Abstract
Since the 1970s, mortality in the hemophilia population has been dominated by human immunodeficiency virus (HIV) and few reports have described mortality in uninfected individuals. This study presents mortality in 6018 people with hemophilia A or B in the United Kingdom during 1977 to 1998 who were not infected with HIV, with follow-up until January 1, 2000. Given disease severity and factor inhibitor status, all-cause mortality did not differ significantly between hemophilia A and hemophilia B. In severe hemophilia, all-cause mortality did not change significantly during 1977 to 1999. During this period, it exceeded mortality in the general population by a factor of 2.69 (95% confidence interval [CI]: 2.37-3.05), and median life expectancy in severe hemophilia was 63 years. In moderate/mild hemophilia, all-cause mortality did not change significantly during 1985 to 1999, and median life expectancy was 75 years. Compared with mortality in the general population, mortality from bleeding and its consequences, and from liver diseases and Hodgkin disease, was increased, but for ischemic heart disease it was lower, at only 62% (95% CI: 51%-76%) of general population rates, and for 14 other specific causes it did not differ significantly from general population rates. There was no evidence of any death from variant Creutzfeldt-Jakob disease or from conditions that could be confused with it.
Introduction
In the late 1960s the United Kingdom Haemophilia Centre Doctors' Organisation (UKHCDO) initiated a nationwide database for planning the care of people with congenital blood coagulation defects. From 1976 to 1998, it included details of all males diagnosed with hemophilia A or hemophilia B regardless of whether they required treatment, and it was updated each year adding newly diagnosed individuals.1 The information held on the database has been used to carry out a study of mortality in the complete hemophilia population of the United Kingdom over a period of 23 years. This paper presents information on people with hemophilia A or B who were not infected with human immunodeficiency virus (HIV). It describes how all-cause mortality varied with type and severity of hemophilia, calendar period, and age. It also presents life expectancy, and mortality from specific causes of death, including deaths involving intracranial hemorrhage or other bleeds. Mortality in those who were infected with HIV has been reported elsewhere,2 as has the influence of inhibitors on mortality.3
Patients, materials, and methods
The present study includes all males with hemophilia A or B who were registered with the UKHCDO database during 1977 to 1998. Participating hemophilia centers are listed in the Appendix at the end of this article. Their vital status on January 1, 2000, was ascertained using information from Haemophilia Centres and the National Health Service Central registers. Death certificates were obtained for those who had died, and the underlying cause of death coded to the ninth revision of the International Classification of Diseases.4 This was done using the standard rules except that if the underlying cause was described as hemophilia due to some other, more specific, cause then the more specific cause was taken. For example, if a death was described on the death certificate as caused by hepatitis due to hemophilia but with no mention of bleeding then it was classified as due to hepatitis. Also, if a death was described as caused by hemorrhage due to hemophilia with no mention of any other condition it was classified as due to hemorrhage. However, deaths described as caused by bleeding and also by another condition, both due to hemophilia, were classified as due to hemophilia. Similarly, deaths described as caused by the late effects of hemophilic bleeds were classified as due to hemophilia.
Information on the cause of death was often available from the hemophilia centers and was added to the information from the death certificate to identify individuals for whom intracranial hemorrhage, other bleeds, liver cancer, or liver disease contributed in causing the death. All available information on cause of death was also examined for neurologic conditions that might indicate variant Creutzfeldt-Jakob disease (vCJD) and, for those with hemophilia B, for a diagnosis of pulmonary embolism or disseminated intravascular coagulation (DIC). Approval for the study was obtained from the Central Oxford Ethics Research Committee. The study was conducted in accordance with the Declaration of Helsinki.
Person-years at risk were calculated as the time from date of registration on the UKHCDO database to date last seen. For most individuals this was the earliest of date of death, date of emigration, or January 1, 2000. However, for the few individuals who could not be traced on the National Health Service Registers, this was the date of last recorded contact with a hemophilia center. HIV testing became available late in 1984 and virtually all hemophilia patients who had received potentially infected blood products were tested in 1985. For each HIV-infected individual, a date of seroconversion was estimated using the dates of all relevant HIV-positive and HIV-negative blood samples.2 The median estimated date of HIV infection was December 1982, and for more than 80% of those who became infected the estimated date of seroconversion was during 1981 to 1983. For many hemophilia patients who died during 1980 to 1985, their HIV status was never determined and it has previously been shown5 that HIV had little impact on mortality prior to 1985. Therefore, those who were subsequently shown to be infected with HIV contributed to the person-years at risk until January 1, 1985, or until their estimated date of seroconversion, if later. Deaths and person-years at ages 85+ years were excluded from the analysis.
Person-years and deaths were subdivided by current age (5-year groups), calendar period (single years), whether the individual had ever developed inhibitors, and type and severity of hemophilia. Death rates were calculated as the ratio of the number of deaths to the number of person-years at risk. Factors influencing mortality were studied using Poisson regression. To compare the death rate from specific causes with national mortality rates, the number of deaths expected for each cause of death of interest was calculated by multiplying the person-years at risk by the corresponding age- and calendar year–specific male national mortality rate, derived from the World Health Organization database (http://www.who.int/whosis/mort/download/en/index.html). Significance tests and confidence intervals assumed that the number of observed deaths had a Poisson distribution and that the number of expected deaths was fixed. Significance tests were 2-sided. Calculations were carried out using version 5.0 of the computer package Stata (http://www.stata.com).
Results
Characteristics of the study population
There were 6018 males with hemophilia A or B registered on the UKHCDO nationwide database during 1977 to 1998 who were either not infected with HIV or who died before January 1, 1985 (Table 1). Eighty-one percent (4874) had hemophilia A and 19.0% (1144) had hemophilia B. The vital status on January 1, 2000, was established for all but 3.2% (194). Among the 1320 people with severe hemophilia, 18.9% (250) had died by the end of the follow-up period, as had 12.0% (177/1476) of people with moderate hemophilia and 13.5% (435/3222) of people with mild hemophilia.
Mortality from all causes
The annual age-specific death rate from all causes for people with severe hemophilia was nearly twice that for people with moderate or mild hemophilia (death rate ratio: 1.82; 95% confidence interval [CI]: 1.54-2.16; P < .001, after adjustment for calendar period, development of inhibitors and type of hemophilia). The all-cause death rates in people with moderate hemophilia and people with mild hemophilia did not differ significantly from each other (adjusted death rate ratio: 1.15 for mild compared with moderate; 95% CI: 0.95-1.39; P = .15), and in subsequent analyses moderate and mild hemophilia were considered together.
Among people with severe hemophilia, those with hemophilia B had a somewhat lower all-cause death rate than those with hemophilia A (adjusted death rate ratio: 0.71), but the difference did not reach statistical significance (95% CI: 0.49-1.04; P = .07), while among those with moderate/mild hemophilia the death rate from all causes was very similar in people with hemophilia A and hemophilia B (adjusted death rate ratio: 0.96; 95% CI: 0.77-1.21; P = .75) (Table 2). The number of people with hemophilia B in the study was not large enough for separate analysis and, in subsequent analyses, hemophilia A and hemophilia B have been combined unless otherwise indicated.
Among people with severe hemophilia the all-cause death rates in calendar periods 1977 to 1984, 1985 to 1992, and 1993 to 1999 were very similar (death rate ratios adjusted for age, development of inhibitors, and type of hemophilia: 1.00, 0.96 [95% CI: 0.71-1.30], and 0.96 [95% CI: 0.71-1.31] in 1977 to 1984, 1985 to 1992, and 1993 to 1999, respectively; P for trend: .78). In contrast, among those with moderate/mild hemophilia the all-cause death rate in 1985 to 1992 and 1993 to 1999 was about 20% lower than in 1977 to 1984 (adjusted death rate ratios: 1.00, 0.81 [95% CI: 0.65-1.01] and 0.79 [95% CI: 0.64-0.98]), and the trend with calendar period reached statistical significance (P for trend: .05).
In view of the calendar period–specific results, analyses of age-specific mortality and life expectancy considered time periods 1977 to 1999 for people with severe hemophilia and 1985 to 1999 for people with moderate/mild hemophilia. In both severe and moderate/mild hemophilia, the death rate from all causes exhibited the same age pattern as that of the general population in that it was relatively high in children younger than 5 years, fell to a minimum at ages 5 to 14 years, and then rose progressively with increasing age (Table 3). At every age, the all-cause death rate in people with severe hemophilia was higher than that in people with moderate/mild hemophilia, and the all-cause death rate in those with moderate/mild hemophilia was higher than that of the general male population of the United Kingdom in 1999 (Table 3).
Comparison with mortality rates in the general population
During 1977 to 1999, the all-cause death rate in severe hemophilia was higher than the corresponding age- and calendar year–specific all-cause death rate in the general male population by a factor of 2.69 (95% CI: 2.37-3.05; P < .001), while for patients with moderate/mild hemophilia it was increased by a factor of 1.19 (95% CI: 1.09-1.29; P < .001) (Table 4).
When specific causes of death were examined, mortality rates in the hemophilia population were significantly increased compared with those for the general male population for coagulation defects and for intracranial hemorrhage (Table 4A “Causes that were significantly increased in the hemophilia population”). In children younger than 5 years, there was a total of 10 deaths from intracranial hemorrhage compared with only 0.013 expected (ratio of observed to expected deaths: 775.19 [95% CI: 371.74-1425.61]). Considering all ages, mortality was also increased compared with the general population for other hemorrhage, for injuries other than suicide and poisoning, for hepatitis and liver disease, and for liver cancer. Of the 42 deaths certified as due to injuries other than suicide or poisoning, either the death certificate or the cause of death information from the hemophilia center indicated that bleeding played a role in 20 (13 severe A, 3 moderate/mild A, 3 severe B, 1 moderate/mild B). Based on death certificate information, mortality was also increased from “ill-defined cerebrovascular disease” and for “diseases of the digestive system other than liver disease or gastrointestinal hemorrhage,” and for most of the deaths in these 2 categories information from the hemophilia centers indicated that the death was partly or wholly due to bleeding. Mortality was increased for Hodgkin disease, based on a total of 4 deaths. For most of the specific causes of death that were increased compared with the general male population the proportionate increase was substantially greater in people with severe than in people with moderate/mild hemophilia (Table 4A “Causes that were significantly increased in the hemophilia population”).
For ischemic heart disease, the mortality rate in the hemophilia population was only 62% of that in the general male population (95% CI: 51%-76%) and the proportionate reduction was similar in patients with severe and moderate/mild hemophilia, although the number of deaths from ischemic heart disease in patients with severe hemophilia was small, so that the deficit was not statistically significant when this group was considered by itself (Table 4B “Ischemic heart disease”).
For 14 other specific causes of death, including non-Hodgkin lymphoma, ischemic stroke, and suicide and poisoning, mortality rates in the hemophilia population did not differ significantly from those in the general male population for either severe hemophilia, or for moderate/mild hemophilia or for both groups combined (Table 4C “Other causes”).
When the analysis shown in Table 4 was repeated separately for those with hemophilia A and B the results were similar. In particular for ischemic heart disease the ratio of observed to expected deaths in hemophilia A was 0.64 (95% CI: 0.52-0.79, based on 89 deaths) while in hemophilia B it was 0.54 (95% CI: 0.30-0.88, based on 15 deaths).
Deaths involving intracranial or other hemorrhage
In both severe and moderate/mild hemophilia, the variation in the death rate with age for deaths from bleeding of any type and for deaths from intracranial hemorrhage resembled that for all causes, with high rates at ages younger 5 years, falling to a minimum at ages 5 to 14 years, and increasing progressively from age 15 years (Table 5). The death rates in people with hemophilia B were similar to those for people with hemophilia A for bleeding of any type and for intracranial hemorrhage, after adjusting for age, calendar period, and inhibitor status (death rate ratios B versus A: severe—bleeding of any type, 0.89 [95% CI: 0.55-1.45] P = .64; intracranial hemorrhage, 0.90 [95% CI: 0.48-1.69] P = .74; moderate/mild—bleeding of any type, 0.94 [95% CI: 0.61-1.45] P = .78; intracranial hemorrhage, 0.79 [95% CI: 0.42-1.49] P = .45).
In severe hemophilia, when all patients (ie, both those who had developed inhibitors and those who had not) were considered together, the annual age-standardized mortality rate for deaths involving any type of bleed decreased progressively from 8.9 per 1000 during 1977 to 1984, to 8.3 during 1985 to 1992, and to 5.6 during 1993 to 1999 (P for trend: .05; Table 6). However, when the analysis was repeated removing those individuals who had developed inhibitors from the time that they were first reported, the annual mortality rate among people with severe hemophilia but who had not developed inhibitors did not decrease during the period studied, with values of 5.5, 4.9, and 5.6 per 1000 during 1977 to 1984, 1985 to 1992, and 1993 to 1999, respectively (P for trend: .90). For deaths involving intracranial hemorrhage, there was little evidence that the mortality rate changed during the period studied either in all patients with severe hemophilia (annual age-standardized mortality rates: 4.8, 4.8, and 3.4 per 1000 during 1977 to 1984, 1985 to 1992, and 1993 to 1999, respectively; P for trend: .40) or when just those who did not develop inhibitors were considered (rates: 3.1, 3.5, and 3.5 per 1000, respectively). Similarly, among patients with moderate/mild hemophilia, there was little evidence that the mortality rate for all deaths involving bleeding or for deaths involving intracranial hemorrhage decreased over the period studied after the exclusion of patients who developed inhibitors (Table 6).
At ages younger than 5 years, intracranial hemorrhage was involved in 11 of a total of 13 deaths from all causes in severe hemophilia at ages younger than 5 years, and only 2 were in patients with inhibitors, while in moderate/mild hemophilia 3 of the 6 deaths at ages younger than 5 years involved intracranial hemorrhage, and none was in a patient with inhibitors (Tables 5–6). The intracranial hemorrhage death rate in age group younger than 5 years did not change significantly with calendar period (P for trend in severe = .99; P for trend in moderate/mild = .68; Table 6). Of the 9 children younger than 5 years who died with intracranial hemorrhage during 1977 to 1992, 2 were younger than one month, one was 10 months, and the remainder were 1.4, 1.7, 2.0, 2.9, 3.9, and 4.7 years, while for the 5 such children dying during 1993 to 1999, 4 were younger than one month and one was aged 1 year. Four (1 severe A at 10 months in 1980, 2 severe A in 1981 and 1988, and 1 moderate A in 1992, all at ages 1-4 years) were recorded as sporadic cases, 1 (severe A at 1 week in 1985) was recorded as coming from a known hemophilia family, and for the remainder no information was available.
Deaths involving vCJD, pulmonary embolism, or DIC
Variant CJD was not mentioned specifically in the cause of death information for any individual. Nor was there any mention of any spongiform encephalopathy, or of any other condition that might be confused with these diseases. Of the 96 deaths that occurred in hemophilia B (44 of which involved bleeding), pulmonary embolism was reported in 2 patients (one in 1981 in a patient with pancreatic cancer, and one in 1987 as a postoperative complication), and DIC was reported in 2 patients (who died in 1980 and 1985).
Life expectancy
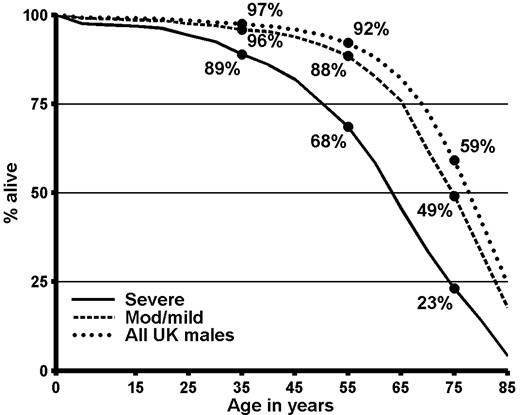
In severe hemophilia, the proportions of individuals surviving to ages 35, 55, and 75 years were 89%, 68%, and 23%, respectively (Figure 1), and median life expectancy was 63 years (Table 7“All causes of death”). In moderate/mild hemophilia, the proportions surviving to ages 35, 45, and 55 were 96%, 88%, and 49%, respectively, and median life expectancy was 75 years. For all United Kingdom males in 1999, median life expectancy was 78 years. Thus, in severe hemophilia median life expectancy was 15 years less than that of the general male population and in moderate/mild hemophilia it was 3 years less. To investigate how much of the reduction in life expectancy compared with the general population could be attributed to liver disease, the life expectancy calculations were repeated omitting all the deaths for which either the information on the death certificate or the information from the hemophilia center mentioned liver disease or liver cancer. In severe hemophilia, omission of the deaths associated with liver disease increased median life expectancy to 66 years, while for moderate/mild hemophilia median life expectancy increased to 77 years.
Survival in men in the United Kingdom with hemophilia who were not infected with HIV and in the general male population of the United Kingdom in 1999. Calculations based on the data summarized in Table 3.
Survival in men in the United Kingdom with hemophilia who were not infected with HIV and in the general male population of the United Kingdom in 1999. Calculations based on the data summarized in Table 3.
Discussion
Study design
This is the largest follow-up study ever performed of people with hemophilia and it covers the longest period of follow-up. The study includes the complete population of United Kingdom residents diagnosed with hemophilia A or B during a period of more than 20 years, thus eliminating the possibility of bias that is present in studies based on cross-sectional surveys of hemophilia populations, where there are inevitably a number of nonrespondents, and in which children who die in the first few years of life tend to be underrepresented, leading to estimates of life expectancy from birth that are higher than the true value. Additionally, the study has made use of the nationwide flagging system available in the United Kingdom via the National Health Service Central Registers to eliminate duplicate records for individuals who attended many hemophilia centers or who changed their name. The central system of flagging has also enabled ascertainment of the appreciable number of deaths that occur in people with hemophilia but without the knowledge of any hemophilia center.
The study makes substantial use of the cause of death as given on the death certificate. Certified causes of death are derived from information recorded at the time of the death and are derived in a similar way for all deaths, regardless of whether the person involved had hemophilia. They thus provide an appropriate basis for assessing the magnitude of the overall death rate in the hemophilia population as a whole, and also for comparisons of cause-specific death rates between people with hemophilia and the general population. For some analyses, the certified cause of death information was supplemented by information on cause of death obtained directly from the hemophilia center involved. Both types of information are subject to limitations. For example, not all conditions present at the time of death may be included. Also, in a person with hemophilia and liver disease it may be difficult to distinguish between bleeding secondary to liver disease and unrelated to hemophilia, and bleeding in which hemophilia plays a role. There is, therefore, inevitably some uncertainly in the specification of the underlying cause of death.
For the majority of the period covered by this report, mortality in the hemophilia population as a whole was dominated by deaths related to HIV. Consequently few reports in recent years have focused on mortality patterns in people with hemophilia who are not infected with HIV and who, at present, form the majority of the hemophilia population in most countries. The current report has made use of the extensive information on HIV test results available for the United Kingdom hemophilia population, and from 1985 onward, which is when HIV-related mortality began to increase in this population,5 each HIV-infected individual has been removed from the population under study on his estimated date of HIV seroconversion, thus enabling the number of person-years at risk in HIV-negative individuals to be computed and creating appropriate denominators for the calculation of age-specific death rates in the HIV-negative hemophilia population. Similarly, the few individuals whose vital status could not be ascertained at the end of the follow-up were removed from the study population on the date they were last known to be alive.
Mortality from all causes and life expectancy
The all-cause mortality rate for the complete population of people with severe hemophilia changed little during the entire period 1977 to 1999 (Table 2), and median life expectancy for those with severe hemophilia was 15 years lower than for the general male population in 1999, at 63 compared with 78 years (Table 7). Most of the reduction in life expectancy in severe hemophilia was due to the consequences of bleeding, with liver disease making only a small contribution.
For moderate/mild hemophilia, all-cause mortality was lower during 1985 to 1999 than it had been during 1977 to 1984 (Table 2). This may either be a real decrease in mortality, or it may simply reflect an increase in the coverage by the database of individuals with moderate or mild clotting defects who do not need regular treatment. During 1985 to 1999, the all-cause mortality rate in moderate/mild hemophilia remained stable (Table 2) and median life expectancy was 75 years, 3 years less than that of the general male population in 1999 (Table 7) and most of the difference was accounted for by liver disease.
Our estimated median life expectancy from birth of 63 years in severe hemophilia is greater than the value of 57 years at birth reported for the 1960s and 1970s in Sweden,6 and similar to the value of 63 years at birth reported during 1973 to 1985 in the Netherlands,7 and 61 years from age 1 year during 1971 to 1980 reported in the United States.8 It is, however, considerably less than the 73 years at age 1 year reported for severe hemophilia in the absence of HIV infection during 1980 to 1995 in Canada9 or the 69 and 70 years from age 1 year reported during 1985 to 1992 and 1992 to 2001, respectively, in the Netherlands.10,11 In the recent Dutch study,11 the age-specific death rate for HIV-negative individuals with severe hemophilia exceeded that for the general population by a factor of 2.8 (95% CI: 1.9-4.2), very similar to the factor of 2.69 (95% CI: 2.37-3.05) seen in the present study (Table 4). It is, however, unclear why the Dutch study found a near normal life expectancy despite an increased death rate compared with the general population, but one possible explanation might be that the Dutch study underestimated the death rate in young people with severe hemophilia. It is notable that in the Dutch study the youngest participant was 4 months old and that no deaths were reported in children aged younger than 10 years,11 compared with 14 deaths at ages younger than 5 years from intracranial hemorrhage in the United Kingdom study (Table 6). It is clear that some deaths were missing in a previous analysis of mortality in people with severe hemophilia in the United Kingdom, which wrongly concluded that there was a “near normal expectation of life.”12 The present study suggests that it is still premature to assume that people with severe hemophilia who do not have HIV infection have a normal life expectancy, as some recent authors have done.13-15
Mortality from intracranial or other hemorrhage
A previous analysis of mortality in this population, focusing on the effect of inhibitors, found that the substantial increase in mortality associated with the development of inhibitors in people with severe hemophilia in the late 1970s had disappeared by the end of the 1990s.3 There has, however, been no corresponding decrease in mortality from intracranial hemorrhage or from other bleeds in the remainder of the hemophilia population. Mortality rates from intracranial hemorrhage and from bleeding of any type were similar in hemophilia A and hemophilia B, both for patients with a severe disorder and for those with a moderate/mild disorder. Thus the present study provides no support for the hypothesis that hemophilia B is less severe clinically than hemophilia A.16
A study of the causes of death in people with hemophilia in Sweden during 1957 to 1980 reported that intracranial hemorrhage accounted for 38% (15/39) of all deaths in people with severe hemophilia,17 and the proportion of deaths involving intracranial hemorrhage was only slightly lower than this in the present study, at 34% (84/250). Intracranial hemorrhage was also found to be the leading cause of death in studies covering this time period of people with hemophilia at all ages in the Netherlands,11 the United States,18,19 and France.20 The French study20 reported 3 deaths from intracranial hemorrhage in neonates during the 1990s.
Primary prophylaxis was not widely used in the United Kingdom until the early 1990s when evidence became available leading to confidence that heat-treated products were free of the risk of viral infections.21,22 and it has never been given on a regular basis to babies younger than about a year, those with significant joint damage, and those with moderate or mild hemophilia. However, from around 1990, children with severe hemophilia but no appreciable joint damage have been started on prophylaxis from the age of about 18 months and, once started, continue to receive it. In view of this, it noteworthy that of the 9 children younger than 5 years old who died with intracranial hemorrhage during 1977 to 1992, 6 were older than one year, while of the 5 such children dying during 1993 to 1999, the oldest was aged one year. Although the numbers involved are small, this indicates that prophylaxis may be reducing death from intracranial hemorrhage in the United Kingdom hemophilia population.
Mortality from cardiovascular disease
There was a clear reduction in the number of deaths from ischemic heart disease compared with the number expected from general population rates, as has previously been observed in studies of mortality in people with hemophilia in the Netherlands7 (1 death observed, 5 expected) and Greece23 (1 death observed, 4 expected). A study of hospital discharge rates in hemophilia in the United States also found a decrease.24 In the present study, the age-specific death rate from ischemic heart disease was 62% of that in the general population. This is a smaller proportionate reduction than was observed in the Dutch and Greek studies. However, as the present study is based on a total of 104 deaths from heart disease compared with only 1 in each of the Dutch and Greek studies, the present estimate is likely to be the more stable one.
The proportionate reductions in mortality from heart disease were virtually identical in severe and in moderate/mild hemophilia, at 38% and 37%, respectively (Table 4) and were similar to the value observed in a recent study of mortality from ischemic heart disease among carriers of hemophilia25 (36%, based on 39 deaths). This argues against a gradually increasing protective effect as concentrations of factor VIII and IX decrease and in favor of a threshold effect. A detailed study of Dutch patients has shown that the reduction in ischemic heart disease in the hemophilia population cannot be attributed to differences in cardiovascular risk factors such as blood pressure or cholesterol level.26 Thus it is likely that the reduced mortality from ischemic heart disease seen in people with hemophilia or carriers of the disease is a consequence of some aspect of hemophilia, although the causal pathway by which this occurs is not yet fully understood.
In the present study, the number of deaths certified as due to ischemic stroke was also lower than the number expected, at 0.63, which would be consistent with hemophilia having a protective effect against ischemic stroke as well as ischemic heart disease (Table 4). However, this estimate is based on only 4 deaths so that the ratio was not significantly different from unity. Also, as in other studies based on death certificates, the death certificate did not specify whether the stroke was ischemic or hemorrhagic for many of the deaths attributed to stroke. Therefore, as yet, no conclusions can be drawn as to the level of ischemic stroke in the hemophilia population compared with the general population.
Mortality from other causes
Mortality from liver diseases, including liver cancer, was substantially increased compared with mortality in the general population, as would be expected given the high rates of infection with hepatitis C in the hemophilia population during the period studied. For causes other than liver diseases, hemorrhage, causes indirectly due to bleeding such as accidents, and cardiovascular disease, mortality rates in the hemophilia population were similar to those of the population as whole (Table 4). The small increase in mortality from Hodgkin disease seems likely to be a chance occurrence, and there was no evidence of an increase in non-Hodgkin lymphoma, despite the high levels of hepatitis C infection in this population, although the numbers involved are small and the data are insufficient to rule out a weak relationship.27,28 There was no evidence of increased mortality from cancer in general or from infections other than hepatitis as have sometimes been suggested,7,29,30 and the suicide level in people with hemophilia was similar to that in the general population. Variant CJD was first described in 1996, some 10 years after the first reports of bovine spongiform encephalopathy in cattle in the United Kingdom.31 While our findings provide some reassurance that people in the United Kingdom with hemophilia who have received large pool concentrates derived from British plasma have not been affected by this condition, we recognize that this study covers only the first few years of the potential latent period, and a separate surveillance study for this condition has been established by UKHCDO.
Concluding remarks
This study has made use of the UKHCDO nationwide database, together with the ability to ascertain vital status on a nationwide basis via the National Health Service central registers and, for those who have died, the certified cause of death. It has, for the first time, characterized life expectancy and cause-specific mortality in a large hemophilia population that was not infected with HIV. The results show that, despite the advances that took place in the treatment of hemophilia during the last 2 decades of the 20th century, mortality from intracranial hemorrhage changed little in the absence of factor inhibitors. They also showed that life expectancy in severe hemophilia was still 15 years lower than that of men in the general population at the end of the 20th century, while in moderate/mild disease was 3 years lower.
The prospects for the future are good. The study confirms that there is a substantial reduction in mortality from ischemic heart disease in people with hemophilia compared with the general population and, more importantly, the results are consistent with a substantial reduction in mortality from intracranial hemorrhage among those receiving prophylaxis. If it is possible to continue studies such as this into the future, then the findings of the present study will form a useful baseline with which future mortality patterns in hemophilia populations can be compared, thus obtaining a quantitative measure of the effect of innovations in hemophilia care on mortality.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the United Kingdom Medical Research Council and Cancer Research United Kingdom. Sarah Darby and Sau Wan Kan are supported by Cancer Research United Kingdom. The UKHCDO National Database was held at Oxford Haemophilia Centre and supported by Oxford Haemophilia Centre while this study was being carried out.
We thank the Office of National Statistics and the General Register Offices in Edinburgh and Belfast for help in establishing the vital status of the population and providing death details, and Patricia Wallace of Oxford Haemophilia Centre for clerical work.
Authorship
Contribution: R.J.S., S.W.K., P.L.F.G., and S.C.D. coordinated data collection; S.C.D. designed the statistical analysis; S.C.D. and S.W.K. carried out the statistical analysis; all authors participated in the preparation of the report.
A complete list of the participating institutions of the UK Haemophilia Centre Doctors' Organisation can be found in the Appendix.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: UKHCDO Secretariat, University Department of Haematology, Manchester Royal Infirmary, Oxford Road, Manchester M13 9WL, United Kingdom; e-mail: lynne.dewhurst@cmmc.nhs.uk.
Appendix
United Kingdom Haemophilia Centres contributing data to this study: Aberdeen: Grampian Area Haemophilia Centre, Aberdeen Royal Infirmary. Ashford: Haematology Laboratory, Ashford Hospital. Bangor: Haemophilia Centre, Ysbyty Gwynedd. Barnstaple: Department of Haematology, North Devon District Hospital. Basingstoke: The North Hampshire Haemophilia Centre, North Hampshire Hospital. Bath: Department of Haematology, Royal United Hospital (North). Bedford: Department of Haematology, Bedford Hospital Trust. Belfast: Northern Ireland Haemophilia Comprehensive Care Centre, Belfast City Hospital; Royal Belfast Hospital for Sick Children. Birmingham: Haemophilia Unit, Queen Elizabeth Hospital; Department of Haematology, The Birmingham Children's Hospital National Health Service Trust. Blackburn: Department of Haematology, Blackburn Royal Infirmary. Bournemouth/Poole: Department of Haematology, Poole General Hospital. Bradford: Bradford Haemophilia Centre; Department of Paediatrics, Bradford Royal Infirmary. Brighton: Department of Haematology, Royal Sussex County Hospital. Bristol: Avon Haematology Unit, Bristol Oncology Centre; Department of Oncology/Bone Marrow Transplantation, Royal Hospital for Sick Children. Bury St. Edmunds: The West Suffolk Hospital. Camberley: Department of Pathology, Frimley Park Hospital. Cambridge: Department of Clinical Haematology, Addenbrooke's Hospital. Canterbury: Haemophilia Centre, Kent and Canterbury Hospital. Cardiff: Department of Haematology, University Hospital of Wales. Carlisle: Department of Pathology, Cumberland Infirmary. Carshalton: Department of Haematology, St Helier Hospital. Chelmsford: Department of Haematology, Broomfield Hospital. Chertsey: Department of Pathology, St Peter's Hospital. Chichester: Haematology Laboratory, St Richard's Hospital. Colchester: Department of Haematology, District General Hospital. Coventry: Department of Haematology, Walsgrave Hospital National Health Service Trust. Derby: Derbyshire Royal Infirmary. Dorchester: Department of Haematology, West Dorset Hospital. Dundee: Haemophilia Unit, Ninewells Hospital. Eastbourne: Department of Haematology, District General Hospital. Edinburgh: Haemophilia Centre, Royal Infirmary; Department of Haematology, Royal Hospital for Sick Children. Epsom: Haematology Laboratory, Epsom General Hospital. Exeter: Department of Haematology, Royal Devon & Exeter Hospital (Wonford). Glasgow: Haemophilia and Thrombosis Centre, Glasgow Royal Infirmary; Department of Haematology, Royal Hospital for Sick Children. Harlow: Department of Haematology, Princess Alexandra Hospital. Harrogate: Harrogate District Hospital. Harrow: Department of Haematology, Northwick Park Hospital. Hereford: Department of Haematology, County Hospital. Hillingdon: Hillingdon Hospital. Huddersfield: Department of Haematology, Huddersfield Royal Infirmary. Hull: Department of Haematology, Kingston General Hospital. Inverness: Department of Haematology, Raigmore Hospital. Ipswich: The Ipswich Hospital. Kettering: General Hospital. Kingston upon Thames: Haematology Laboratory, Kingston Hospital. Lancaster: Department of Haematology, Royal Lancaster Infirmary. Leeds: Haemophilia Unit; Department of Paediatric Haematology, St James' University Hospital. Leicester: Haemophilia Centre, Leicester Royal Infirmary. Lincoln: Lincoln County Hospital. Liverpool: Haematology Laboratories, Royal Liverpool University Hospital; Department of Haematology, Royal Liverpool Children's Hospital, Alder Hey. London: Department of Haematology, Imperial College School of Medicine, Hammersmith Hospital; Department of Haematology, St Mary's Hospital; Department of Haematology, Great Ormond Street Hospital for Sick Children; Department of Haematology, Barts and The London Haemophilia Centre, Royal London Hospital; Haemophilia Centre, Royal Free Hospital; Department of Haematology, University College Hospital; Department of Haematology, King's College Hospital; Department of Haematology, Lewisham Hospital; Haemophilia Centre, St Thomas' Hospital; Department of Haematology, St George's Hospital. Luton: Department of Pathology, Luton and Dunstable Hospital. Manchester: University Department of Haematology, Manchester Royal Infirmary; Department of Haematology, Royal Manchester Children's Hospital. Medway: Medway Maritime Hospital. Milton Keynes: Department of Haematology, Milton Keynes Hospital. Middlesborough: Department of Clinical Pathology, Middlesborough General Hospital. Newcastle upon Tyne: Haemophilia Centre, Royal Victoria Infirmary. Newport: Department of Haematology, Royal Gwent Hospital. Northampton: Department of Haematology, Northampton General Hospital National Health Service Trust. Norwich: Department of Haematology, Norfolk and Norwich Hospital. Nottingham: Department of Haematology, University Hospital, Queen's Medical Centre. Oxford: Oxford Haemophilia Centre, Churchill Hospital. Peterborough: Peterborough District Hospital. Plymouth: Derriford Hospital. Portsmouth: Central Laboratory, East Wing, St Mary's General Hospital. Salisbury: Department of Pathology, Salisbury District Hospital. Sheffield: Sheffield Haemophilia and Thrombosis Centre, Royal Hallamshire Hospital; The Roald Dahl Paediatric Haematology Centre, The Children's Hospital. Shrewsbury: Department of Pathology, Shrewsbury Hospital (Copthorne North). Southampton: South Hampshire Haemophilia Centre, South Hampshire General Hospital. Southend: Department of Haematology, Southend Hospital. St Leonards-On-Sea: Conquest Hospital. Stoke on Trent: Central Pathology Laboratory, North Staffordshire Hospital. Sunderland: The District General Hospital. Swansea: Swansea Haemophilia Centre, Singleton Hospital. Taunton/Yeovil: Department of Haematological Medicine, Taunton and Somerset Hospital. Thornton Heath: Haematology Laboratory, Mayday Hospital. Torquay: Department of Haematology, Torbay Hospital. Truro: Department of Haematology, Treliske Hospital. Tunbridge Wells: Pembury Hospital. Whitehaven: West Cumberland Hospital. Winchester: Pathology Laboratory, Royal Hampshire County Hospital. Wolverhampton: Department of Haematology, New Cross Hospital. Worcester: Department of Haematology, Worcester Royal Infirmary National Health Service Trust. Worthing: Haematology Laboratory, Worthing Hospital. York: York District Hospital.