Abstract
Pulmonary hypertension is prevalent in adult patients with sickle cell disease and is strongly associated with early mortality and markers of hemolysis, in particular, serum lactate dehydrogenase (LDH). Intravascular hemolysis leads to impaired bioavailability of nitric oxide (NO), mediated by NO scavenging by plasma oxyhemoglobin and by arginine degradation by plasma arginase. We hypothesized that serum LDH may represent a convenient biomarker of intravascular hemolysis and NO bioavailability, characterizing a clinical subphenotype of hemolysis-associated vasculopathy. In a cohort of 213 patients with sickle cell disease, we found statistically significant associations of steady-state LDH with low levels of hemoglobin and haptoglobin and high levels of reticulocytes, bilirubin, plasma hemoglobin, aspartate aminotransferase, arginase, and soluble adhesion molecules. LDH isoenzyme fractionation confirmed predominance of LD1 and LD2, the principal isoforms within erythrocytes. In a subgroup, LDH levels closely correlated with plasma cell-free hemoglobin, accelerated NO consumption by plasma, and impaired vasodilatory responses to an NO donor. Remarkably, this simple biomarker was associated with a clinical subphenotype of pulmonary hypertension, leg ulceration, priapism, and risk of death in patients with sickle cell disease. We propose that LDH elevation identifies patients with a syndrome of hemolysis-associated NO resistance, endothelial dysfunction, and end-organ vasculopathy.
Introduction
Pulmonary hypertension is an increasingly recognized complication of sickle cell disease and other chronic hereditary and acquired hemolytic anemias. Doppler echocardiography screening reveals a tricuspid regurgitant jet velocity (TRV) of 2.5 m/s or greater in 33% of adults with sickle cell disease, indicative of pulmonary hypertension.1 Pulmonary hypertension in these patients is associated with a 10-fold increased risk for early mortality. Elevated pulmonary artery pressures in patients with sickle cell disease have been associated with low hemoglobin concentration, high levels of serum lactate dehydrogenase (LDH), elevated systolic systemic blood pressure, history of priapism, renal insufficiency, and markers of iron overload. Of the several independent epidemiologic factors associated with pulmonary hypertension, an elevated level of serum LDH has drawn particular interest and controversy. In addition to a link with pulmonary hypertension, we and others have observed a link between LDH and a generalized state of endothelial activation, reflected by elevated blood plasma levels of soluble adhesion molecules, especially vascular cell adhesion molecule (VCAM-1).2-10 Both pathologic endothelial activation and pulmonary hypertension are associated with more severe hemolytic anemia, reflected by low hemoglobin levels and high reticulocyte counts.1,2,8 These data begin to suggest that LDH elevation may be a marker of hemolysis-associated endothelial dysfunction and pulmonary hypertension.
LDH has long been considered a useful clinical marker of intravascular hemolysis. Its serum levels are mildly elevated in extravascular hemolysis, such as immune hemolytic anemia, but are substantially elevated with intravascular hemolysis, such as thrombotic thrombocytopenic purpura and paroxysmal nocturnal hemoglobinuria.11 Although in sickle cell disease two thirds of hemolysis occurs extravascularly, the remaining one third of red cells hemolyze intravascularly, potentially releasing as much as 10 g hemoglobin per day into blood plasma.12 This robust hemolytic rate increases even further during vasoocclusive pain crisis (VOC). Elegant biochemical studies performed 35 years ago have demonstrated significant increases in serum LDH and plasma hemoglobin levels, the gold standard marker of intravascular hemolysis, during VOC.13,14 This hyper-hemolysis recently has been confirmed by labeled red blood cell studies that reveal further decreases in red blood cell survival during VOC in patients with sickle cell disease.15
We hypothesized that LDH elevation serves as a surrogate marker of intravascular hemolysis in patients with sickle cell disease, associated with decompartmentalization of erythrocyte hemoglobin and arginase into blood plasma. In this model, a state of nitric oxide (NO) resistance develops due to consumption of NO by plasma cell-free hemoglobin.16,17 Arginase depletes plasma arginine, the substrate for NO production by NO synthase, further compromising NO bioavailability.18-20 NO also appears to be consumed by reactive oxygen species generated by the high levels of xanthine oxidase activity and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity that accompany sickle cell disease.21-24 This state of reduced NO bioavailability is associated with impaired blood flow physiology in patients and mice with sickle cell disease, and also in healthy dogs with induced intravascular hemolytic anemia.17,25-30
Considering the strong epidemiologic associations between LDH elevation and both endothelial activation and pulmonary hypertension, and the mechanistic links between plasma hemoglobin level and endothelial dysfunction, we have hypothesized that serum LDH may be a clinically useful prognostic factor in patients with sickle cell disease. In addition, LDH elevation may serve as an indirect marker of a hemolysis-endothelial dysfunction syndrome that may characterize a clinical subphenotype of patients with sickle cell disease. We hypothesized that the clinical features of this syndrome would include pulmonary hypertension, cutaneous leg ulceration, priapism, and risk of death.
Patients, materials, and methods
Patient population
The patient population consists of the 195 patients with sickle cell disease characterized in a previous report, supplemented with continued follow-up, and by an additional 18 patients enrolled since the date of that report.1 All patients provided written informed consent, in accordance with the Declaration of Helsinki, for research protocols approved by the institutional review boards of the National Institutes of Health (NIH). Eligibility criteria included all forms of sickle cell disease, with patients at least 18 years of age. All patients provided medical histories, using a standardized questionnaire, provided blood samples, and underwent physical examination and Doppler echocardiography. The details of the echocardiographic examination have been described and included 3-chamber view assessment of the TRV.1 Clinical laboratory testing was performed in the Clinical Center of the NIH. Transcutaneous oxygen saturation was measured by standard clinical pulse oximeter devices. Patients were contacted at least every 6 months to document continued survival, and copies of death certificates were obtained to document dates of deaths. The cutoff date for survival analysis was May 1, 2005.
A subset of 33 patients from this cohort consented to additional blood sampling at baseline or during VOC pain crisis for measurement of LDH isoenzymes and other clinical laboratory testing. A separate subset of 26 patients from this cohort volunteered for studies of forearm blood flow by strain gauge venous occlusion plethysmography. The characteristics of this protocol and other data from the overlapping patient cohort have been reported elsewhere.17 Briefly, a catheter was placed in the brachial artery, and blood was obtained by gentle withdrawal in a syringe and transferred to heparinized tubes for low-speed centrifugation without braking. The intra-arterial catheter was connected to an infusion pump that delivered 5% dextrose-in-water at 0.5 mL/min. After 20 minutes of rest, baseline forearm blood flow was measured. Sodium nitroprusside was infused at 0.8, 1.6, and 3.2 μg/min, each for 5 minutes. After 3 minutes of each infusion dose, forearm blood flows were measured.
ELISA
Plasma hemoglobin was measured on dilutions of patients' plasma using an enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's directions (Bethyl Laboratories, Montgomery, TX). Soluble VCAM-1, intercellular adhesion molecule-1 (ICAM-1), E-selectin, and P-selectin were measured on dilutions of patients' plasma performed in accordance with the manufacturer's directions (R&D Systems, Minneapolis, MN).
Arginase activity
After the first 153 patients had been enrolled, plasma arginase activity was measured in a batch for all patients for whom a sufficient quantity of stored frozen plasma was available (n = 140). Arginase activity was determined as the conversion of [14C-guanidino]-l-arginine to [14C]urea, which was converted to 14CO2 by urease and trapped as Na214CO3 for scintillation counting as previously described.20 Briefly, aliquots of plasma or red blood cell lysate were spun down on collection and frozen at –80°C. Thawed samples were later incubated for 10 minutes at 55°C in complete assay mixture lacking arginine. The reaction was initiated by addition of labeled arginine and incubation was continued at 37°C for 2 hours. The reaction was terminated by heating at 100°C for 3 minutes. Samples were incubated with urease at 37°C for 45 minutes, and Na214CO3 was trapped on sodium hydroxide-soaked filters following acidification of the samples with hydrochloric acid to volatilize the 14CO2. Values of arginase activity are reported as μmol/mL/h.
NO consumption assay
The assay has been described in detail previously.16 Briefly, all assays were performed within a custom-made, glass, 2.1-mL reaction chamber maintained at 20°C. NO was generated in situ (150-300 nM) by the decay of PROLINONOate (Cayman Chemical, Ann Arbor, MI) in an argon-sparged, essentially anaerobic, phosphate-buffered, isotonic saline solution, pH 7.4. NO was continuously monitored with an ISO-NO Mark II NO meter and an amperometric, NO-specific microchip-based electrode (World Precision Instruments, Sarasota, FL). Plasma samples (10 μL) were added into the reaction chamber by means of a gas-tight Hamilton syringe (Hamilton, Reno, NV) after NO production by the NO donor ceased. NO consumption was quantified by dividing the measured instantaneous decrease in electrode current produced on the addition of 10 μL sample by the slope of a standard curve.
Statistical analysis
Analysis of continuous variables was performed with nonparametric (Spearman) correlations. To compare the degree of elevation of each isoenzyme fraction to each other, each of the 50 isoenzyme values was expressed as a ratio to the upper limit of normal, such that a ratio of 1 indicates a value at the upper limit of normal. These ratios were compared for each isoenzyme fraction by 2-tailed, unpaired Student t test, and P values less than or equal to .05 were considered significant. To compare changes in forearm blood flow by LDH values, the patient population was divided into those with LDH above and below the median LDH value for that group of 26 patients. The change in forearm blood flow induced by 3 different doses of sodium nitroprusside was compared between the 2 groups by analysis of variance (ANOVA) with repeated measures. Results were considered significant if P was less than or equal to .05. To analyze the effect of LDH levels on categorical variables, the patient population was divided into 3 groups, the first comprising those patients with LDH values at least 1 SD below the mean, the second comprising patients with LDH values within 1 SD above or below the mean, and the third comprising patients with LDH values at least 1 SD above the mean. The relationships among these 3 groups and specific categorical variables were tested for significance using a χ2 test for trend. Results were considered significant if P was less than or equal to .05. LDH values were compared among 3 groups of patients with increasing TRV values using the Kruskal-Wallis test. Results were considered significant if P was less than or equal to .05. Mortality rates were compared between the group of patients with LDH values below the median and those above the median, using the log-rank test. Results were considered significant if P was less than or equal to .05. Analysis was performed using Prism 4.03 (Graphpad Software, San Diego, CA).
Results
LDH is strongly associated with markers of hemolysis and levels of soluble endothelial adhesion molecules in the cohort
Patient characteristics of the cohort and 2 subgroups of the cohort are detailed in Table 1. We investigated the association of LDH with markers of hemolysis, organ dysfunction, and endothelial activation in a population of 213 patients with all forms of sickle cell disease, using blood samples obtained while in steady state. LDH was highly correlated with multiple markers of hemolytic severity (Table 2). LDH was inversely correlated with hemoglobin and haptoglobin levels. LDH correlated significantly with plasma hemoglobin levels, reticulocyte counts, concentration of hemoglobin S, and plasma arginase activity, another enzyme found in abundance in sickle erythrocytes and associated with markers of hemolytic severity. LDH was strongly associated with markers normally elevated in either hemolysis or liver disease, including aspartate aminotransferase (AST) and direct and indirect bilirubin. A significant correlation was also observed with alanine aminotransferase (ALT), a highly specific marker of hepatocellular injury, although this association was weaker than that observed with markers of hemolytic rate. Interestingly, an association was observed between LDH and leukocyte count, likely associated with turnover of the high numbers of leukocytes produced in patients with sickle cell disease. The relationship between LDH and other markers of organ injury, including creatinine, alkaline phosphatase, and creatine kinase, was very weak. LDH significantly correlated with plasma levels of soluble VCAM-1, a marker of endothelial cell activation. Weak but significant correlations were observed with other adhesion molecules, including soluble E-selectin, ICAM-1, and P-selectin. Finally, LDH was inversely correlated with transcutaneous oxygen saturation. Virtually all of these associations remained significant after application of a Bonferroni correction for multiple comparisons, which would reduce the P value threshold for significance to .001. Concerns regarding multiple comparisons are greatly diminished by this correction and by the fact that the LDH value correlated with multiple markers of hemolysis. The correlations shown in Table 2 were essentially the same if patients with sickle cell hemoglobin C (HbSC) disease were excluded from analysis (data not shown). Fetal hemoglobin levels did not correlate with LDH, although this correlation was reduced by hydroxyurea. Most other correlations were qualitatively similar in patients taking or not taking hydroxyurea (Table S1 on the Blood website; see the Supplemental Materials link at the top of the online article).
LDH isoenzyme distribution suggests erythrocyte origin
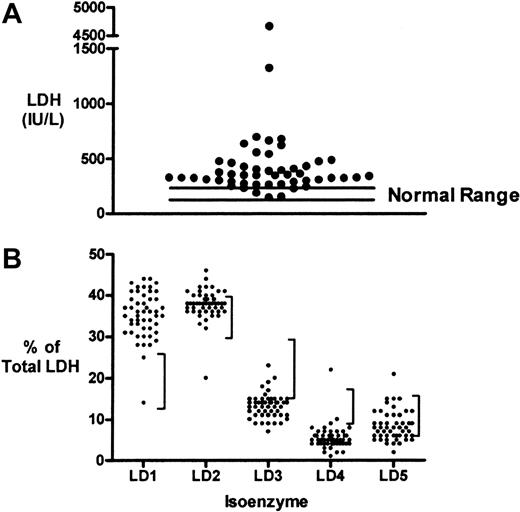
To ascertain the relative contributions of LDH activity from different tissue sources to total serum LDH level in patients with sickle cell disease, blood samples were collected from 33 patients with sickle cell disease on 50 occasions. The patient characteristics of this subgroup are shown in Table 1 and included a large number of patients with pulmonary hypertension. We find that LDH levels (518 ± 97 IU/L, mean ± SEM) were elevated above the normal range (113-226 IU/L) in most patients in this subgroup (Figure 1A). The percentage of total serum LDH contributed by each of LDH isoenzymes 1 to 5 was measured for each blood sample (Figure 1B). The percentages of LD1 and LD2 were elevated above the normal range for most samples. These are the isoenzyme fractions known to dominate in red blood cells, cardiac muscle, and kidney.31 In contrast, the percentages of LD3 (lymphoid cells and platelets), and LD4 and LD5 (both dominant in liver and skeletal muscle) tended to fall below the lower limits of normal. To test the statistical significance of these observations, the LDH isoenzyme values for each sample were expressed as a ratio to the upper limit of normal (Table 3). LD1 and LD2 ratios were each significantly higher than LD3, LD4, and LD5 (2-tailed Student t test, P < .001 in each case). The LD1 ratio was somewhat higher than the LD2 ratio (P = .01). This suggests that red blood cells, cardiac muscle, or kidney are the tissue sources of serum LDH in these patients with sickle cell disease. Qualitatively similar results that remained statistically significant were obtained for LDH isoenzyme distribution if the analysis was limited to one specimen per patient.
Serum LDH levels and isoenzyme distribution in patients with sickle cell disease. (A) Distribution of LDH levels on 50 occasions in 33 patients with sickle cell disease. Nearly all results are above the normal range (113-226 IU/L). (B) LDH isoenzyme fractionation of each sample suggests a tendency for the patient values to fall above the normal range (vertical bars to the right of each distribution) for LD1 and LD2 and below the normal range for LD3, LD4, and LD5 isoenzymes.
Serum LDH levels and isoenzyme distribution in patients with sickle cell disease. (A) Distribution of LDH levels on 50 occasions in 33 patients with sickle cell disease. Nearly all results are above the normal range (113-226 IU/L). (B) LDH isoenzyme fractionation of each sample suggests a tendency for the patient values to fall above the normal range (vertical bars to the right of each distribution) for LD1 and LD2 and below the normal range for LD3, LD4, and LD5 isoenzymes.
Association of LDH with plasma hemoglobin and NO resistance
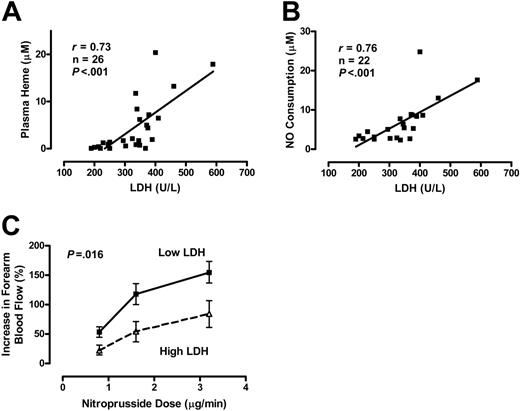
The relationship of serum LDH levels to plasma hemoglobin and vascular reactivity was assessed in another subgroup of patients with homozygous sickle cell disease who volunteered for forearm blood flow studies.17 The specimen collection in this subgroup was performed under highly controlled conditions, including blood draws via indwelling arterial catheters, in a manner that minimized artifactual hemolysis. This yielded a very high correlation between serum LDH and plasma heme levels (Figure 2A). LDH was similarly associated with the level of NO consumption activity in blood plasma (Figure 2B). In this stringently characterized group, the following values were obtained: LDH 328 ± 90 IU/L (mean ± SD), plasma hemoglobin 4.2 ± 4.6 μM, and NO consumption 6.7 ± 5.6 μM. Because high LDH values appear to predict high plasma heme and NO consumption levels, we evaluated the vascular responsiveness to the exogenous NO donor sodium nitroprusside in patients with below median LDH values (< 338 IU/L) to those with higher than median value. Patients with high LDH values demonstrated a blunted vasodilation response to 3 different doses of the NO donor sodium nitroprusside (P = .016, ANOVA with repeated measures; Figure 2C), consistent with NO resistance. Although the statistical power was limited for sex analysis, in general the association of high LDH level with NO resistance was stronger in men than in women (Figure S1). These data suggest that LDH is an accurate biomarker of hemoglobinemia, NO consumption, and NO resistance in patients with sickle cell disease.
Serum LDH as a marker of intravascular hemolysis and NO resistance in 26 patients with sickle cell disease. Serum LDH levels correlate with plasma heme levels (A) and the amount of plasma NO consumption (B) as measured by an in vitro assay (Spearman correlation). (C) Patients with higher LDH levels (340-589 IU/L, n = 13) have NO resistance compared to patients with lower LDH levels (190-337 IU/L, n = 13) as indicated by the blunted degree of increased forearm blood flow induced by brachial artery infusion of the NO donor sodium nitroprusside at 0.8, 1.6, and 3.2 μg/min, measured by venous occlusion plethysmography (P = .016, ANOVA with repeated measures). Results are depicted as mean ± SEM.
Serum LDH as a marker of intravascular hemolysis and NO resistance in 26 patients with sickle cell disease. Serum LDH levels correlate with plasma heme levels (A) and the amount of plasma NO consumption (B) as measured by an in vitro assay (Spearman correlation). (C) Patients with higher LDH levels (340-589 IU/L, n = 13) have NO resistance compared to patients with lower LDH levels (190-337 IU/L, n = 13) as indicated by the blunted degree of increased forearm blood flow induced by brachial artery infusion of the NO donor sodium nitroprusside at 0.8, 1.6, and 3.2 μg/min, measured by venous occlusion plethysmography (P = .016, ANOVA with repeated measures). Results are depicted as mean ± SEM.
Association of LDH with clinical outcomes and mortality
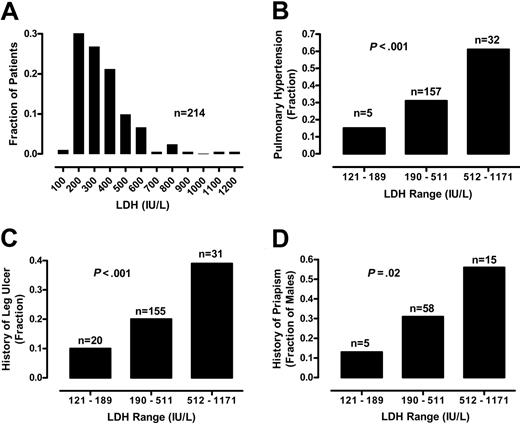
We assessed the possible association between LDH levels and a series of clinical outcomes, including prevalence of pulmonary hypertension, cutaneous leg ulceration, and priapism. To compare these categorical variables to LDH levels, we divided the 213 patients into 3 groups. The low LDH group comprises patients with LDH values (group range, 121-189 IU/L; group mean, 166 IU/L; n = 20) at least 1 SD below the mean, the medium LDH group includes LDH values (group range, 190-511 IU/L; group mean, 317 IU/L; n = 162) falling within 1 SD of the mean, and the high LDH group includes patients with LDH values (group range, 512-1171 IU/L; group mean, 647 IU/L; n = 31) at least 1 SD above the mean. The prevalence of pulmonary hypertension, defined as TRV 2.5 m/s or greater, was closely related to LDH group (χ2 13.45, df 1, P < .001, χ2 test for trend; Figure 3). These results are consistent with our previous analysis of LDH in an overlapping patient population.1
Relationship of serum LDH levels and history of vasculopathic complications. (A) The frequency distribution of 213 patients with sickle cell disease by LDH level in hundreds is indicated by the vertical bars. For comparison of the prevalence of selected sickle cell complications, data from 213 patients are divided into 3 groups according to serum LDH levels. The low LDH is defined by LDH levels lower than 1 SD below the overall mean (range, 121-189 IU/L), medium LDH by overall mean LDH level plus or minus 1 SD (range, 190-511 IU/L), and high LDH higher than 1 SD above the overall mean (range, 512-1171 IU/L). The prevalences of pulmonary hypertension (B), leg ulcers (C), and in men, priapism (D) are also related to LDH group. All statistics are significant by χ2 test for trend. The number of patients in each group (n) is indicated above each bar.
Relationship of serum LDH levels and history of vasculopathic complications. (A) The frequency distribution of 213 patients with sickle cell disease by LDH level in hundreds is indicated by the vertical bars. For comparison of the prevalence of selected sickle cell complications, data from 213 patients are divided into 3 groups according to serum LDH levels. The low LDH is defined by LDH levels lower than 1 SD below the overall mean (range, 121-189 IU/L), medium LDH by overall mean LDH level plus or minus 1 SD (range, 190-511 IU/L), and high LDH higher than 1 SD above the overall mean (range, 512-1171 IU/L). The prevalences of pulmonary hypertension (B), leg ulcers (C), and in men, priapism (D) are also related to LDH group. All statistics are significant by χ2 test for trend. The number of patients in each group (n) is indicated above each bar.
Two other important LDH associations include the patient history of leg ulcers and priapism. At the time of screening for pulmonary hypertension, patients provided medical history information regarding medical complications of sickle cell disease. The percentage of patients with a past history of leg ulcers was significantly related to LDH group (χ2 6.71, df 1, P < .001; Figure 3). The history of priapism in men was also significantly related to LDH group (χ2 5.32, df 1, P = .02, n = 94; Figure 3). Similar trends were obtained even when patients with hemoglobin SC disease were excluded from analysis of the relationship of LDH group to pulmonary hypertension (χ2 8.60, df 1, P = .003, n = 175), leg ulceration (χ2 5.43, df 1, P = .02, n = 175), and priapism (χ2 3.13, df 1, P = .08, n = 67; Figure S2). We did not find a significant relationship of serum LDH to patient-provided history of stroke or frequency of VOC pain crisis or acute chest syndrome, although the strength of this conclusion is limited by the unavailability of medical chart documentation of these frequencies. These results suggest that LDH may predict risk for a constellation of specific vascular complications, including pulmonary hypertension, cutaneous leg ulceration, and priapism.
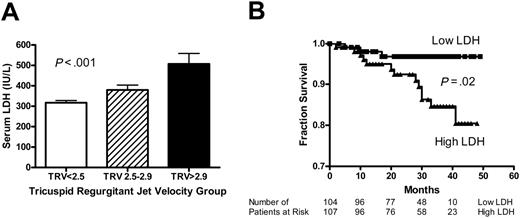
To further evaluate the relationship of LDH to severity of pulmonary hypertension, we compared the LDH values for 3 groups of patients: (1) no pulmonary hypertension, defined as TRV less than 2.5 m/s; (2) mild pulmonary hypertension, TRV 2.5 to 2.9 m/s; and (3) moderate to severe pulmonary hypertension, TRV greater than 2.9 m/s. This classification has been previously validated.1 LDH values rise with severity of pulmonary hypertension (317 ± 11 versus 380 ± 24 versus 508 ± 51 IU/L, mean ± SEM, P < .001, Kruskal Wallis test; Figure 4A). Qualitatively similar results were obtained when patients with hemoglobin SC were excluded from analysis (344 ± 12 versus 395 ± 25 versus 537 ± 52 IU/L, mean ± SEM, P = .001, Kruskal Wallis test; Figure S3A), and with subgroup analysis according to hydroxyurea treatment (Figure S4A-B). These results suggest that steady-state LDH values may be related to severity of pulmonary hypertension.
We also examined the mortality rate in patients with higher or lower than median LDH values. Information on deaths was collected during a follow-up period of up to 49 months. Follow-up of at least 4 months was available for 211 patients with a median duration of 30 months (range, 2-49 months). Since enrollment in the study, 19 patients with sickle cell disease had died as of April 2005, with median survival time of 19 months (range, 2-41 months). Median follow-up was 31 months for the 195 patients who survived (range, 3-49 months). Nine patients had not responded to attempts to contact them and were considered lost to follow-up. Confirmation of all deaths with death certificates and the absence of available death certificates in the United States for patients lost to follow-up suggest that we have not missed any deaths and all patients lost to follow-up were alive at the time of data analysis. Patients with LDH values higher than the median (range, 121-315 IU/L) had reduced survival compared to those with LDH values lower than the median (range, 316-1171 IU/L; log-rank test, χ2 5.32, df 1, P = .02; Figure 4B). Using the same LDH cutoff, similar results were obtained when patients with hemoglobin SC were excluded (log-rank test, χ2 5.17, df 1, P = .02; Figure S3B). In subgroup analysis, the mortality differences remained significant in patients not taking hydroxyurea and showed a trend in the same direction for patients on hydroxyurea therapy (Figure S4C-D). These data suggest that high steady-state LDH values may predict early mortality.
Discussion
Thirty-five years ago, Neely and colleagues elegantly demonstrated in patients with sickle cell disease that serum LDH was elevated, that it is related to plasma hemoglobin levels, and that both increased during VOC crisis.14 They also demonstrated an LDH isoenzyme pattern supporting intravascular hemolysis as the principal source of elevated total serum LDH levels.13,14,32 Revisiting their work with modern biochemical methods, we have confirmed and extended their results. Although LD1 and LD2 isoenzymes can be released also by cellular injury to cardiac muscle or kidney,31 the strong correlation with clinical markers of hemolytic severity in our results (and poor correlation with creatine kinase or creatinine) strongly implicates intravascular hemolysis as the dominant source of serum LDH in patients with sickle cell disease. This conclusion is further supported by association of elevated serum LDH levels with low levels of hemoglobin and high levels of reticulocytes and bilirubin. The disproportionate correlation to AST more than ALT is consistent with the higher concentration of AST compared to ALT in red blood cells, released during intravascular hemolysis.33
Serum LDH levels are associated with pulmonary hypertension and early mortality. (A) Compared to patients without pulmonary hypertension (TRV < 2.5 m/s, n = 138), higher mean LDH values are seen with mild pulmonary hypertension (TRV 2.5-2.9 m/s, n = 51) and moderate-severe pulmonary hypertension (TRV > 2.9 m/s, n = 21; P < .001, Kruskal-Wallis test). (B) Kaplan-Meier plot of survival of patients with lower or higher than median steady-state LDH levels (defined as < 315 IU/L or ≥ 315, respectively; P = .02, log-rank test).
Serum LDH levels are associated with pulmonary hypertension and early mortality. (A) Compared to patients without pulmonary hypertension (TRV < 2.5 m/s, n = 138), higher mean LDH values are seen with mild pulmonary hypertension (TRV 2.5-2.9 m/s, n = 51) and moderate-severe pulmonary hypertension (TRV > 2.9 m/s, n = 21; P < .001, Kruskal-Wallis test). (B) Kaplan-Meier plot of survival of patients with lower or higher than median steady-state LDH levels (defined as < 315 IU/L or ≥ 315, respectively; P = .02, log-rank test).
Our data indicate that steady-state serum LDH is a convenient biomarker for the pathologic accumulation of hemoglobin and arginase in blood plasma, with consequently impaired NO bioavailability. The red cell membrane normally serves as a physical and diffusional barrier that segregates erythrocyte proteins from plasma and endothelium. Intravascular hemolysis disrupts this protective compartmentalization and allows pathologic biochemical reactions to occur.34 At least 2 such reactions are known. The first involves the stoichiometric inactivation of NO by cell-free plasma hemoglobin, associated with impairment of NO-dependent blood flow in patients with sickle cell disease.16,25-27,30,35,36 The second involves the release of erythrocyte arginase, which converts plasma l-arginine into ornithine, resulting in depletion of plasma l-arginine, the required substrate for NO production by NO synthase, with associated pulmonary hypertension.19,20,37 LDH also correlates with endothelial-derived soluble adhesion molecules, considered markers of endothelial activation that are normally repressed by NO.2,4,5,8,38-44
Most significant from a clinical perspective, steady-state LDH elevation furthermore identifies a subset of patients in our cohort with sickle cell disease at risk for pulmonary hypertension, cutaneous leg ulceration, priapism, and early death. This supports the concept, first advanced by Ballas and more recently by Alexander and colleagues, that a subgroup of patients with sickle cell disease suffers frequent leg ulcers, independent of the frequency of VOC crisis.45,46 Our data suggest expansion of the leg ulcer phenotype to include pulmonary hypertension, priapism, severe hemolytic anemia, and NO resistance.1 These clinical features have also been reported in thalassemia intermedia, hereditary spherocytosis, and other forms of severe hemolytic anemia.47-62 This suggests that sickling is not required for this subphenotype and instead implicates the severe hemolysis of sickle cell disease in its pathobiology. Consistent with this model, the Jamaican sickle cell population, characterized by severe hemolysis, has a much higher prevalence of leg ulcers and priapism than those in Greece and India, who have less severe hemolysis, but comparable rates of VOC pain crisis.63-65 Recent analysis from the Cooperative Study of Sickle Cell Disease confirms the link of priapism to LDH and other markers of hemolytic severity.66
It is interesting that LDH elevation also is associated with low transcutaneous oxygen saturation. This is consistent with publications from 2 other groups, who have found in patients with sickle cell disease the association of desaturation with anemia and reticulocytosis, suggesting the same link between hemolysis and hypoxemia.44,67-69 We speculate that this link might involve pulmonary hypertension and ventilation-perfusion mismatch (secondary to dysregulation of lung perfusion), but further investigations are required to understand this observation.
It is remarkable that LDH, a readily available clinical laboratory test, appears to predict a syndrome of hemolysis-associated endothelial dysfunction, a subphenotype of sickle cell disease that includes pulmonary hypertension, priapism, cutaneous leg ulceration, and risk of death. Although this study is exploratory in nature, and certainly requires further validation in other patient populations, the findings may be generalizable to a host of chronic hemolytic diseases. LDH appears to hold great promise as an effective prognostic indicator and biomarker of a hemolytic mechanism of vascular pathobiology in patients with sickle cell disease.
Prepublished online as Blood First Edition Paper, November 15, 2005; DOI 10.1182/blood-2005-06-2373.
Supported by intramural funds from the National Heart, Lung and Blood Institute and the Clinical Center, National Institutes of Health (M.T.G.); by National Institute of Health grants RO1 GM57384 (S.M.M.); and by grants HL-04386-05 and M01-RR01271, Pediatric Clinical Research Center (C.R.M.).
G.J.K. and M.T.G. designed research; G.J.K., V.M., R.M., J.L., J.T., J.S.N., X.W., M.P., and C.R.M. performed research; G.J.K., M.P., and S.M.M. analyzed data; and G.J.K. and M.T.G. wrote the paper.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mary Hall for invaluable protocol management and clerical support. We thank Dr Oswaldo Castro for clinical support and Lori Hunter, RN, and Wynona Coles, RT, for clinical research assistance.