Abstract
We evaluated the efficacy of umbilical cord blood (UCB) in the setting of a nonmyeloablative regimen consisting of fludarabine (200 mg/m2), cyclophosphamide (50 mg/kg), and a single fraction of total body irradiation (200 cGy) with cyclosporine and mycophenolate mofetil for posttransplantation immunoprophylaxis. The target cell dose for the UCB graft was 3.0 × 107 nucleated cells/kg, resulting in the selection of a second partially human leukocyte antigen-matched UCB unit in 85%. One hundred ten patients with hematologic disease were enrolled. Neutrophil recovery was achieved in 92% at a median of 12 days. Incidences of grades III and IV acute and chronic graft-versus-host disease (GVHD) were 22% and 23%, respectively. Transplantation-related mortality was 26% at 3 years. Survival and event-free survival (EFS) at 3 years were 45% and 38%, respectively. Favorable risk factors for survival were absence of high-risk clinical features (Karnofsky 50-60, serious organ dysfunction, recent fungal infection, P < .01) and absence of severe GVHD (P = .04), and favorable risk factors for EFS were absence of high-risk clinical features (P < .01) and use of 2 UCB units (P = .07). These findings support the use of UCB after a nonmyeloablative conditioning as a strategy for extending the availability of transplantation therapy, particularly for older patients.
Introduction
Umbilical cord blood (UCB) transplantation has emerged as an effective therapy for treating patients with advanced or high-risk hematologic diseases who have no suitable related or unrelated blood or bone marrow donor.1-3 Conventional myeloablative conditioning regimens, however, are associated with significant morbidity and mortality, particularly in older patients or those with extensive prior therapy or other clinical feature associated with transplantation-related mortality (TRM).1,2,4-6 In such patients, nonmyeloablative regimens have been used to reduce the risk of regimen-related toxicity and TRM.7-14 To date, only pilot studies on the use of UCB transplantation using various reduced-intensity conditioning regimens have been reported.15-23 This report establishes the safety profile of one such regimen that consists of fludarabine (FLU; 200 mg/m2), cyclophosphamide (CY; 50 mg/kg), and single fraction of total body irradiation (TBI; 200 cGy) in 110 consecutive adult patients with hematologic disease who were recipients of UCB.
Patients, materials, and methods
Inclusion criteria
Patients with advanced or high-risk hematologic disease were eligible for UCB transplantation if they had no related donor matched at 5-6/6 human leukocyte antigen (HLA) loci (A, B, and DRB1). Patients were eligible for nonmyeloablative therapy if they met any of the following criteria: aged greater than 45 years, preexisting high-risk clinical features for TRM (serious organ dysfunction [n = 8]; invasive mold infection within 4 months before transplantation [n = 14]; Karnofsky performance score 50 to 60 [n = 3]), or history of extensive prior therapy (defined as > 12 months alkylator-based chemotherapy; > 6 months alkylator-based chemotherapy plus extensive radiation; or history of autologous transplantation). Serious organ dysfunction was defined as a hyperbilirubinemia greater than 2 times the upper limit of normal, elevated hepatic transaminases greater than 2 times the upper limit of normal, corrected carbon monoxide diffusing capacity (DLCO) no greater than 50% of predicted, or left ventricular ejection fraction (LVEF) less than 45%. Patients who were more than 70 years of age or had end-stage organ dysfunction (DLCO < 30% predicted or LVEF < 35%), active serious infection, or HIV infection were not eligible. Treatment protocols were approved by the Institutional Review Board of the University of Minnesota and registered at http://www.cancer.gov (protocol IDs: UMN-2000LS039 and UMN-2005LS036). As part of a pilot study of reduced-intensity conditioning, 22 patients were previously reported.24 All patients or legal guardians provided written informed consent in accordance with the Declaration of Helsinki before enrollment.
UCB unit selection algorithm
UCB units were required to be matched at greater than 4 of 6 HLA antigens based on antigen-level HLA-A and -B typing and allele-level HLA-DRB1 typing. Matching at HLA-C, DQ, and DP were not considered. Over the duration of the study, UCB units were required to have a minimum cryopreserved total nucleated cell (TNC) dose of 2.0 × 107/kg. However, the target cell dose was greater than or equal to 3.0 × 107 TNC/kg, resulting in the selection of a second partially HLA-matched UCB unit if available. In those for whom a second UCB unit could be identified, the second unit also had a minimum of 4 of 6 antigens matched with the first unit. Methodology of HLA typing has been detailed elsewhere.25
Treatment
One hundred ten patients received a single dose of 50 mg/kg of CY on day −6, 40 mg/m2 of FLU daily on days −6 to −2, and a single fraction of 200 cGy TBI without shielding on day −1. Equine antithymocyte globulin (ATG, ATGAM; Pharmacia, Kalamazoo, MI) at 15 mg/kg every 12 hours on days −3 to −1 was added in a subpopulation of patients who had received less than 2 cycles of multiagent chemotherapy within the 3 months before enrollment (and no history of autologous transplantation). All patients received cyclosporine (CsA) twice daily from day −3 for at least 3 months with target trough levels of 200 ng/mL to 400 ng/mL and mycophenolate mofetil (MMF) at 1 g intravenously or orally twice daily from day −3 to +30.
Patients were hospitalized in single rooms ventilated with high-efficiency particulate air filtration systems. Patients at risk for the recurrence of herpes simplex virus received prophylactic low dose acyclovir until day 100. Patients seropositive for cytomegalovirus (CMV) received prophylactic high dose acyclovir until neutrophil recovery followed by acyclovir until day 100. Documented CMV reactivation or infection demonstrated by antigenemia or DNA polymerase chain reaction testing after transplantation was treated with therapeutic doses of ganciclovir + intravenous immunoglobulin. Broad-spectrum antibiotics were administered for fever during neutropenia, and antifungal coverage was added for persistent fever unresponsive to antibiotic therapy. All patients received fluconazole or voriconazole for prophylaxis of fungal infections for 100 days and trimethoprim-sulfamethoxazole for prophylaxis of Pneumocystis carinii after engraftment for 12 months after transplantation and extended spectrum fluoroquinolones for prophylaxis of Gram-positive organisms during treatment of GVHD. Granulocyte-colony stimulating factor (G-CSF; 5 μg/kg per day) from day 0 was administered to all patients until the absolute neutrophil count (ANC) was greater than 2500/μL for 2 consecutive measurements.
Cryopreserved units of UCB were transported to the transplantation center via overnight delivery in a dry shipper previously cooled by liquid nitrogen (temperature less than −150°C) before the initiation of the preparative regimen and then maintained in the vapor phase of liquid nitrogen until the day of transplantation. Units were thawed using the method described by Rubinstein et al.26 Second units were infused within 1 hour of the prior UCB infusion.
Study endpoints
The primary end point was the incidence of neutrophil recovery, defined as neutrophil recovery (ANC ≥ 500/μL) in association with partial or complete chimerism by 42 days after transplantation. Partial chimerism was defined as marrow reconstitution of 10% to 90% donor cells, and complete chimerism was defined as greater than 90%. Event times for neutrophil recovery were measured from the date of transplantation and were censored for death or disease progression before day 21 without neutrophil recovery. Primary graft failure was defined as lack of neutrophil recovery (ANC ≥ 500/μL) at day 42 or less than 10% marrow reconstitution of donor origin. Secondary graft failure was defined as severe neutropenia of more than 1 week in duration or autologous recovery after primary engraftment. Engraftment in double-unit recipients was defined as the percentage of cells derived from both donors. Five patients were not evaluable for engraftment because they died or relapsed within 21 days after transplantation.
Secondary endpoints included the cumulative incidence of platelet recovery at 6 months, acute GVHD at day 100, chronic GVHD at 1 year, relapse of malignancy at 3 years, TRM at 6 months, and the probabilities of event-free (relapse, progression, or death) and overall survival at 3 years. Diagnosis of acute and chronic GVHD was based on standard clinical criteria with histopathologic confirmation where possible.27 The analysis of relapse included only 106 patients with malignant diseases.
Statistical analysis
Variables related to patient, disease, and transplantation characteristics were compared using the chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. Probabilities of overall and EFS were calculated using the Kaplan-Meier estimator.28 For analyses of survival, death from any cause was considered an event and for EFS, relapse, progression, or death. Data on surviving patients were censored at last follow-up for analyses of EFS. Patients alive in continuous remission were censored at last follow-up. Probabilities and 95% confidence intervals (CI) of neutrophil and platelet recovery, acute and chronic GVHD, transplantation-related mortality, and relapse were calculated using the cumulative incidence function.28 For neutrophil and platelet recovery and GVHD, death without the event was the competing hazard. For transplantation-related mortality, relapse was the competing event, and for relapse, transplantation-related death was the competing event.
Statistical comparison of time-to-event curves was completed by log-rank test. Continuous factors, such as chimerism and cell dose, were compared between recipients of 1 and 2 units by the nonparametric Wilcoxon test.29 Comparison of proportions was performed by the chi-square test or Fisher exact test if the expected number was less than or equal to 5. In order to study variables associated with unit predominance in recipients of double-unit grafts, a matched pairs t test was used for continuous factors and a McNemar test was used for categorical factors.29
Logistic regression analysis29 was used to model factors influencing sustained donor engraftment, and Cox proportional hazards regression30 modeled potential predictors of acute and chronic GVHD, TRM, and event-free and overall survival. The following variables were evaluated in regression analysis of sustained donor engraftment: interval from multiagent chemotherapy or prior autologous transplantation, number of UCB donor units, recipient age, diagnosis, UCB donor-recipient HLA match, CD34+ cell dose, conditioning including ATG, recipient CMV serostatus, and history of prior autologous transplantation. In the Cox regression models for grades II-IV acute GVHD, TRM, EFS, and overall survival we considered number of UCB donor units, recipient age, weight, sex, donor-recipient HLA match, TNC dose, CD34+ cell dose, CD3+ cell dose, interval from prior chemotherapy, conditioning with ATG, presence of preexisting high-risk clinical features (poor organ function, Aspergillus in prior 4 months, Karnofsky ≤ 60, extensive prior therapy) and recipient CMV serostatus. In Cox regression models for TRM, EFS, and overall survival, the following additional variables were evaluated: diagnosis, disease risk, and development of acute GVHD.
Results
Patient and graft characteristics
One hundred ten patients received transplants between October 2001 and September 2005. Median age and weight of patients was 51 (range, 17-69) years and 76 (range, 50-134) kg, respectively; other patient characteristics are detailed in Table 1. Fifty-one (46%) patients received nonmyeloablative therapy based solely on age of more than 45 years whereas 44 (40%) patients had 2 or more eligibility criteria. The median follow-up of surviving patients was 19 (range, 4.8-51) months.
Graft characteristics are described in Table 2. Notably, most patients received 2 UCB units (n = 93) to achieve the required cryopreserved cell dose. For the entire group of patients, the median cell doses infused were 3.7 × 107 TNC/kg (range, 1.1-5.3), 4.7 × 105 CD34/kg (range, 0.7-18.8), and 1.1 × 107 CD3/kg (range, 0.2-3.0). Cell doses were similar between recipients of single versus double UCB transplantation (3.3 vs 3.7 × 107 TNC/kg, P = .21, and 3.8 vs 4.9 × 105 CD34/kg, P = .60) except for higher CD3 dose in recipients of 2 UCB units (1.2 vs 0.6 × 107/kg, P < .01). Among the 203 infused UCB units, 12(6%) were 6/6, 67 (33%) were 5/6, and 124 (61%) were 4/6HLA-matched to the recipient. Sixty-five percent of patients transplanted with 1 UCB unit received a 2-HLA antigen mismatched unit whereas 79% of those transplanted with 2 UCB units received at least 1 2-HLA antigen mismatched unit.
Hematopoietic recovery and chimerism
Primary neutrophil recovery occurred in 92% at a median of 12 days (range, 0-32) after UCB infusion (Figure 1A). Recovery was similar in those receiving 1 or 2 units (94% vs 91%, respectively). Primary and secondary graft failure occurred in 7 and 8 patients, respectively. For the entire cohort, the cumulative incidence of sustained engraftment (ie, neutrophil recovery with complete chimerism) was 85% (95% CI, 77%-92%). Logistic regression analysis failed to reveal any independent predictors of graft failure (Table 3).
Cumulative incidence of neutrophil recovery by day 42 and unsupported platelet recovery greater than 50 × 109/L at 6 months after nonmyeloablative umbilical cord blood transplantation. (A) Neutrophil recovery. (B) Unsupported platelet recovery.
Cumulative incidence of neutrophil recovery by day 42 and unsupported platelet recovery greater than 50 × 109/L at 6 months after nonmyeloablative umbilical cord blood transplantation. (A) Neutrophil recovery. (B) Unsupported platelet recovery.
The cumulative incidence of platelet recovery (> 50 × 109/L) was 65% (95% CI, 54%-76%) by day 180 and occurred at a median of 49 days (range, 0-134) (Figure 1B).
Chimerism was mixed in early time points (Figure 2). The median bone marrow chimerism in 98 evaluable patients was 89% (range, 8%-100%) at day 21. At day 100 and 1 year, chimerism was 100% (range, 34%-100%) and 100% (range, 87%-100%), respectively. Of the 81 patients with sustained chimerism after receiving 2 UCB units, only 1 unit contributed to long-term hematopoiesis in all patients. Double chimerism (ie, contribution from each UCB unit) was detectable within the bone marrow in 43% of patients at day 21, 9% at day 100, 3% at day 180, and 0% at 1 year. The median percent of donor-derived cells attributable to the dominant unit was 83% (range, 8%-100%) at day 21 and 100% (range, 34%-100%) beyond day 100. Neither total nucleated, CD34+, and CD3+ cell doses; HLA matching; nucleated cell viability; ABO typing; gender match; or order of unit infusion was predictive of which unit eventually dominated.
Bone marrow chimerism 21, 100, 180, and 365 days after nonmyeloablative umbilical cord blood transplantation. The solid horizontal line indicates the median chimerism; ◇, patients with sustained donor engraftment; and ▴, patients who developed graft failure.
Bone marrow chimerism 21, 100, 180, and 365 days after nonmyeloablative umbilical cord blood transplantation. The solid horizontal line indicates the median chimerism; ◇, patients with sustained donor engraftment; and ▴, patients who developed graft failure.
Acute and chronic GVHD
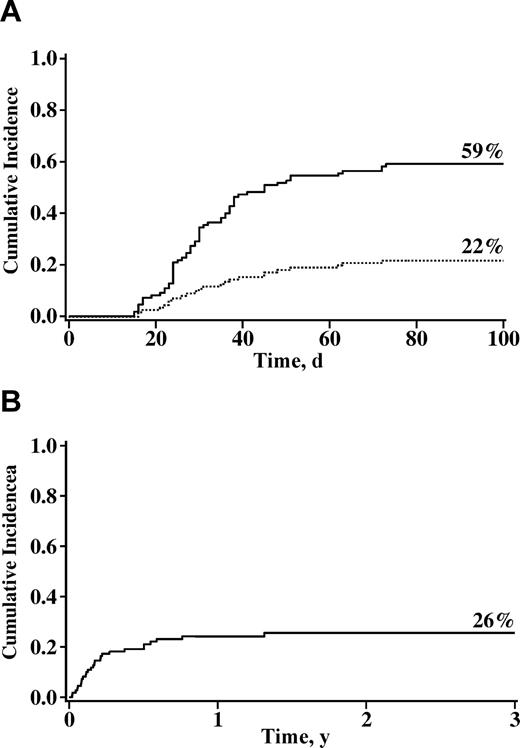
The cumulative incidences of grades II-IV and grades III and IV acute GVHD at day 100 were 59% (95% CI, 49%-69%) and 22% (95% CI, 14%-30%), respectively (Figure 3A). Twenty one patients had grade III and 3 had grade IV GVHD. In univariate analysis, patients who received 2 UCB units had a trend toward a higher risk of grades II-IV acute GVHD (62% vs 41%, P = .09). Cox regression analysis (Table 3) revealed that patients receiving double-unit UCB grafts and those not receiving ATG had a higher relative risk of GVHD. Neither presence of mixed chimerism (relative risk [RR] 0.8; 95% CI, 0.3-1.3; P = .30) nor time to neutrophil recovery (RR 0.8; 95% CI, 0.7-1.1; P = .22) were associated with risk of acute GVHD. The cumulative incidence of chronic GVHD was 23% (95% CI, 15%-31%) at 1 year with no factor identified as predictive of chronic GVHD.
Cumulative incidence of grades II-IV (––) and grades III and IV (----) acute GVHD and 3-year treatment-related mortality after nonmyeloablative umbilical cord blood transplantation. (A) Incidences of GVHD. (B) Incidence of 3-year treatment-related mortality.
Cumulative incidence of grades II-IV (––) and grades III and IV (----) acute GVHD and 3-year treatment-related mortality after nonmyeloablative umbilical cord blood transplantation. (A) Incidences of GVHD. (B) Incidence of 3-year treatment-related mortality.
Transplantation-related mortality
The incidence of TRM was 19% (95% CI, 12%-26%) at day 180 and 26% (95% CI, 18%-34%) at 3 years (Figure 3B). Factors associated with a higher risk of TRM at 180 days in univariate analysis were recipient age of 45 years or younger (32% vs 15%, P = .02), recipient CMV-negative serostatus (29% vs 9%, P < .01), use of ATG (31% vs 13%, P = .02), presence of preexisting high-risk clinical features (38% vs 12%, P < .01), and history of fungal infection within 4 months of transplantation (36% vs 17%, P = .05). For patients in whom the only indication for a nonmyeloablative conditioning was age of greater than 45 years (n = 20), TRM was 10% (95% CI, 0%-23%) at 6 months and 15% (95% CI, 7%-23%) at 2 years. In Cox regression analysis, the presence of one or more preexisting high-risk clinical features was the only independent predictor of increased TRM (Table 3).
Survival and relapse
The incidence of disease relapse or progression for the 106 patients transplanted for malignant diseases was 31% (95% CI, 21%-41%) at 3 years, with a trend toward a reduced relapse among double UCB graft recipients (30% vs 41%, P = .07). The probability of EFS was 38% (95% CI, 28%-48%) at 3 years and was better using two 39% (27%-51%) versus one 24% (4%-44%) UCB units (P = .05; Figure 4A). EFS was significantly better for patients without preexisting high-risk clinical features at the time of transplantation (45% vs 17%, P < .01). In Cox regression analysis, improved EFS was associated with absence of preexisting high-risk clinical features (P < .01) with a trend toward better EFS in recipients of 2 UCB units (P = .07) (Table 3).
Cumulative proportion of 3-year event-free survival and overall survival for patients receiving either 1 (—) or 2 (---) UCB unit transplantation after a nonmyeloablative conditioning regimen. (A) Event-free survival. (B) Overall survival.
Cumulative proportion of 3-year event-free survival and overall survival for patients receiving either 1 (—) or 2 (---) UCB unit transplantation after a nonmyeloablative conditioning regimen. (A) Event-free survival. (B) Overall survival.
Three-year survival was 45% (95% CI, 34%-56%) with 57 patients surviving 5 to 50 months after transplantation (Figure 4B). Notably, for patients greater than 45 years of age (n = 20) and no other indication for a nonmyeloablative conditioning, survival was 49% (95% CI, 25%-73%) at 3 years. The most frequent causes of death were disease relapse or disease progression (n = 27) and infection (n = 12) and GVHD (n = 6). All 3 patients with performance status between 50 and 60 died: 1 with relapse at day 195 and 2 of multiorgan failure at days 20 and 46 after transplantation. In Cox regression analysis, patients with preexisting high-risk clinical features and grades II and IV acute GVHD had a significantly higher relative risk of death (Table 3).
Discussion
The present study supports the use of UCB transplantation after a nonmyeloablative therapy in adults with hematologic disease. This therapeutic approach was associated with rapid neutrophil recovery, low incidence of TRM, and survival rates similar to those reported with other HSC sources.7-9,11,13,14 In addition, we also observed the following: (1) transient mixed chimerism (ie, presence of host cells) at early time points after transplantation; (2) transient double chimerism (ie, presence of cells from each donor unit) in recipients of 2 UCB units; (3) a higher incidence of grade II acute GVHD in recipients of 2 partially HLA-matched UCB units; and (4) a trend toward lower relapse rate and higher EFS in recipients of 2 UCB units. Although larger patient numbers and longer follow-up are needed to verify the significance of these observations, it is clear that use of the double UCB platform in the setting of a nonmyeloablative therapy extends the availability of transplantation to those who cannot find a suitably HLA-matched adult volunteer marrow or peripheral blood donor and who are at increased risk of regimen-related toxicity and TRM, such as older or heavily treated patients.
Rapid neutrophil recovery after nonmyeloablative therapy has been previously reported with other stem-cell sources.7-9,11,12,14 However, it was previously unknown whether UCB provides an adequate number of alloreactive T cells to establish life-long chimerism. As reported in several smaller series, mixed chimerism is established early after nonmyeloablative UCB transplantation with most becoming complete chimeras by day 60 and all by day 100.17,21 Of interest, transient double chimerism was also observed similar to that seen after a myeloablative therapy, with chimerism derived from 1 donor unit at day 100. As in the myeloablative setting,25 no factor reliably predicted which of the 2 UCB units would predominate long term. Further, with a few exceptions, the predominating unit was readily identifiable by day 21. In contrast, Ballen et al21 observed an association between infusion order and unit predominance, with the first UCB unit infused predominating 76% of the time. UCB infusion techniques may play a role. While at the University of Minnesota, the 2 UCB units are infused over 1 hour sequentially with no interval between infusions; Ballen et al typically allow 3.5 and 4.5 hours between UCB unit infusions.
The observed incidence of sustained engraftment was high even compared with reports of adults receiving a myeloablative UCB transplantation.1,2 We found only a trend toward HLA matching as a risk factor associated with sustained donor-derived hematopoietic recovery, as reported by others.31,32 Although we have speculated that the increased cell dose achieved with 2 UCB units may assist in engraftment and partially compensate for the adverse effect of HLA mismatch in the myeloablative setting, neither the nucleated cell dose nor the number of UCB units in the graft were identified as independent risk factors for engraftment after a nonmyeloablative regimen. This is possibly because of the limited range of graft cell doses used and paucity of graft failure events. In fact, no demographic or treatment factors were associated with sustained engraftment. Potential approaches for improving upon engraftment may include better conditioning or posttransplantation immunosuppressive regimens. For example, Maris et al33 have observed better engraftment rates after increasing the dose and treatment duration with MMF.
Our use of 2 partially HLA-matched UCB units25 was first established as a strategy to offer UCB as a stem cell source for patients who did not have an adequate single unit. In this report, most patients were older and larger, significantly skewing the proportion receiving 2 UCB units. Due to the small number of patients transplanted with a single UCB unit, we were not able to detect any potential differences in rate of neutrophil recovery and engraftment in recipients of 1 or 2 UCB units. Although a multicenter randomized study might be considered to verify the true benefit of single- versus double-unit UCB transplantation, such a study is not likely to occur in adults and adolescents because of the limited number of UCB units that would meet the cell dose requirements proposed in this study.
Notably, the incidence of acute GVHD in our cohort was higher than in our prior experience with single-unit UCB transplantation34 yet similar to what we have observed in recipients of 2 UCB units after myeloablative conditioning.25 Our higher incidence of acute GVHD was mostly due to grade II (37%), and GVHD itself was infrequently the primary cause of death (5%). As in other reports of adult UCB transplantation, HLA matching was not associated with the incidence of acute GVHD.32 The effect of HLA matching on acute GVHD might have been obscured because nearly all units were mismatched at 1 (62/203) or 2 (124/203) HLA loci. Alternatively, a higher CD3 cell dose in recipients of 2 units and older recipient age contribute to the risk of GVHD. Published reports on the incidence of acute GVHD in adults after UCB transplantation are variable, ranging from an incidence of less than 30%18,19,22 to an incidence from 40% to 70%.17,21,32,35,36 The lower incidence of acute GVHD among patients who received ATG as part of the conditioning regimen was not surprising because ATG probably provided some degree of in vivo T-cell depletion.
Only a small proportion of patients (23%) developed chronic GVHD, and most did not require extended immunosuppression. In contrast, other adults series of UCB17,19 and adult donor7-9,11-14 transplantations after reduced-intensity conditioning report an incidence of extensive chronic GVHD between 30% and 70%. No risk factor, including use of ATG, was associated with differing risks of chronic GVHD.
Despite a high incidence of acute GVHD, TRM was low and similar to that reported in other studies of nonmyeloablative therapies with UCB18,19,21,23 and other sources of HSCs.7,10,12-14 In Cox regression analysis, preexisting high-risk clinical features were the only factors associated with increased risk of TRM. Notably, older patients without preexisting high-risk clinical features had very low TRM. Furthermore, the data suggest that UCB transplantation after a nonmyeloablative therapy may be a particularly safe option because risks of severe acute and chronic GVHD are limited.
In this series as in others,34,37 the presence of preexisting high-risk clinical features and the development of severe GVHD but not age were associated with poorer overall survival and EFS. Therefore, our data support the use of nonmyeloablative, UCB transplantation in patients 45 years old and older who do not have other high-risk clinical features. However, the data also suggest that patients with certain preexisting clinical conditions should not be routinely considered for transplantation, because TRM remains unacceptably high even with a nonmyeloablative approach. Of note, Sorror et al37 also observed high TRM and poor survival in patients with a comorbidity risk score greater than 3 but only when recipients of myeloablative and nonmyeloablative therapy were analyzed together. Notably, the same group did not find an association between the comorbidity the risk score and risk of TRM or survival in the nonmyeloblative setting.9 Although transplantations might still be indicated, those with high-risk clinical features need to be counseled appropriately about the high risks of regimen-related toxicity and TRM.
Our study's heterogeneous population prevents us from drawing any conclusion regarding the suitability of nonmyeloablative UCB transplantation for any specific diseases. As the experience with nonmyeloablative UCB transplantation grows, the availability of reports of disease-specific outcomes will help identify patient populations that benefit the most from this treatment modality. We speculate that additional strategies are needed to further enhance engraftment in the nonmyeloablative setting, in particular for patients who have not received recent chemotherapy, such as patients with MDS, myelofibrosis, and CML.
In summary, we have demonstrated the safety and efficacy of UCB transplantation after nonmyeloablative preparative therapy with 45% of patients alive at a median of 19 months. This approach substantially extends the availability of transplantation to patient populations previously excluded on the basis of older age and lack of an HLA-matched donor. Furthermore, we have confirmed that the use of 2 partially HLA-matched units is a feasible strategy for potentially extending UCB transplantation to more patients. Except for patients with preexisting high-risk clinical features, survival rates reported are similar to those described for recipients of sibling or unrelated donors grafts after reduced-intensity transplantation.7-9,11,13,14 Low TRM was particularly notable in older patients without other risk factors, suggesting that age alone should not be a barrier to transplantation as a therapeutic option. These data support the conclusion that older patients with high-risk hematologic diseases can now be offered UCB transplantation with nonmyeloablative conditioning as a potentially curative treatment option.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the National Cancer Institute (PO1-CA65493) (J.E.W., T.E.D., P.B.M., and J.S.M.) and the Children's Cancer Research Fund (J.E.W. and T.E.D.).
The authors acknowledge Michael Franklin for editorial assistance.
National Institutes of Health
Authorship
Contribution: C.G.B. and J.S.M. performed research, analyzed data, and wrote the paper; J.N.B. and B.R.B. designed research, performed research, and wrote the paper; D.J.W. designed research, performed research, analyzed data, and wrote the paper; T.E.D. analyzed data and wrote the paper; P.B.M. performed research and wrote the paper; and J.E.W. designed research, performed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Claudio G. Brunstein, Department of Medicine, Mayo Mail Code 480, 420 Delaware Street, SE, Minneapolis, MN 55455; e-mail: bruns072@umn.edu.