Abstract
CD38 is a surface receptor able to induce activation, proliferation, and survival of human and mouse lymphocytes; this molecule is expressed on the surface of both mature and immature B cells. In this work, the function of CD38 in the maturation of murine B lymphocytes in the spleen was analyzed. The results showed that CD38 is highly expressed on Transitional 2 (T2) B lymphocytes with an intermediate expression on Transitional 1 (T1) and mature follicular B cells (M). Correlating with a high expression of CD38, T2 cells are also larger and more granular than T1 or M B cells. T2 cells also showed high levels of other molecules, which indicate an activated phenotype. CD38 crosslinking induced proliferation and maturation of T2 B lymphocytes; in contrast, T1 subset died by apoptosis. Finally, CD38 stimulation of T2 B lymphocytes obtained from Btk-, Lyn-, or Fyn-deficient mice showed a defective differentiation; similarly, drugs interfering with PI3K or ERK decreased the proliferation or differentiation of this subset. This suggests that these molecules participate in the CD38 signaling pathway. As a whole, the results indicate that CD38 plays an important role in the regulation of B-cell maturation in the spleen.
Introduction
CD38 is a 42-kilodalton (kDa) transmembrane glycoprotein widely expressed in hematopoietic tissue. This protein possesses ectoenzymatic and cellular receptor functions.1,2 The incubation of splenic B lymphocytes with anti-CD38 agonistic antibodies induces diverse effects, such as proliferation, calcium mobilization, protein phosphorylation, migration, and apoptosis.3-9 CD38 is expressed from early stages in the bone marrow,10 and its level of expression is regulated during maturation of B or T lymphocytes in both humans and mice.4,11,12 For this reason, CD38 is considered a marker of differentiation and activation of T and B lymphocytes.13-15 All these events may be regulated “in vivo” by putative ligands present in diverse cellular types.16-18 Although the structural requirements for their function are not clear, it has been suggested that CD38 may associate with distinct receptors, including the B-cell receptor (BCR), T-cell receptor, and CD19.19-23 However, since the initial description of the anti-CD38 monoclonal antibody (mAb) NIM-R5, no functional association between CD38 with the BCR was noticed. Simultaneous, or step-by-step, addition of both reagents did not demonstrate additive or costimulatory effects.4 Previous reports have shown that anti-CD38 stimulation of mature splenic B cells induces their proliferation, which requires Bruton Tyrosine Kinase (Btk),24,25 Src family kinases Lyn and Fyn26 or phosphatidylcholine-phospholipase C(PC-PLC)/phospholipase D but not phospholipase C-gamma 2 (PLC-γ2)27 to activate the nuclear factor-kappa B (NF-κB).28 In addition, there is some evidence that CD38 stimulation activates the extracellular signal-regulated kinase (Erk) in both B and T lymphocytes.9,22
Previous reports have shown that anti-CD38 stimulation induces apoptosis of normal and progenitor human B cells.9,29,30 The analysis of a murine pro-B leukemic cell line (Ba/F3) demonstrated that anti-CD38 stimulation induces apoptosis independently of their CD38 enzymatic activity. This independence was demonstrated by either the use of a blocking drug or catalytically inactive CD38.8 CD38-deficient mice have some defects in its humoral response; some of these defects have been explained through a deficient antigen presentation by dendritic cells; nevertheless, defects in B cells have not been fully explored.31 It has been shown that CD38 is expressed selectively during maturation of murine T and B cells, but the role of the molecule during these processes has not been analyzed.10,12
B-cell development has been recently described in more detail,32-34 and immature B lymphocytes in the spleen have been subdivided into 2 subpopulations: transitional 1 (T1) and transitional 2 (T2), which can be differentiated from mature follicular B lymphocytes (M).35 These subsets can be separated by the expression of surface markers, such as CD24, CD21, and CD23.35-37 T1s differentiate into T2s and then become mature B lymphocytes.35 These subsets differentially respond to anti-IgM stimulation or to B-cell activating factor (BLyS, TALL-1, THANK, zTNF4).38-42 In response to BCR crosslinking, T1s die by apoptosis, whereas T2s proliferate and differentiate toward mature B lymphocyte.40 In addition, B-cell activating factor is required for the T1 to T2 transition.39,40 The signaling pathways for this maturation have been partially reported.41,43
The main objective of this work was to analyze the role of CD38 during B-cell development, particularly in the last stages taking place in the spleen. First, the expression of CD38 was evaluated among T1, T2, and M subsets. Then, the proliferation, differentiation, and apoptosis in response to anti-CD38 stimulation were studied. In each case, the enzymatic activity of CD38 was also analyzed. Second, expression and functions of CD38 were evaluated in mice with defects in the kinases Lyn, Fyn, and Btk or through the use of drugs inhibiting phosphatidylinositol 3-kinase (PI3K), Erk and PLC-γ2. The data demonstrated differences in T1 and T2 responsiveness to anti-CD38 stimulation. T2 subset proliferated and differentiated into mature B cells and required PI3K, Btk, Lyn, Fyn, and Erk signaling pathways; meanwhile the T1 subset died by apoptosis. In addition, the enzymatic activity of CD38 was not required for these phenomena. Together, these results indicate that CD38 signaling plays a critical role in B-cell maturation.
Methods
Mice
C57Bl/6 wild-type, C57Bl/6 CD38−/−, B6.129 wild-type, B6.129 Lyn−/−, B6.129 Fyn−/−, BALB/c wild-type, and BALB/c XID were bred and maintained in the Centro de Investigación y de Estudios Avanzados (CINVESTAV) animal facility. All experiments were approved by the Animal Care and Use Committee of CINVESTAV. Female 6- to 8-week-old mice were used for all experiments.
Flow cytometry
Cell suspensions were prepared from splenocytes. Erythrocytes were depleted with 0.85% ammonium chloride solution. One million cells in phosphate-buffered saline (PBS) containing 1% bovine serum albumin and 0.01% NaN3 were stained with the following antibodies: anti-CD21-fluorescein isothiocyanate (FITC), anti-B220-Sprd, anti-CD24-biotin, and anti-CD38-PE (Southern Biotechnology Associates, Birmingham, AL). Then the cells were washed with PBS containing 1% bovine serum albumin and 0.01% NaN3 and stained with streptavidin-APC (BD Biosciences PharMingen, San Diego, CA). After washing, the cells were fixed with 1% formaldehyde in PBS. Data were collected in FACSCalibur cytometer (BD Biosciences, San Jose, CA) and analyzed using the FlowJo software (Tree Star, Ashland, OR). Other molecules were analyzed with the following antibodies: anti–gM-PE, anti–IgD-PE, anti–CD5-PE, anti–MHC-II-PE, anti–CD44-PE, anti–CD25-PE, anti–CD23-PE (Southern Biotechnology Associates), and streptavidin-APC.
Purification of B-cell subpopulations
Spleen cell suspensions (2 × 108) from each strain of mice were stained with the following antibodies: anti–B220-PerCP (Miltenyi Biotec, Auburn, CA), anti–CD21-FITC, anti–CD23-PE, and anti–CD24-biotin. After washing with RPMI 1640 (Invitrogen, Carlsbad, CA), the cells were stained with streptavidin-APC. Then, the cells were sorted in a FACSVantage cell sorter (BD Biosciences). Each purified population was collected in 0.5 mL of cold 10% fetal calf serum (Invitrogen). Viability evaluated by Trypan blue exclusion (Invitrogen) was above 95%. Cell numbers were adjusted and the cells were used immediately.
Proliferation, differentiation, and apoptosis assays
After sorting, 1 to 2 × 105 of each B subset was cultured in RPMI 1640 medium containing 10% fetal bovine serum, 100 μM nonessential amino acids (Sigma-Aldrich, St Louis MO), 1 mM sodium pyruvate (Invitrogen), 0.2 mg/mL penicillin (Invitrogen), 0.5 mg/mL streptomycin (Invitrogen), and 5.5 × 10−5 M β-mercaptoethanol (Invitrogen). In some experiments, cells were preincubated 30 minutes with each of the following drugs: 30 μM LY294002 (PI3K inhibitor), 25 μM PD98059 (Erk inhibitor), 200 nM U-73 122 (PLC-γ2 inhibitor), or 200 nM of the inactive analog U-73 343 (all from Calbiochem, San Diego, CA), 100 μM 8-bromo-cyclic adenosine diphosphate ribose (8-Br-cADPR; Sigma-Aldrich), or vehicle alone. Cells in triplicate wells were stimulated with rat-IgG (50 μg/mL), rat-IgG2a (50 μg/mL; Zymed Laboratories, South San Francisco, CA), anti-CD38 (NIM-R5; 50 μg/mL), F(ab′)2 anti–mouse IgM (10 μg/mL; Cappel Laboratories, Durham, NC), or lipopolysaccharide (LPS, from Escherichia coli 055:B5; 20 μg/mL), with or without 200 U/mL interleukin-4 (IL-4; Genzyme, Cambridge, MA). The plates were incubated 24 and 48 hours at 37°C 8 hours before harvesting, the cells were pulsed with 1 μCi of [3H]-thymidine (25 Ci/mmol; GE Healthcare, Little Chalfont, United Kingdom) and then harvested. [3H]-Thymidine incorporated to DNA was quantified in the scintillation counter (LS 6500; Beckman Coulter, Fullerton, CA). To evaluate differentiation, the cells were stimulated as described for the proliferation assay. In some experiments, a lower concentration of anti-CD38 (25 μg/mL) or F(ab′)2 anti–mouse IgM (5 μg/mL) was used. After incubation, the cells were harvested and washed with medium. Finally, they were restained with the primary antibodies previously described. For apoptosis, T1 B cells were stimulated as described for the proliferation and differentiation assays. They were harvested 12 and 24 hours after incubation and stained with annexin V-biotin (BD Biosciences PharMingen) according to the manufacturer's instructions. After incubation, the cells were washed and stained with streptavidin-APC and propidium iodide (PI; Sigma-Aldrich). In other experiments, the cells were permeabilized 2 hours with cold 90% methanol (Mallinckrodt Baker, Phillipsburg, NJ), and stained 15 minutes with PI. The data were collected in the FACSVantage cell sorter and analyzed by FlowJo software.
Western blot
A total of 2 million T2 B cells were stimulated 1, 5, and 10 minutes with rat-IgG (50 μg/mL), anti-CD38 (50 μg/mL), or F(ab′)2 anti-mouse IgM (20 μg/mL). After stimulation, the cells were lysed with a buffer containing 10 mM Tris/HCl (pH 7.3; Merck, Darmstadt, Germany), 2 mM Na3VO4 (Sigma-Aldrich), 0.4 mM EDTA (Mallinckrodt Baker), 10 mM NaF (Sigma-Aldrich), 1 mM phenylmethanesulfonyl fluoride (Sigma-Aldrich), 2 μg/mL aprotinin (Sigma-Aldrich), 2 μg/mL leupeptin (Roche Diagnostics, Indianapolis, IN) and 1% Nonidet P-40 (v/v) (Sigma-Aldrich). Equivalent protein quantities were loaded into a 10% gel (sodium dodecyl sulfate-polyacrylamide gel electrophoresis). After separation, proteins were transferred to nitrocellulose membranes and then incubated with anti-phospho-p42/44 MAPK (Thr202/Tyr204; Cell Signaling Technology, Danvers, MA) or anti-ERK-2 (Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were developed with anti-mouse-HRP (Zymed) or anti–rabbit-HRP antibodies (DakoCytomation, Dako North America, Carpinteria, CA). Finally, proteins were detected using ECL-Plus (GE Healthcare). In some experiments, the cells were preincubated 30 minutes with 30 μM LY294002, 25 μM PD98059 or the vehicle (dimethylsulfoxide, DMSO).
Erk phosphorylation
A total of 1 million splenocytes were stimulated 10 minutes, as described in “Western blot.” After stimulation, cells were fixed in 10% formaldehyde and permeabilized with cold 90% methanol. The cells were stained with mouse IgG1 anti-phospho-p42/44 MAPK and then goat anti–mouse IgG1-PE (Southern Biotechnology Associates). The cells were restained with anti-CD21-FITC, anti-CD24-biotin, and then APC-streptavidin. Data were collected in the FACSVantage cell sorter and analyzed by FlowJo software (Tree Star). In some experiments, the cells were preincubated 30 minutes with 25 μM PD98059 or the vehicle (DMSO).
Statistical analysis
The statistical significance of all the indicated assays was assessed with the Student t test (P < .05 was considered significant).
Results
CD38 is differentially expressed during development of splenic B lymphocytes
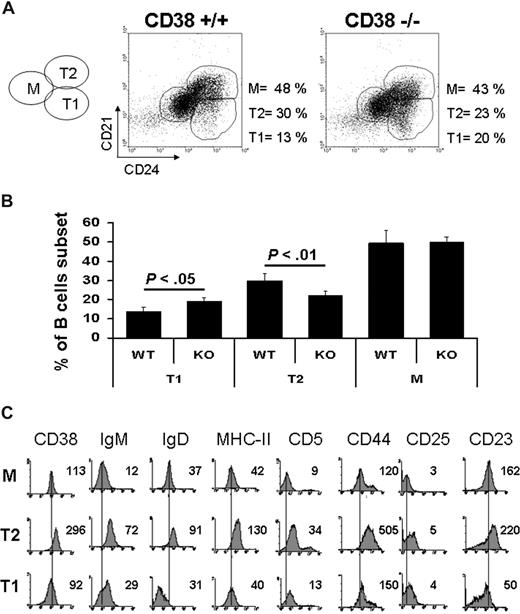
A region containing B220-positive lymphocytes was created, and the percentage of T1 (CD24high, CD21low), T2 (CD24high, CD21high), and M (CD24low, CD21intermediate) B lymphocytes was evaluated according to Loder et al.35 The results showed that the percentages of each subset in the wild-type mice were similar to previous reports (Figure 1A).35,40 The numbers of T2 B cells from CD38-deficient mice were diminished, whereas those from T1 B cells were higher compared with their wild-type mice counterpart (Figure 1A,B); both differences were statistically significant. CD38 was highly expressed on T2 cells compared with its expression on T1 and M B lymphocytes (Figure 1C). In addition, T2 B cells were larger and more granular (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article) than T1 and M B lymphocytes. The expression of IgM, IgD, MHC-II, CD5, CD44, CD25, and CD23 was also evaluated (Figure 1C). The results showed that all these molecules were highly expressed on the T2 B-cell subset. As a whole, these results suggested that CD38 may participate during B-lymphocyte maturation, particularly in the transition from T1 to a mature phenotype.
Transitional 1 B cells are increased and Transitional 2 B cells are decreased in CD38-deficient mice. Total splenic cells were gated on B220-positive cells (100%). Percentage of each subset was calculated according to the expression of CD21 and CD24. (A) Representative dot plots from wild-type and CD38-deficient mice. (B) Percentages of each population from 10 independent experiments. Results are expressed as mean plus or minus SD (P < .05 or P < .01 as indicated, Student t test). (C) A 4-color stain was used to analyze the expression of different surface markers. Each histogram shows the mean fluorescence intensity for each marker in every subpopulation.
Transitional 1 B cells are increased and Transitional 2 B cells are decreased in CD38-deficient mice. Total splenic cells were gated on B220-positive cells (100%). Percentage of each subset was calculated according to the expression of CD21 and CD24. (A) Representative dot plots from wild-type and CD38-deficient mice. (B) Percentages of each population from 10 independent experiments. Results are expressed as mean plus or minus SD (P < .05 or P < .01 as indicated, Student t test). (C) A 4-color stain was used to analyze the expression of different surface markers. Each histogram shows the mean fluorescence intensity for each marker in every subpopulation.
CD38 engagement induces proliferation of T2 and M B lymphocytes
T1, T2, and M B cells were purified as reported35,40 to evaluate the proliferative response of B-cell subsets to anti-CD38 stimulation. The sorting analysis revealed 95% purity for each subset (Figure S2A). T1 B cells did not proliferate after anti-CD38 stimulation, but, in clear contrast, T2 and M B lymphocytes showed proliferation at 48 hours (Figure 2A). This effect increased in T2 and M B subpopulations with the addition of IL-4, whereas T1 subset did not respond even with this addition (Figure S2B).
Anti-CD38 stimulation induces proliferation of transitional 2 B cells. Splenocytes from wild-type or CD38−/− mice were stained for B220, CD21, and CD24. The cells were gated on B220-positive cells, and each population was purified according to its expression of CD21 and CD24. The purity of each subpopulation (T1, T2, or M) was more than 90%. (A) In 96-well plates, triplicates of each subset from wild-type mice were stimulated as indicated. (B) T1 B cells from wild-type or CD38−/− mice were preincubated 30 minutes with 8-Br-cADPR or only medium. The cells, without washing, were then stimulated with rat-IgG2a (□), rat anti–mouse CD38 (rat IgG2a, ■), F(ab′)2 anti–mouse IgM (▒), or LPS (▨). (C) T2 B cell subsets from wild-type or CD38−/− mice were stimulated as in panel B except that LPS was not included. The plates were incubated 48 hours at 37°C. Each well was pulsed with 1 μCi of [3H] thymidine 8 hours before being harvested. Results are expressed as mean plus or minus SD from 3 independent experiments (P < .01 as indicated, Student t test).
Anti-CD38 stimulation induces proliferation of transitional 2 B cells. Splenocytes from wild-type or CD38−/− mice were stained for B220, CD21, and CD24. The cells were gated on B220-positive cells, and each population was purified according to its expression of CD21 and CD24. The purity of each subpopulation (T1, T2, or M) was more than 90%. (A) In 96-well plates, triplicates of each subset from wild-type mice were stimulated as indicated. (B) T1 B cells from wild-type or CD38−/− mice were preincubated 30 minutes with 8-Br-cADPR or only medium. The cells, without washing, were then stimulated with rat-IgG2a (□), rat anti–mouse CD38 (rat IgG2a, ■), F(ab′)2 anti–mouse IgM (▒), or LPS (▨). (C) T2 B cell subsets from wild-type or CD38−/− mice were stimulated as in panel B except that LPS was not included. The plates were incubated 48 hours at 37°C. Each well was pulsed with 1 μCi of [3H] thymidine 8 hours before being harvested. Results are expressed as mean plus or minus SD from 3 independent experiments (P < .01 as indicated, Student t test).
CD38 enzymatic activity mediates cell migration.7,44 With the aim to analyze whether CD38 enzymatic activity has a role in the proliferation induced by the anti-CD38 mAb, T1 and T2 B cells were preincubated with 100 μM 8-bromo-cyclic adenosine diphosphate ribose (8-Br-cADPR). This concentration has been demonstrated to block efficiently CD38 enzymatic functions without affecting the viability of the cells.8 As shown in Figure 2B, T1 B cells did not proliferate with or without the addition of 8-Br-cADPR. Despite the lack of proliferation with anti-CD38 or anti-IgM, T1 B cells were perfectly able to proliferate with LPS, which demonstrated that they were viable and ready to proliferate to an appropriate stimulus. In Figure 2B, T1 B cells from CD38-deficient mice show the same behavior than their counterpart from wild-type mice. The analysis of T2 B cells (Figure 2C) similarly showed that the cells can equally proliferate to anti-CD38 or BCR stimulation, which indicates that CD38 enzymatic activity was dispensable for the proliferation induced by the anti-CD38 mAb. This figure also shows that T2 B cells from CD38-deficient mice are similarly able to proliferate to BCR stimulation without any evident defect compared with their wild-type counterpart. These results suggest that CD38 is working as a receptor in the induction of proliferation of T2 B cells. As described for other systems, CD38 receptorial activity seems to be independent of its enzymatic activity.8,45 The results also show that the behavior of T1 and T2 B cells from CD38-deficient mice is similar to that of their wild-type counterparts, at least in these assays.
Consistent with previous reports showing that splenic immature B lymphocytes exhibit differential responsiveness to BCR stimulation,40 our data show that CD38 promotes proliferation of immature T2 B cells. However, CD38 stimulation is not able to induce the proliferation of T1 B lymphocytes. Of note, simultaneous, or step-by-step, addition of anti-CD38 and anti-IgM reagents did not show additive or costimulatory effects (data not shown), which indicates that these molecules may work independently.
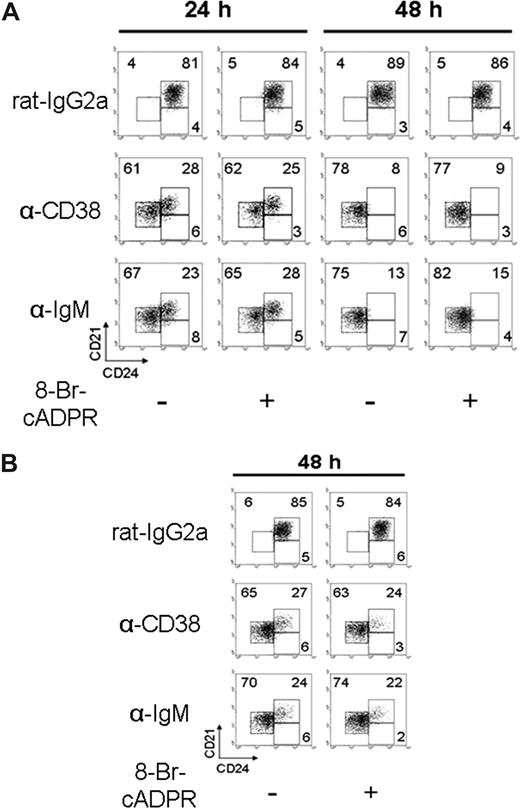
Anti-CD38 stimulation promotes differentiation of T2 B lymphocytes
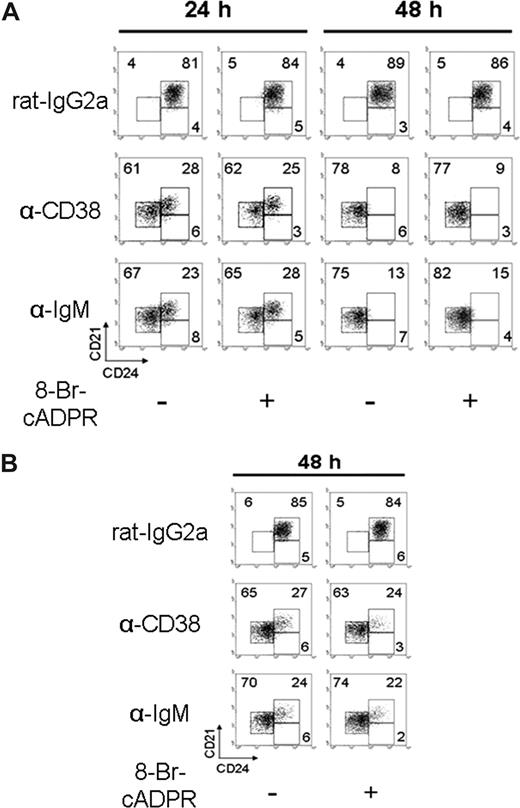
For these experiments, the in vitro assay previously described for B-cell differentiation was used.40 Purified T1, T2, and M cells were cultured for 24 or 48 hours in the presence or absence of anti-CD38 mAb. Anti-CD38 stimulation induced the differentiation of T2 B cells toward a mature phenotype. The differentiation was observed after 24 hours of incubation, and it was clearly established at 48 hours (Figure 3A; Table 1). This effect was further improved with the addition of IL-4 (Figure S3A). Nevertheless, the differentiation was not observed in the T1 B cells (Figure S3B; Table 1); additionally, highly purified T2 B cells, lacking marginal zone B cells (Figure S4A), showed that CD38 induced their differentiation (Figure S4B). The addition of 8-Br-cADPR had no effect in the differentiation of T2 B cells induced by either anti-CD38 mAb or BCR stimulation (Figure 3A), which indicates that CD38-enzymatic activity is dispensable for this effect. Figure 3B shows similar results when half of the anti-CD38 dose was added to the culture and the cells were examined 48 hours later. Here it can be seen that the differentiation induced by the anti-CD38 mAb, although slightly less efficient, cannot be inhibited by 8-Br-cADPR. These results also show that, in these cells and for the proliferation and differentiation assays, the function CD38 is independent of its enzymatic activity.
Anti-CD38 stimulation promotes T2 B lymphocyte differentiation. (A) T2 subsets, obtained as in Figure 2, were preincubated 30 minutes with 8-Br-cADPR or medium and activated with rat-IgG2a (50 μg/mL), anti-CD38 (50 μg/mL), or anti-IgM (10 μg/mL). Cells were harvested at 24 or 48 hours and restained to evaluate differentiation. (B) T2 B cells were preincubated as in panel A, activated with rat-IgG2a or anti-CD38 at (25 μg/mL) or anti-IgM (5 μg/mL; “Proliferation, diferentiation, and apoptosis assays”) for 48 hours, and restained as in panel A. Numbers in each dot plot indicate the percentage of cells and are representative from 3 independent experiments with similar results.
Anti-CD38 stimulation promotes T2 B lymphocyte differentiation. (A) T2 subsets, obtained as in Figure 2, were preincubated 30 minutes with 8-Br-cADPR or medium and activated with rat-IgG2a (50 μg/mL), anti-CD38 (50 μg/mL), or anti-IgM (10 μg/mL). Cells were harvested at 24 or 48 hours and restained to evaluate differentiation. (B) T2 B cells were preincubated as in panel A, activated with rat-IgG2a or anti-CD38 at (25 μg/mL) or anti-IgM (5 μg/mL; “Proliferation, diferentiation, and apoptosis assays”) for 48 hours, and restained as in panel A. Numbers in each dot plot indicate the percentage of cells and are representative from 3 independent experiments with similar results.
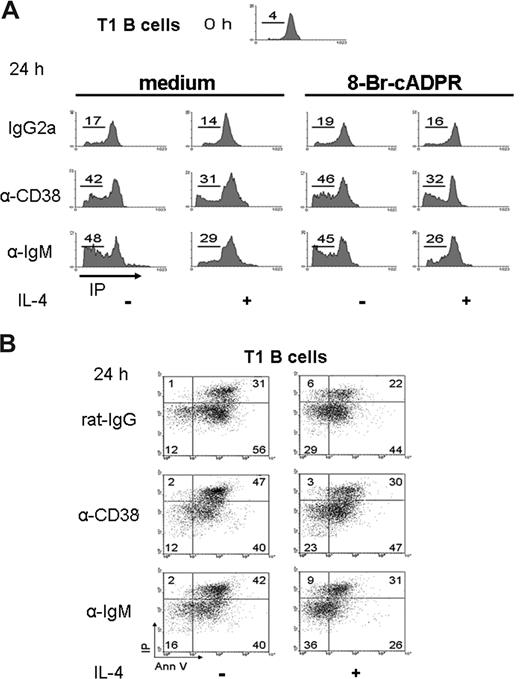
CD38 stimulation promoted apoptosis of T1 B cells
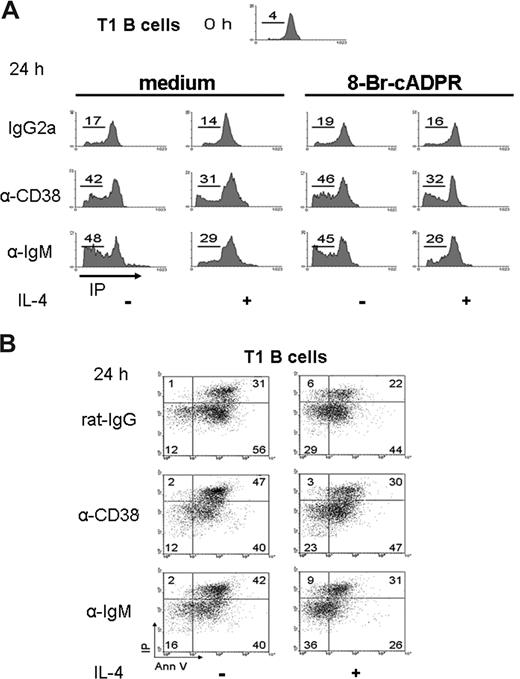
Early reports showed that anti-CD38 stimulation of human immature B cells induces apoptosis.9 Because immature T1 cells did not proliferate or differentiate after the anti-CD38 stimulation, the apoptosis of these cells was evaluated. Purified T1 cells were cultured 24 hours with anti-CD38. The results showed a high number of T1 B lymphocytes in the sub/G0 area (Figure 4A), or annexin V/PI-positive cells (Figure 4B), which indicated apoptosis. T1 cells were also evaluated at 12 hours with similar results (Figure S5A). The numbers of apoptotic T1 cells were reduced with the addition of IL-4; however, IL-4 did not sufficiently rescue the T1 subset from the apoptosis induced by CD38 stimulation (Figures 4A,B,S5A,B). The percentage of apoptosis of T1 B cells was significantly different (P < .01) in the absence or presence of anti-CD38 stimulation (Figure S5B). These results showed that anti-CD38 induced apoptosis of T1 B cells, similarly to what the anti-IgM did.40 As described earlier, these experiments were also done in the presence of 8-Br-cADPR. As shown in Figure 4A, the addition of the inhibitor did not modify the results obtained in their absence. Thus, the ability of anti-CD38 mAb to induce the apoptosis of T1 B cells also seems to be independent of CD38 enzymatic activity.
Apoptosis of T1 B lymphocytes on CD38 crosslinking. (A) T1 B cells, obtained as in Figure 2, were preincubated with 8-Br-cADPR or medium for 30 minutes, stimulated as indicated, and 24 hours later, permeabilized and stained with PI. The histograms show the content of DNA for each condition. The numbers represent the percentage of T1 B cell in SubG0 region. (B) T1 B cells were activated as above and harvested and stained with annexin V and PI. Numbers indicate the percentage of T1 B cells in each region. The dot plots are representative from 3 independent experiments with similar results.
Apoptosis of T1 B lymphocytes on CD38 crosslinking. (A) T1 B cells, obtained as in Figure 2, were preincubated with 8-Br-cADPR or medium for 30 minutes, stimulated as indicated, and 24 hours later, permeabilized and stained with PI. The histograms show the content of DNA for each condition. The numbers represent the percentage of T1 B cell in SubG0 region. (B) T1 B cells were activated as above and harvested and stained with annexin V and PI. Numbers indicate the percentage of T1 B cells in each region. The dot plots are representative from 3 independent experiments with similar results.
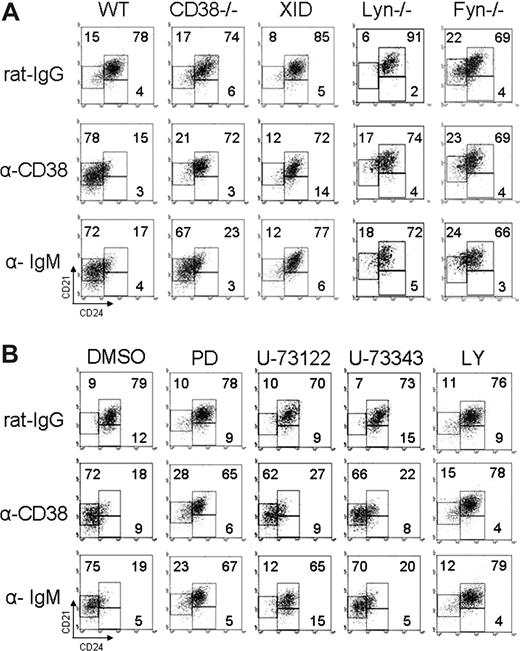
CD38 stimulation requires Lyn, Fyn, PI3K, and Erk to induce proliferation of T2 B lymphocytes
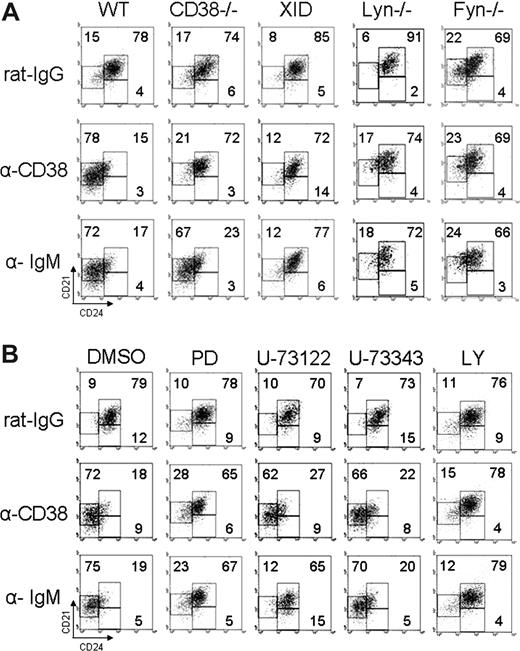
The CD38 signaling pathway has been partially described.24-27 To evaluate whether these signaling molecules participate in the proliferation induced by anti-CD38, T2 B cells were purified from mice deficient in the expression of Lyn, Fyn, or wild-type mice. In addition, T2 B cells from wild-type mice were treated with the drugs LY294002 or PD98059, which interferes with PI3K or Erk activation, respectively. It was found that the proliferation induced after CD38 crosslinking was less effective in T2 B cells from Lyn- or Fyn-deficient mice (Figure 5A), which is consistent with previous reports that analyze whole splenic B cells.24 Moreover, incubation of T2 B cells from wild-type mice with LY294002 or PD98059 also inhibited the proliferation of T2 B cells stimulated with anti-CD38 (Figure 5B).
CD38 signaling requires Lyn, Fyn, PI3K, and Erk to induce proliferation of T2 B lymphocytes. (A) T2 B cells from Lyn−/−, Fyn−/−, or wild-type mice were stimulated 48 hours as indicated. (B) T2 B cells from wild-type mice were preincubated 30 minutes with DMSO, LY294002 (LY), or PD98059 (PD). Cells with the drug were stimulated as indicated above. Each well was pulsed with 1 μCi [3H] thymidine 8 hours before being harvested. Results are expressed as mean plus or minus SD from 3 independent experiments (P < .05 or P < .01 as indicated, Student t test).
CD38 signaling requires Lyn, Fyn, PI3K, and Erk to induce proliferation of T2 B lymphocytes. (A) T2 B cells from Lyn−/−, Fyn−/−, or wild-type mice were stimulated 48 hours as indicated. (B) T2 B cells from wild-type mice were preincubated 30 minutes with DMSO, LY294002 (LY), or PD98059 (PD). Cells with the drug were stimulated as indicated above. Each well was pulsed with 1 μCi [3H] thymidine 8 hours before being harvested. Results are expressed as mean plus or minus SD from 3 independent experiments (P < .05 or P < .01 as indicated, Student t test).
CD38 requires Lyn, Fyn, Btk, PI3K, and Erk, but not PLC-γ2 to promote the differentiation of T2 B lymphocytes
CD38 stimulation requires several signaling molecules, but not PLC-γ2, to promote B-cell proliferation.27 Purified T2 B lymphocytes were induced to differentiate by CD38 or BCR stimulation. The results showed that T2 B lymphocytes from mice deficient in the expression of Lyn, Fyn, or mice with defective Btk (Xid) did not differentiate on anti-CD38 stimulation (Figure 6A; Table 2). In addition, LY294002 or PD98059 interfered with the differentiation induced by anti-CD38 of T2 B cells from wild-type mice. Nevertheless, the PLC-γ2 inhibitor U-73 122 did not affect T2 B cell differentiation promoted by CD38, although it almost completely inhibited the differentiation induced by the BCR (Figure 6B; Table 3).
CD38 requires Lyn, Fyn, Btk, PI3K, and Erk, but not PLC-γ2 to promote differentiation of T2 B lymphocytes. (A) T2 B cells from CD38−/−, Lyn−/−, Fyn−/−, Btk-deficient (Xid), or wild-type mice were stimulated 48 hours as indicated. (B) T2 B cells from wild-type mice were preincubated 30 minutes with inhibitory drugs and then stimulated as indicated, harvested at 48 hours, and restained with anti-B220, anti-CD21, and anti-CD24. The numbers show the percentage in each region and are representative from 3 independent experiments with similar results.
CD38 requires Lyn, Fyn, Btk, PI3K, and Erk, but not PLC-γ2 to promote differentiation of T2 B lymphocytes. (A) T2 B cells from CD38−/−, Lyn−/−, Fyn−/−, Btk-deficient (Xid), or wild-type mice were stimulated 48 hours as indicated. (B) T2 B cells from wild-type mice were preincubated 30 minutes with inhibitory drugs and then stimulated as indicated, harvested at 48 hours, and restained with anti-B220, anti-CD21, and anti-CD24. The numbers show the percentage in each region and are representative from 3 independent experiments with similar results.
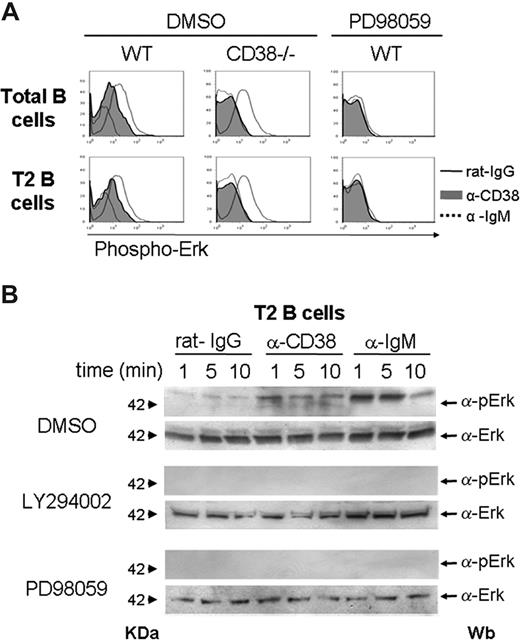
Anti-CD38 stimulation induces Erk phosphorylation in T2 B lymphocytes
Erk phosphorylation was evaluated by flow cytometry and Western blot assays. The results showed that CD38 crosslinking induced the phosphorylation of Erk in splenic B cells from wild-type mice, but no phosphorylation was seen in mice deficient of CD38 (Figure 7A). The addition of the drug PD98059 inhibited the phosphorylation induced by anti-CD38, which suggested that Erk was activated in the T2 B-cell subset. Moreover, Western blot assays showed that CD38 crosslinking induced the phosphorylation of Erk at 1, 5, or 10 minutes in purified T2 B lymphocytes from wild-type mice (Figure 7B). The incubation with the drugs LY294002 or PD98059 inhibited the phosphorylation of Erk (Figure 7B).
Erk is phosphorylated in T2 B lymphocytes on anti-CD38 stimulation, and this effect requires PI3-kinase activity. Total splenocytes from CD38−/− or wild-type mice were preincubated with DMSO or PD98059 for 30 minutes and then activated 10 minutes with rat-IgG (black line), anti-CD38 (gray histogram), or anti-IgM (dotted line). After activation, the cells were stained for B220, CD21, CD24, and pErk as described in “Erk phosphorylation.” (A) Phosphorylation of total or purified T2 B lymphocytes. (B) T2 B cells from wild-type mice were preincubated with DMSO, LY294002, or PD98059 for 30 minutes and then activated 1, 5, or 10 minutes with rat-IgG, anti-CD38, or anti-IgM. Erk phosphorylation was analyzed by Western blot as described in “Erk phosphorylation.”
Erk is phosphorylated in T2 B lymphocytes on anti-CD38 stimulation, and this effect requires PI3-kinase activity. Total splenocytes from CD38−/− or wild-type mice were preincubated with DMSO or PD98059 for 30 minutes and then activated 10 minutes with rat-IgG (black line), anti-CD38 (gray histogram), or anti-IgM (dotted line). After activation, the cells were stained for B220, CD21, CD24, and pErk as described in “Erk phosphorylation.” (A) Phosphorylation of total or purified T2 B lymphocytes. (B) T2 B cells from wild-type mice were preincubated with DMSO, LY294002, or PD98059 for 30 minutes and then activated 1, 5, or 10 minutes with rat-IgG, anti-CD38, or anti-IgM. Erk phosphorylation was analyzed by Western blot as described in “Erk phosphorylation.”
Discussion
The observation that CD38-deficient mice have decreased levels of T2, increased levels of T1, and stable M subsets was one of the key observations to begin this work. B-cell compartments have not been fully characterized in these mice; thus, the first task was to analyze the expression levels of CD38 among immature B cells. Once the differential levels of CD38 were observed among the 3 stages (Figure 1), the next step was to analyze whether those cells were able to respond to CD38 crosslinking. The data showed that purified T1 B cells did not proliferate or differentiate to T2 or M B lymphocytes after anti-CD38 stimulation; instead, they died by apoptosis. In contrast, purified T2 B lymphocytes proliferated and differentiated to mature B lymphocytes after CD38 crosslinking. None of these events required the enzymatic activity of CD38, which indicates that CD38 was acting only as a receptor. Thus, these results show a differential activity for CD38 in immature transitional B lymphocytes, which suggests that CD38 plays an important role in the generation of splenic mature B cells. In addition, the data show that purified T2 B cells from mice deficient in expression of Btk, Lyn, or Fyn have reduced ability to proliferate and, more importantly, they were impaired to mature after anti-CD38 stimulation. Similar results were obtained with the addition of drugs that inhibit PI3K or Erk signaling molecules. Together, these results suggest that CD38 participates in the development of immature B lymphocytes by inducing apoptosis of T1 but proliferation and maturation of T2 B lymphocytes.
CD38 is expressed during B-cell development, although a detailed analysis of its expression in immature B cells from the spleen has not been reported. In this regard, the data herein show that CD38 is highly expressed on T2 B cells compared with its expression on T1 or M B lymphocytes. Interestingly, the data showed that CD38-deficient mice have a reduced percentage of T2 B cells compared with that from wild-type mice. In addition, the number of T1 B lymphocytes was incremented in the CD38-deficient mice. This “arrest” in maturation suggests that CD38 participates in the proliferation and differentiation of immature B lymphocytes. An extensive analysis of the properties of T1s and T2s from the CD38-deficient mice is incomplete; however, the initial set of results, presented here, suggests that these cells do not behave differently compared with the same populations derived from wild-type mice. However, more work is needed to fully support the role of CD38 in these processes.
The results showed that T1 B cells did not proliferate or differentiate in response to CD38 crosslinking, but, as reported for human immature B cells,9 they died by apoptosis. In sharp contrast, CD38 stimulation induced proliferation of T2 B lymphocytes (Figures 2A,C,S2B) and their differentiation to a mature phenotype (Figures 3A,B,S3A). Interestingly, these results correlate with studies in which immature B lymphocytes, stimulated through BCR proliferate and differentiate if they are more mature (T2) or with the same stimulus, die by apoptosis if they are more immature (T1).39,40
The ontogeny of B lymphocytes has been widely studied, and several reports have shown that 2 × 107 IgM+ B cells are daily generated in bone marrow; however, only 10% to 15% migrate to the spleen where they mature and differentiate.46,47 Several reports suggest that negative selection occurs at the immature B-cell stage. This negative selection has been studied in vitro by BCR cross-linking.39 Other groups have shown evidence of positive selection in marginal zone or B1 B cells48,49 ; nevertheless, the requirements for B cell maturation are not completely elucidated. The pre-sence of CD38 during B-cell ontogeny may suggest a role of this molecule in the maturation of B lymphocytes; however, apart from few studies in human B cells,9 its role in differentiation has not been explored. As also mentioned in “Introduction” and “Results,” anti-CD38 stimulation did not show additive or costimulatory effects. This apparent inconsistency represents another area of research because both molecules seem to regulate the same phenomena.
The signaling pathway driving maturation of T2 B cells after CD38 stimulation was analyzed. These results showed that CD38 stimulation partially requires Lyn, Fyn, and Erk to induce T2 B-cell proliferation; in addition, the data suggest that PI-3K is entirely required for CD38 to induce proliferation of this subset. Furthermore, CD38 promotes T2 B-cell maturation and requires Lyn, Fyn, PI3K, Btk, and Erk, but not PLC-γ2. Consistent with these results, several reports have shown that signaling defects in Btk, Lyn, PLC-γ2, Fyn, or PI3K prevent maturation of B lymphocytes.41,43 These mice have reduced numbers of mature B cells but apparently intact T2 B-cell pools.50-55 Thereby, this work demonstrates that CD38 exploits some of the molecules used by BCR signaling to induce proliferation and differentiation of T2 B lymphocytes; however, it also complements the observation that PLC-γ2 is not required by CD38 signaling and this fact sustains that, although BCR and CD38 may use similar signaling pathways, there are some differences that may explain a differential or complementary role during B-cell activation and differentiation.
Finally, the MAPK Erk has been recently explored in CD38-dependent activation of human B cells by Deaglio et al.22 The present report shows that Erk is required to induce proliferation and differentiation of T2 B lymphocytes after CD38 stimulation. Consistent with previous reports,56 this work shows that Erk is possibly activated downstream PI3K in T2 B cells after CD38 activation. CD38 may regulate differentiation in critical points of B-cell selection possibly by collaborating with the BCR20 or other molecules, such as CD19.22 An area that requires further research is the mechanism through which CD38 “transmits” the signal from the cell surface to the nucleus. As shown in this and other articles,1,5,20-22,27 CD38 activates several signaling pathways. So far it has not been possible to clearly demonstrate physical association of CD38 with transduction-signaling molecules, although several candidates have been already reported.20,22 Although the participation of CD38 in B-cell development was analyzed in this work, it is necessary to elucidate the location of its “natural ligand” so these effects can be induced “in vivo.” The study of the CD38 and its “ligand” will contribute to the understanding of the biologic role of these molecules during the ontogeny of B lymphocytes. CD38 may also represent a target for treatment when an inappropriate B-cell response, such as some forms of autoimmunity or some B-cell lymphomas, is established.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Hector Romero-Ramírez for his help and technical assistance, Ricardo Gaxiola-Centeno for taking care of the mice, and Dr Claudia González-Espinosa for providing B6.129 wild-type, B6.129 Lyn−/−, and B6.129 Fyn−/− mice.
This work was supported by grants from Consejo Nacional de Ciencia y Tecnología, México (40 218 and 56 836). This work was submitted in partial fulfillment of the requirements for the DSc degree of J.C.R.-A. at Doctorado en Ciencias Biomedicas, Universidad Nacional Autonoma de Mexico.
Authorship
Contribution: J.C.R.-A. performed research, collected and analyzed data, and wrote the paper; M.E.M.-G. participated at the beginning of this research; C.S.-M. and V.H.R.-G. performed some flow cytometry and sorting experiments; L.S.-A. designed research, analyzed data, and wrote the paper; all the authors read and agree with the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Leopoldo Santos-Argumedo, Departamento de Biomedicina Molecular, CINVESTAV-IPN, Av IPN 2508, Col Zacatenco, México City, México; e-mail: lesantos@cinvestav.mx.

![Figure 2. Anti-CD38 stimulation induces proliferation of transitional 2 B cells. Splenocytes from wild-type or CD38−/− mice were stained for B220, CD21, and CD24. The cells were gated on B220-positive cells, and each population was purified according to its expression of CD21 and CD24. The purity of each subpopulation (T1, T2, or M) was more than 90%. (A) In 96-well plates, triplicates of each subset from wild-type mice were stimulated as indicated. (B) T1 B cells from wild-type or CD38−/− mice were preincubated 30 minutes with 8-Br-cADPR or only medium. The cells, without washing, were then stimulated with rat-IgG2a (□), rat anti–mouse CD38 (rat IgG2a, ■), F(ab′)2 anti–mouse IgM (▒), or LPS (▨). (C) T2 B cell subsets from wild-type or CD38−/− mice were stimulated as in panel B except that LPS was not included. The plates were incubated 48 hours at 37°C. Each well was pulsed with 1 μCi of [3H] thymidine 8 hours before being harvested. Results are expressed as mean plus or minus SD from 3 independent experiments (P < .01 as indicated, Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/7/10.1182_blood-2007-08-107714/5/m_zh80070817550002.jpeg?Expires=1769273411&Signature=GtlDEilsZFhg0NpBuSZzOt~ydeFZmi0cI-0DIVXvHS6MunLm~c-9tZQA99NFuBNFKDqkvdvLuWKO0ZHH~Opnm9pL4xkN~nudt1NN4VtZhMByC33IHTcGZLagdmLzY1avMntr4S1FsO7bn-YfSrQhpCbzWz04tx6kfw84i88Ontt6QxxmelSMT76zvDLBJU4kQnmAKV7tkt~k4EG2wptx3-VKTy-Y4txTSCVHiyCeHsf1ECJFQy4S4vitZ3tGNl65rCaKjNb~pktzlPF0POebWt5kThiX8379q~ejoBgek7Dzi4M3-d~cjhmCAytTCDAlgfSOvvKkWPUecfszp1yr5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. CD38 signaling requires Lyn, Fyn, PI3K, and Erk to induce proliferation of T2 B lymphocytes. (A) T2 B cells from Lyn−/−, Fyn−/−, or wild-type mice were stimulated 48 hours as indicated. (B) T2 B cells from wild-type mice were preincubated 30 minutes with DMSO, LY294002 (LY), or PD98059 (PD). Cells with the drug were stimulated as indicated above. Each well was pulsed with 1 μCi [3H] thymidine 8 hours before being harvested. Results are expressed as mean plus or minus SD from 3 independent experiments (P < .05 or P < .01 as indicated, Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/7/10.1182_blood-2007-08-107714/5/m_zh80070817550005.jpeg?Expires=1769273411&Signature=1B365vwO9yP~Db9a6UQtvbonudo4RQ~AlDmyQgmVOcalaprbg73tM6hzuA04eRMtq~d41-T-gFWPY7wcOkJFT3GDEvzR-VMUeAY7d3qI~2rFAAb7nogvrMZPF6Ino9cn-lX3gDr-5Ahv1BWPUUkQUFhT0bC3fUbDQJsyiMfx1lCvqbK3cql8718YMqgD2eSeX5oiPwD~lqPYWCdC35A5TQpTx07ybB5UsUxqcL0DjbmgGTlcLRg-~XJpciF0v4Y8nH0ei1dYwjQLT8FStuxHquQlPlbktfVdgUhDK5TjuyQBdNs2LEEmHiiS2hIutRiS8IoIeA4riyGfYNnFurTy2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 2. Anti-CD38 stimulation induces proliferation of transitional 2 B cells. Splenocytes from wild-type or CD38−/− mice were stained for B220, CD21, and CD24. The cells were gated on B220-positive cells, and each population was purified according to its expression of CD21 and CD24. The purity of each subpopulation (T1, T2, or M) was more than 90%. (A) In 96-well plates, triplicates of each subset from wild-type mice were stimulated as indicated. (B) T1 B cells from wild-type or CD38−/− mice were preincubated 30 minutes with 8-Br-cADPR or only medium. The cells, without washing, were then stimulated with rat-IgG2a (□), rat anti–mouse CD38 (rat IgG2a, ■), F(ab′)2 anti–mouse IgM (▒), or LPS (▨). (C) T2 B cell subsets from wild-type or CD38−/− mice were stimulated as in panel B except that LPS was not included. The plates were incubated 48 hours at 37°C. Each well was pulsed with 1 μCi of [3H] thymidine 8 hours before being harvested. Results are expressed as mean plus or minus SD from 3 independent experiments (P < .01 as indicated, Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/7/10.1182_blood-2007-08-107714/5/m_zh80070817550002.jpeg?Expires=1770166254&Signature=ggKvaMxhZosURDAu85np6B25-IfA9emw6LBeLIQJx1mPpwZOkToGqsaY7u6NEGWhVDzbpYQp7qXYKfe~bJ8kIQzv7F8w-ICkeQToxQbZe5XYVb14HniyeLb6m3m25AxJYeejoJ2rlsPr9eItCw~1oZ~8h8zx-9-o0Xt36QCqcaEaiCkpBD8ERJBcRqixb8jprpjRE8q20ZNhb-hjQDIhflInTUFpbvOLRpZojvwPY~oXP1oLLXAtSjw3-SK01qkGWHPoz6RthhdHLLj7mRiXlGs-fUzcqrhqQT4~KzCQr31jmvUydB571NhASAXRsLybT4o0YjIfS98e-PrMT3fu~g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. CD38 signaling requires Lyn, Fyn, PI3K, and Erk to induce proliferation of T2 B lymphocytes. (A) T2 B cells from Lyn−/−, Fyn−/−, or wild-type mice were stimulated 48 hours as indicated. (B) T2 B cells from wild-type mice were preincubated 30 minutes with DMSO, LY294002 (LY), or PD98059 (PD). Cells with the drug were stimulated as indicated above. Each well was pulsed with 1 μCi [3H] thymidine 8 hours before being harvested. Results are expressed as mean plus or minus SD from 3 independent experiments (P < .05 or P < .01 as indicated, Student t test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/111/7/10.1182_blood-2007-08-107714/5/m_zh80070817550005.jpeg?Expires=1770166254&Signature=eqK7KgKNtV6s01GT-b7B5JvxpF3kkFZpgupw0fOY3poa0xmwpNYXrlEE50PTmKRSf9cURTh9p0~ofWK1tcctJq2Dwj6vwKZ3I~ahXbNFaEDqZNpOF~1rQJfU8vFgXO4x7aCks4~WlB36076bHF-ubBPBxCwny51CwAV~-1qtnfbbRCDRu25qhoT489Uon3jMzHDD2zRgmKFGU3raDhtQU6IecGCI5eMmzrk17j8Sag2Cho8gMNh4w3UCs02By22IAgDY8IwjsEjtttAhF77eK~T~W66Xy7jZocZfWbucLhy2vvauLzCKGLMNPlG2mR4Eexb6E9fjppNkDuOqjVqhGg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)