable mutant clones (DN-Syk/HL, cl3 and cl8) were isolated. Syk-siRNA or control siRNA was also transferred into HL60 cells and stable mutant clones (Syk-siRNA/HL, cl2 and cl6; control siRNA/HL, cl1 and cl4) were isolated. Flag-rescue–Syk was transferred into Syk-siRNA/HL cl6 and stable mutant was isolated. Protein expression was examined in these mutant clones and parental HL60 cells. (A) Expression of transferred Flag-tagged DN-Syk protein in DN-Syk/HL (cl3 and cl8) cells was confirmed by immunoblotting analysis using anti-Syk polyAb and anti-Flag monoAb (top panel). Macrophage-like differentiated Day3-HL60 and DN-Syk/HL cells were incubated with serum-treated zymosan. Cell lysates were immunoprecipitated with antiphosphotyrosine monoAb and immunoblotting analysis was performed with anti-Syk polyAb. As to the whole cell lysates, immunoblotting analysis was also done with anti-Syk polyAb (bottom panel). (B) Expression of endogenous Syk was examined in Syk-siRNA/HL (cl2 and cl6), control siRNA/HL (cl1 and cl4), and HL60 cells by immunoblotting analysis using anti-Syk polyAb (top panel). Expression of Flag-rescue–Syk was examined by immunoblotting analysis using anti-Syk polyAb and anti-Flag monoAb (bottom panel). (C) Cell-surface expression of CR3 (integrin αMβ2) on macrophage-like differentiated Day3-HL60, DN-Syk/HL (cl3 and cl8), Syk-siRNA/HL (cl2 and cl6), control siRNA/HL (cl1 and cl4), and Flag-rescue–Syk/Syk-siRNA/HL cells were analyzed by flow cytometry with anti-CR3 monoAb (thick line) or with control mouse IgG (thin line).
Neither DN-Syk nor Syk-siRNA affects the expression of the complement receptor CR3. DN-Syk was transferred into HL60 cells and stable mutant clones (DN-Syk/HL, cl3 and cl8) were isolated. Syk-siRNA or control siRNA was also transferred into HL60 cells and stable mutant clones (Syk-siRNA/HL, cl2 and cl6; control siRNA/HL, cl1 and cl4) were isolated. Flag-rescue–Syk was transferred into Syk-siRNA/HL cl6 and stable mutant was isolated. Protein expression was examined in these mutant clones and parental HL60 cells. (A) Expression of transferred Flag-tagged DN-Syk protein in DN-Syk/HL (cl3 and cl8) cells was confirmed by immunoblotting analysis using anti-Syk polyAb and anti-Flag monoAb (top panel). Macrophage-like differentiated Day3-HL60 and DN-Syk/HL cells were incubated with serum-treated zymosan. Cell lysates were immunoprecipitated with antiphosphotyrosine monoAb and immunoblotting analysis was performed with anti-Syk polyAb. As to the whole cell lysates, immunoblotting analysis was also done with anti-Syk polyAb (bottom panel). (B) Expression of endogenous Syk was examined in Syk-siRNA/HL (cl2 and cl6), control siRNA/HL (cl1 and cl4), and HL60 cells by immunoblotting analysis using anti-Syk polyAb (top panel). Expression of Flag-rescue–Syk was examined by immunoblotting analysis using anti-Syk polyAb and anti-Flag monoAb (bottom panel). (C) Cell-surface expression of CR3 (integrin αMβ2) on macrophage-like differentiated Day3-HL60, DN-Syk/HL (cl3 and cl8), Syk-siRNA/HL (cl2 and cl6), control siRNA/HL (cl1 and cl4), and Flag-rescue–Syk/Syk-siRNA/HL cells were analyzed by flow cytometry with anti-CR3 monoAb (thick line) or with control mouse IgG (thin line).
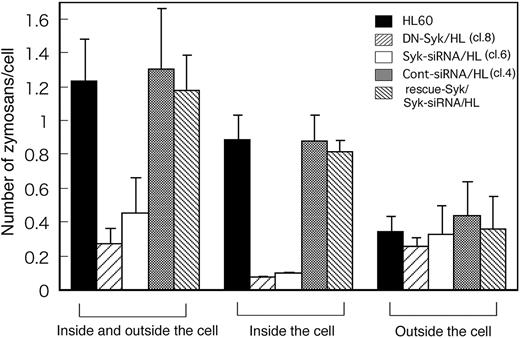
Figure 5 shows that before quenching, Day3-HL60, DN-Syk/HL, Syk-siRNA/HL, control siRNA/HL, and Flag-rescue–Syk/Syk-siRNA/HL cells captured 1.24 ± 0.24, 0.28 ± 0.08, 0.46 ± 0.20, 1.31 ± 0.35, and 1.18 ± 0.21 C3bi-opsonized zymosan particles per cell, respectively. To determine whether capture was impaired due to reduced binding to CR3 or reduced engulfment after binding, cells were stained with trypan blue and only intracellular zymosan particles were counted. The numbers of C3bi-opsonized zymosan particles inside the parental Day3-HL60, DN-Syk/HL, Syk-siRNA/HL, control siRNA/HL, and Flag-rescue–Syk/Syk-siRNA/HL cells were 0.9 ± 0.2, 0.1 ± 0, 0.1 ± 0, 0.9 ± 0.2, and 0.8 ± 0.1 per cell, respectively. These results indicated that most of the C3bi-zymosan attached to the parental HL60 cells was rapidly engulfed and that the number of C3bi-zymosan outside the cells was approximately the same among the parental HL60 cells, DN-Syk/HL cells, and Syk-siRNA/HL cells (Figure 5). These data indicated that the defective complement-mediated phagocytosis of DN-Syk/HL cells and Syk-siRNA/HL cells was not due to decreased C3bi binding to CR3 but rather to loss of internalization of the CR3-bound zymosan.
Syk affects actin dynamics around the C3bi-mediated phagosomes
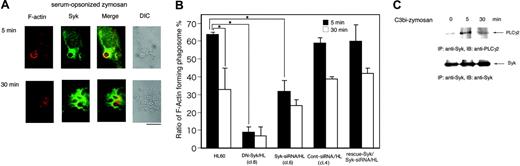
Because the engulfment of particles by phagocytosis is induced by actin polymerization at the forming phagosome, we analyzed the effect of Syk on actin dynamics during complement-mediated phagocytosis. To synchronize phagocytosis, cells were incubated with C3bi-zymosan for 10 minutes, washed once, and then the phagocytic reaction was restarted. At 5 minutes after synchronization, actin fibers obviously accumulated in the region of the forming phagosome that surrounded the C3bi-zymosan particles in HL60 cells (Figure 6A) as reported.5 However, Syk also accumulated at the same time point around phagosomes (Figure 6A-B). At 30 minutes after synchronization, actin began to disassemble, but the accumulation of Syk around phagosomes was sustained (Figure 6A-B).
To understand the role of Syk in actin dynamics, we analyzed actin accumulation around the phagosomes of the parental Day3-HL60, DN-Syk/HL, Syk-siRNA/HL, control siRNA/HL, and Flagrescue–Syk/Syk-siRNA/HL cells. In both DN-Syk/HL cells and Syk-siRNA/HL cells, the numbers of phagosomes surrounded by actin were clearly decreased at 5 minutes after synchronization, but the accumulation of actin was sustained even at 30 minutes. Transfer of control siRNA did not affect the actin accumulation around the phagosomes and transfer of Flag-rescue–Syk restored the accumulation (Figure 6B).
Syk activates the RhoA pathway in C3bi-CR3 signaling
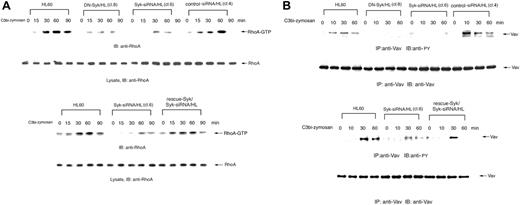
Rho family GTPases are thought to control actin polymerization and the extension of membrane protrusions to form a closed phagosome. We therefore further analyzed whether Syk regulates RhoA signaling in complement-mediated phagocytosis using GST-rhotekin RBD pull-down assays to detect GTP-bound RhoA. Incubation of Day3-HL60 cells with C3bi-opsonized zymosan led to the prompt activation of RhoA but transfer of DN-Syk or Syk-siRNA suppressed RhoA activation, whereas transfer of control siRNA did not affect RhoA activation and transfer of Flag-rescue–Syk restored the activation (Figure 7A), indicating that Syk acts as an activator of the RhoA pathway in C3bi-CR3 signaling, which might lead to engulfment of the particles.
Transfer of DN-Syk or Syk-siRNA results in the inhibition of phagocytosis. Macrophage-like differentiated Day3-HL60 cells were incubated with zymosan particles that were pretreated with PBS, serum, IgG-removed serum, serum containing compstatin (10 μM), or the control peptide to compstatin (10 μM) for 30 minutes at 37°C and then analyzed by flow cytometry to detect phagocytosis of fluorescent zymosan. (A) Representative histogram patterns of the treated cells by flow cytometry are shown. M2 region includes zymosan-positive cells. (B) The percentage of zymosan-positive cells (percentage of M2 region as shown in panel A) in parental HL60 cells incubated with zymosan particles that were pretreated with serum, IgG-removed serum, serum containing compstatin, or the control peptide to compstatin is presented. The mean values and SD of triplicate experiments are shown. The statistically significant difference was assessed by the Student t test; *P < .05. (C) The percentage of zymosan-positive cells (percentage of M2 region shown in panel A) in HL60 cells and primary monocytes is presented. Cells were treated with serum-opsonized or nonopsonized fluorescent zymosan for 10 minutes at 0°C or 37°C, washed with PBS, and further incubated for 10 minutes at 37°C. The mean values and SD of triplicate experiments are shown (left panel). Cell-surface expression of CR3 (integrin αMβ2) on macrophage-like differentiated Day3-HL60 cells and primary monocytes was analyzed by flow cytometry with anti-CR3 monoAb (thick line) or with control mouse IgG (thin line; right panel). (D) The percentage of zymosan-positive cells (percentage of M2 region shown in panel A) in HL60 cells and the mutant clones treated with serum-opsonized or nonopsonized fluorescent zymosan for 30 minutes at 37°C is presented. The mean values and SD of triplicate experiments are shown.
Transfer of DN-Syk or Syk-siRNA results in the inhibition of phagocytosis. Macrophage-like differentiated Day3-HL60 cells were incubated with zymosan particles that were pretreated with PBS, serum, IgG-removed serum, serum containing compstatin (10 μM), or the control peptide to compstatin (10 μM) for 30 minutes at 37°C and then analyzed by flow cytometry to detect phagocytosis of fluorescent zymosan. (A) Representative histogram patterns of the treated cells by flow cytometry are shown. M2 region includes zymosan-positive cells. (B) The percentage of zymosan-positive cells (percentage of M2 region as shown in panel A) in parental HL60 cells incubated with zymosan particles that were pretreated with serum, IgG-removed serum, serum containing compstatin, or the control peptide to compstatin is presented. The mean values and SD of triplicate experiments are shown. The statistically significant difference was assessed by the Student t test; *P < .05. (C) The percentage of zymosan-positive cells (percentage of M2 region shown in panel A) in HL60 cells and primary monocytes is presented. Cells were treated with serum-opsonized or nonopsonized fluorescent zymosan for 10 minutes at 0°C or 37°C, washed with PBS, and further incubated for 10 minutes at 37°C. The mean values and SD of triplicate experiments are shown (left panel). Cell-surface expression of CR3 (integrin αMβ2) on macrophage-like differentiated Day3-HL60 cells and primary monocytes was analyzed by flow cytometry with anti-CR3 monoAb (thick line) or with control mouse IgG (thin line; right panel). (D) The percentage of zymosan-positive cells (percentage of M2 region shown in panel A) in HL60 cells and the mutant clones treated with serum-opsonized or nonopsonized fluorescent zymosan for 30 minutes at 37°C is presented. The mean values and SD of triplicate experiments are shown.
The Vav family of guanine nucleotide exchange factors (GEFs) plays an essential role in coupling integrin signaling with Rho GTPases.33 We therefore analyzed tyrosine phosphorylation of the Vav proteins in complement-mediated phagocytosis in Day3-HL60 and mutant cells. Tyrosine phosphorylation of Vav was obviously increased in HL60 cells but was attenuated in DN-Syk/HL and Syk-siRNA/HL cells. Transfer of control siRNA did not affect tyrosine phosphorylation of Vav and transfer of Flag-rescue–Syk restored the phosphorylation (Figure 7B). These results suggest that Vav acts as a GEF downstream of Syk-mediated CR3 (αMβ2 integrin) signaling.
Discussion
Studies of Syk-deficient murine macrophages have found that Syk plays a central role in Fcγ receptor–mediated phagocytosis.12,13 Syk also plays a crucial role in multiple integrin-mediated effector functions of the innate immune system.15 Considering that the main complement receptor CR3 is integrin αMβ2, we investigated whether Syk is required for complement-mediated phagocytosis using macrophage-like differentiated HL60 cells. First, we determined that our system is based on binding between C3bi and its receptor CR3. We examined the binding of C3bi to zymosan by flow cytometry and observed the process of phagocytosis by time-lapse microscopy. The results confirmed that incubating zymosan with human serum at 37°C but not at 4°C resulted in C3bi binding to zymosan and that only C3bi-bound zymosan induced accelerated phagocytosis in Day3-HL60 cells (Figure 1B-C; data not shown). In addition, the serum-induced phagocytosis was not affected by the removal of IgG from serum but was completely inhibited by the pretreatment of the complement-specific inhibitor compstatin (Figure 4A-B). These results indicated that our phagocytosis system is exclusively mediated by C3bi binding to CR3 and not by the Fcγ receptor.
We then generated mutant HL60 cell clones stably expressing DN-Syk or Syk-siRNA and analyzed the effects of Syk inhibition on complement-mediated phagocytosis. Decreased phagocytosis of C3bi-zymosan in DN-Syk– or Syk-siRNA–expressing mutant cells (Figure 4D) indicated that Syk is required for complement-mediated phagocytosis. The previous report12 showed that Syk is required only for Fcγ receptor–but not for CR3-mediated phagocytosis. Binding study of C3bi-zymosan to CR3 was performed at a lower temperature in their system,12 unlike our data obtained at 37°C. Considering that binding of C3bi to CR3 (integrin αMβ2) is temperature dependent,34 our experimental condition may be appropriate for studying the behavior of integrin receptor CR3-mediated phagocytosis. In fact, phagocytic activity of C3bi-zymosan was absolutely higher when the binding was performed at 37°C than at 0°C in both primary monocytes and macrophage-like differentiated HL60 cells (Figure 4C). But there may be some differences between cells used because Kiefer et al used murine macrophages.12 In addition, difference of determination methods may have affected the results. They determined phagocytic activity by coengulfment of lucifer yellow with zymosan12 but we directly analyzed fluorescent zymosan.
Syk is essential for the engulfment of zymosan but not for its attachment to CR3. Macrophage-like differentiated Day3-HL60, DN-Syk/HL (clone 8), Syk-siRNA/HL (clone 6), control siRNA/HL (clone 4), or Flag-rescue–Syk/Syk-siRNA/HL cells were treated with serum-opsonized fluorescent zymosan for 30 minutes at 37°C and observed with a fluorescence microscope before and after trypan blue staining. The cells treated with zymosan were examined by the quenching assay described above. In each culture plate, more than 100 cells and total zymosan particles (inside and outside the cell; seen before quenching) and bright zymosan particles (inside the cell; seen after quenching) were counted and the average number of zymosan particles per cell was calculated together with SD of triplicate experiments. The counts of outside the cell were obtained as the result of subtraction (inside and outside minus inside) in each plate.
Syk is essential for the engulfment of zymosan but not for its attachment to CR3. Macrophage-like differentiated Day3-HL60, DN-Syk/HL (clone 8), Syk-siRNA/HL (clone 6), control siRNA/HL (clone 4), or Flag-rescue–Syk/Syk-siRNA/HL cells were treated with serum-opsonized fluorescent zymosan for 30 minutes at 37°C and observed with a fluorescence microscope before and after trypan blue staining. The cells treated with zymosan were examined by the quenching assay described above. In each culture plate, more than 100 cells and total zymosan particles (inside and outside the cell; seen before quenching) and bright zymosan particles (inside the cell; seen after quenching) were counted and the average number of zymosan particles per cell was calculated together with SD of triplicate experiments. The counts of outside the cell were obtained as the result of subtraction (inside and outside minus inside) in each plate.
Syk affects actin dynamics around the C3bi-mediated phagosomes. Macrophage-like differentiated Day3-HL60, DN-Syk/HL (clone 8), Syk-siRNA/HL (clone 6), control siRNA/HL (clone 4), or Flag-rescue–Syk/Syk-siRNA/HL cells were incubated with serum-opsonized zymosan for indicated times and treated for morphologic studies (A-B) and immunoblotting analysis (C). (A) The parental HL60 cells were washed twice with culture medium and incubated for an additional 5 minutes or 30 minutes. The cells were fixed, stained with anti-Syk polyAb followed by AlexaFluor 488–labeled secondary antibody (green) and AlexaFluor 594–labeled phalloidin (red), and observed with a confocal laser-scanning microscope. Zymosan-containing phagosomes surrounded by F-actin are presented. A 63 ×/1.4 NA oil objective was used to visualize the images. DIC indicates differential interference contrast. (B) Zymosan-containing phagosomes existing in Day3-HL60, DN-Syk/HL, Syk-siRNA/HL, control siRNA/HL, or Flag-rescue–Syk/Syk-siRNA/HL cells were counted and the percentage of the phagosomes surrounded by F-actin to total phagosomes was calculated. One hundred cells were examined. Mean values and SD of triplicate experiments are shown. The statistically significant difference was assessed by the Student t test; *P < .05. (C) The cell lysates were immunoprecipitated with anti-Syk monoAb, and coprecipitated PLCγ2 was detected by immunoblotting analysis with anti-PLCγ2 polyAb. The reprobing analysis was also done with anti-Syk polyAb.
Syk affects actin dynamics around the C3bi-mediated phagosomes. Macrophage-like differentiated Day3-HL60, DN-Syk/HL (clone 8), Syk-siRNA/HL (clone 6), control siRNA/HL (clone 4), or Flag-rescue–Syk/Syk-siRNA/HL cells were incubated with serum-opsonized zymosan for indicated times and treated for morphologic studies (A-B) and immunoblotting analysis (C). (A) The parental HL60 cells were washed twice with culture medium and incubated for an additional 5 minutes or 30 minutes. The cells were fixed, stained with anti-Syk polyAb followed by AlexaFluor 488–labeled secondary antibody (green) and AlexaFluor 594–labeled phalloidin (red), and observed with a confocal laser-scanning microscope. Zymosan-containing phagosomes surrounded by F-actin are presented. A 63 ×/1.4 NA oil objective was used to visualize the images. DIC indicates differential interference contrast. (B) Zymosan-containing phagosomes existing in Day3-HL60, DN-Syk/HL, Syk-siRNA/HL, control siRNA/HL, or Flag-rescue–Syk/Syk-siRNA/HL cells were counted and the percentage of the phagosomes surrounded by F-actin to total phagosomes was calculated. One hundred cells were examined. Mean values and SD of triplicate experiments are shown. The statistically significant difference was assessed by the Student t test; *P < .05. (C) The cell lysates were immunoprecipitated with anti-Syk monoAb, and coprecipitated PLCγ2 was detected by immunoblotting analysis with anti-PLCγ2 polyAb. The reprobing analysis was also done with anti-Syk polyAb.
Syk activates the RhoA pathway in C3bi-CR3 signaling. Macrophage-like differentiated Day3-HL60, DN-Syk/HL (clone 8), Syk-siRNA/HL (clone 6), control siRNA/HL (clone 4), or Flag-rescue–Syk/Syk-siRNA/HL cells were incubated with serum-opsonized zymosan for indicated times and immunoblotting analysis was performed. (A) The cell lysates were incubated with GST-rhotekin RBD immobilized on glutathione-Sepharose. GTP-bound RhoA was detected by immunoblotting analysis with anti-RhoA polyAb. The same amounts of cell lysates were also immunoblotted with anti-RhoA polyAb. (B) The cell lysates were immunoprecipitated with anti-Vav polyAb and immunoblotted with antiphosphotyrosine monoAb. The reprobing analysis was also done with anti-Vav polyAb. These blots are representative of 3 independent experiments.
Syk activates the RhoA pathway in C3bi-CR3 signaling. Macrophage-like differentiated Day3-HL60, DN-Syk/HL (clone 8), Syk-siRNA/HL (clone 6), control siRNA/HL (clone 4), or Flag-rescue–Syk/Syk-siRNA/HL cells were incubated with serum-opsonized zymosan for indicated times and immunoblotting analysis was performed. (A) The cell lysates were incubated with GST-rhotekin RBD immobilized on glutathione-Sepharose. GTP-bound RhoA was detected by immunoblotting analysis with anti-RhoA polyAb. The same amounts of cell lysates were also immunoblotted with anti-RhoA polyAb. (B) The cell lysates were immunoprecipitated with anti-Vav polyAb and immunoblotted with antiphosphotyrosine monoAb. The reprobing analysis was also done with anti-Vav polyAb. These blots are representative of 3 independent experiments.
The involvement of Syk in Dectin-1 (β-glucan receptor)–mediated phagocytosis has recently been defined together with the role of Syk in cytokine induction or reactive oxygen production in the system.35,36 However, other studies indicated that Dectin-1–mediated phagocytosis is independent of Syk.37 Since β-glucans are the major carbohydrate components of the yeast cell wall including zymosan, we could not eliminate the possibility that Dectin-1 is involved in our system. Judging from the quantitative analysis of phagocytosis by microscopic observation and flow cytometry (Figures 1C and 4), Dectin-1 seemed to have little impact. Nonetheless, the affinity of C3bi for CR3 was probably so high that our system might hardly detect the phagocytosis of nonopsonized zymosan by the β-glucan–Dectin-1 pathway. In fact, after longer incubation (more than 4 hours) of the cells with nonopsonized zymosan, slight uptake of zymosan was detected (data not shown). Therefore, Syk might play an essential role in both CR3- and Dectin-1–mediated phagocytosis in innate immunity in vivo, but our experimental system exclusively focused upon phagocytosis mediated by C3bi-CR3.
We further investigated the critical step where Syk plays an indispensable role in the process of CR3-mediated phagocytosis. In Figure 3C, we certainly showed that almost the same number of CR3 molecules exist on the cell surface among several mutants, but even if the same number of CR3 are expressed on the surface, the different change in the affinity or avidity of integrin αMβ2 might occur. In addition, once phagocytosis starts by the C3bi-CR3 binding, turnover of CR3 molecules begins dynamically such as internalization of CR3 by the engulfment of CR3-bound zymosan or delivery of the cytoplasmic membrane components. Syk might affect the affinity, avidity, the process of engulfment, or dynamic turnover of CR3. Figure 5 shows that Syk plays a crucial role in the engulfment of C3bi-zymosan in complement-mediated phagocytosis, but the precise molecular mechanism that leads to pathogen engulfment downstream of Syk remains to be determined.
A recent study has reported that both actin assembly for the formation of nascent phagosomes and disassembly for proper sealing of phagosomes are required for phagosome completion.5 Hoppe and Swanson38 analyzed the detailed kinetics of Cdc42 and Rac1 during Fcγ receptor–mediated phagocytosis and showed that the activation of both Cdc42 and Rac1 occur early at the tips of extending pseudopodia. But actin disassembly does not correlate with loss of Rac1 and Cdc42 activity.3,5 We then observed the assembly of actin around the phagosomes during complement-mediated phagocytosis (Figure 6A-B). In agreement with the previous report, actin accumulated around the phagosomes at the early stage and decreased at 30 minutes (Figure 6A-B). Syk also accumulated around the phagosomes at the early stage (Figures 2 and 6A) and remained there until actin disassembly. As recent reports suggest that the conversion of PI (4,5) P2 to other chemical species contributes to the termination of actin assembly during the late stage of phagosome completion5 and because Syk is the main tyrosine kinase that activates PLCγ in Fc receptors, Syk might initiate actin disassembly upstream of PLCγ, facilitate phagosome enclosure, and support the release of the phagosomes from just beneath the membrane. In fact, PLCγ2 rapidly associated with Syk after the onset of C3bi-CR3–mediated phagocytosis (Figure 6C).
Next, we analyzed the activation of RhoA signaling to further understand the mechanism through which cells engulf C3bi-bound zymosan. RhoA controls retraction via actin-myosin contractility.39 With respect to phagocytosis, Olazabal et al40 reported that the Rho effector ROCK controls phagocytic cup formation mediated by complement receptor but not by Fcγ receptor and that inhibition of the Rho-ROCK myosin II pathway decreases accumulation of the Arp2/3 complex and leads to a reduction in complement receptor–mediated phagocytic engulfment. In addition, recent reports have shown that the Vav proteins, Rho guanine nucleotide exchange factors, play an essential role in coupling integrin β2 to Rho GTPases and regulate multiple integrin-induced events including phagocytosis.33 Figure 7A-B indicates that the transfer of DN-Syk or Syk-siRNA into HL60 cells remarkably reduced both the activity of RhoA and the phosphorylation of Vav in complement-mediated phagocytosis and that Syk is a crucial upstream regulator of Vav-RhoA signaling that generates contractile force. Taken together, both the PLCγ2 and RhoA pathways are under the control of Syk in complement-mediated phagocytosis.
Together with the previous reports, we therefore propose a mechanism of complement-mediated phagocytosis as follows. (1) The ligation of pathogen-bound C3bi to the receptor CR3 directly aggregates CR3 (integrin αMβ2); this ligation induces actin polymerization at the forming phagosome that might be controlled by GTPases of the Rho family Rac1 and Cdc42 (not RhoA), which are known to be essential for the extension of the pseudopods around the phagocytic particle in Fcγ receptor–mediated phagocytosis.3,38 (2) After the accumulation of actin, disappearance of PI (4,5) P2 after hydrolysis by PLCγ2 has a critical role in the termination of actin assembly and in disassembly from the phagocytic cup that liberates elements of the cytoskeletal machinery for assembly elsewhere.5 (3) Contractile force created by the downstream of Vav-RhoA signaling might engulf the enclosed phagosome.
Namely, the ligation of pathogen-bound C3bi to the receptor CR3 directly aggregates CR3 (integrin αMβ2) and this ligation induces the formation of the signaling complex composed of CR3/integrin αMβ2, Syk, and adaptor proteins that bind these signaling molecules to the cytoskeletal proteins. In this complex, Syk surely acts as a key player and activates both PLCγ2 and Vav-RhoA pathways. Further investigation would clarify precisely how Syk regulates these pathways, resulting in engulfment.
In conclusion, we have demonstrated that Syk plays an indispensable role in complement-mediated phagocytosis by regulating both actin dynamics and the Vav-RhoA activation pathway and that these functions of Syk lead to phagosome formation and pathogen engulfment.
Prepublished online as Blood First Edition Paper, January 31, 2006; DOI 10.1182/blood-2005-09-3616.
Supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, the 21st Century Centers of Excellence (COE) Program of the Ministry of Education, and the Osaka Medical Research Foundation for Incurable Diseases.
Y.S., T.K., J.H., S.M.S.M., R.H., and C.T. performed research; Y.T. designed research, analyzed data, and wrote the paper; K.T. analyzed data; and H.Y. organized this research project and analyzed data.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.