Abstract
Tissue factor (TF) and thrombin are involved in intimal hyperplasia (IH) and remodelling following vascular injury. Because many neointimal smooth muscle cells (VSMCs) derive from circulating vascular progenitors (VPs), we investigated how thrombin influences VP phenotype and function. Following wire-induced carotid artery injury in mice, the majority of circulating VPs expressed TF, were capable of initiating clotting in vitro, and had protease-activated receptors (PAR)–1, –2, and –4. Thrombin, through PAR-1, inhibited apoptosis and caused proliferation, resulting in the outgrowth of VP coexpressing markers of activated endothelial cells and VSMCs, even in the presence of growth factors. These mixed-phenotype VPs circulated as a minority population after injury and shared a similar phenotype with many neointimal cells. Labeled CD34+ cells, injected up to 2 weeks after injury, could be detected in the injured vessel wall, suggesting that continued recruitment may contribute to progressive IH. Finally, CD34+ cells incubated with thrombin prior to injection promoted florid neointimal lesions, whereas those incubated with PAR antagonists inhibited IH and promoted regenerative repair characterized by the development of a quiescent endothelium. We conclude that IH after vascular injury is due to the direct actions of thrombin on mobilized VPs.
Introduction
Mechanical or inflammatory injury to blood vessels results in intimal hyperplasia (IH) and vascular remodeling, which eventually compromises blood flow and leads to critical ischemia of downstream organs. In humans, these processes underpin the manifestations of a number of highly significant cardiologic and vascular diseases. Coagulation proteases, in particular thrombin, promote IH after vascular injury and have been shown in animal models to be important in the pathophysiology following different injuries, including balloon angioplasty1 and allograft vasculopathy.2 Because injury in protease-activated receptor-1 (PAR-1)–deficient mice is followed by an attenuated neointimal response,3 signaling through PAR-1 is an important mechanism by which thrombin acts
There is increasing evidence that a significant proportion of neointimal cells are bone marrow (BM)–derived progenitor cells,4-8 so it is valid to ask what these cells are and how they are involved in the disease process. In humans, vascular progenitors (VPs) capable of differentiating into endothelial cells (ECs) or vascular smooth muscle cells (VSMCs) arise from a common progenitor9 and can be outgrown in vitro from different fractions of circulating peripheral blood mononuclear cells, either a CD34+ population expressing vascular endothelial growth factor receptor-2 (VEGF-R2) or a CD34− fraction.10-14 In the mouse, where ECs and VSMCs are also known to derive from a common embryonic progenitor expressing VEGF-R2 (Flk-1),15 the phenotype of circulating EC or VSMC progenitors in adult animals is ill defined. ECs have been isolated from CD34+ Flk-1+ progenitors16,17 whereas VSMC progenitors (that contribute to neointima formation) have been defined as being either c-kit+18 or c-kit−.19 EC and VSMC progenitors can also be derived from different fractions of murine BM in vitro, including those enriched for cKit+CD34+ or cKit+CD34− cells.18,20
In a previous study, we showed that expression of anticoagulant fusion proteins (based on tissue factor pathway inhibitor [TFPI] or hirudin) on BM-derived cells could completely inhibit neointimal formation after wire-induced endovascular injury.21 In that study, arteries were repaired to a preinjured state by circulating CD34+ cells, recruited into the damaged vessel before switching on the expression of the transgenic (Tg) fusion protein, which was under the control of an α-smooth muscle actin (α-SMA) promoter.
We hypothesized for this study that IH was due entirely to the actions of thrombin, acting through PAR, on CD34+ VPs. Our results support this hypothesis and indicate that the expanding neointima may consist of proliferating progenitors expressing both EC and VSMC markers, similar to the “intermediate” phenotype cells described by Yamashita.15 These cells appear unable to respond to differentiation cues provided by the injured vessel wall unless thrombin is inhibited or PAR is antagonized on their cell surface. Moreover, the data indicate that neointimal expansion may be due in part to continued recruitment of VPs from the circulation, as well as proliferation of existing neointimal cells.
These data significantly enhance our understanding of vascular disease and suggest that regenerative repair by circulating progenitor cells can be promoted by inhibiting the action of thrombin on cell-surface PAR.
Methods
Animals and the vascular injury model
Wild-type (WT) C57BL/6 and heterozygous α-TFPI–Tg and α-Hir–Tg21 mice weighing 25 to 35 g were used for all experiments (α-Tg mice express cell-tethered anticoagulants (TFPI and hirudin, respectively) under the control of an α-SMA promoter directing constitutive expression on cells expressing α-SMA). Enhanced yellow fluorescent protein targeted to the ROSA-26 locus (ROSA-EYFP)22 mice were obtained from Alexandre Potocnik (MRC at Mill Hill, London, United Kingdom). All animal procedures were approved by the UK Home Office. A mouse vascular injury model was performed as previously described.21 Simply, under appropriate anesthesia, a 100-μm-diameter wire was introduced into the left common carotid via the external carotid artery and withdrawn/reinserted 3 times. After confirming restoration of normal blood flow, animals were allowed to recover. Some mice underwent BM reconstitution 4 weeks prior to injury as previously described.21
Manipulation and phenotyping of CD34+ cells
Whole blood was collected before (day 0) and at variable times after injury (n = 12 per group). For immunophenotyping, CD34+ cells were isolated using magnetic beads (StemCell Technology, London, United Kingdom), plated into a 24-well plate at 5 × 103 cells/well, centrifuged at 178g, fixed with 4% paraformaldehyde, washed with PBS for 3 times, centrifuged at 320g for 2 minutes, and then labeled with one of following antibodies (Abs): rat anti-CD34 (Serotec, Oxford, United Kingdom), anti–Sca-1, anti–C-kit (Abcam, Cambridge, United Kingdom), anti-CD31 and anti-CD105 (BD Bioscience Pharmingen, Oxford, United Kingdom); polyclonal rabbit anti–tissue factor (TF),23 anti–VEGF-R2 (ABR-Affinity BioReagents, Golden, CO), anti-CD133, anti-CXCR4 (Abcam), anti-SM22, anti–PAR-1, and anti–PAR-4 (AutogenBioclear, Wiltshire, United Kingdom); mouse monoclonal anti–PAR-2 (Zymed, South San Francisco, CA), and anti–human α-SMA (Sigma-Aldrich, St Louis, MO). Anti-PAR reagents recognize both cleaved and noncleaved forms of the receptor. Second layer staining was with a goat anti-rat IgG-FITC, goat anti-rabbit IgG-FITC, sheep anti-mouse IgG-FITC (all from Sigma-Aldrich), or horse anti-mouse IgG–Texas red (Vector Laboratories, Burlingame, CA). All wells were stained with DAPI. Labeled cells were analyzed using an immunofluorescence (IF) microscopy (Axiovert S100 TV; Zeiss, Welwyn Garden City, United Kingdom). To determine subpopulation densities, a total of 300 cells were counted at ×200 magnification from 3 different wells.
For flow cytometry, CD34+ cells were incubated with the same antibodies or appropriate controls for 30 minutes each layer, washed and then analyzed on a FACSCAN (Becton Dickinson, San Jose, CA).
For functional experiments, CD34+ cells were purified from mice 2 days after injury, seeded in a 24-well plate at 2 × 104 cells/mL and cultured in Iscove modified Dulbecco medium (IMDM; Sigma-Aldrich) supplemented with 2% fetal bovine serum (FBS; StemCell Technology) for 1 to 5 days. Cells were incubated with either thrombin (Enzyme Research Laboratories, Swansea, United Kingdom) or active site-inhibited thrombin (Cambridge Bioscience, Cambridge, United Kingdom), both at 50 nM; mercaptopropionyl-Phe-Cha-Arg-Lys-Pro-Asn-Asp-Lys-NH2 (PAR-1 antagonist) or trans-cinnamoyl YPGKF-NH2 (PAR-4 antagonist; both from Peptides International, Louisville, KY) at 10 μM; and VEGF or platelet-derived growth factor (PDGF; both from Insight Biotechnology, Middlesex, United Kingdom) at 50 ng/mL or combinations of these, before IF.
For in vitro clotting assays, 105 CD34+ cells were resuspended in 50 μL Tris-buffered saline (TBS) before mixing with WT plasma in a glass tube (Corning, Corning, NY). Tubes were incubated at 37°C in a water bath after adding 10 μL 250 mM CaCl2 and 90 μL phospholipids (Diagnostic Reagents, Oxford, United Kingdom) in TBS, and the time to clot was measured.
For in vivo work, CD34+ cells were purified from mice injured 2 days previously and cultured in either medium, 50 nM thrombin, or thrombin with PAR-1 and PAR-4 antagonists (both at 10 μM) for 1 hour, before washing 3 times and injection of 7.5 × 105 cells into a second mouse at the time of injury.
Immunohistology
Frozen sections were prepared exactly as previously described.21 The following additional Abs were used: rabbit anti–mouse P-selectin (BD Bioscience Pharmingen), chicken anti–mouse E-selectin (R&D Systems, Minneapolis, MN), and rabbit anti–chicken IgG-FITC (Sigma-Aldrich). Some sections were counterstained with DAPI. Sections were examined under the immunofluorescence microscope with Plan-NEOFLUAR objectives using a KTL/CCD-1300/Y/HS camera from Princeton Instruments (Trenton, NJ). Images were analyzed using the MetaMorph imaging system (Universal Imaging, Downingtown, PA).
Morphometric analyses
Sections were prepared and examined as previously described.21 A total of 3 sections from each of 5 different animals were analyzed. The contralateral, noninjured artery was used as a control in all cases.
Isolation of neointimal cells
Carotid arteries were removed 28 days after injury or from uninjured control mice. Under an operating microscope, the luminal layer of cells, along with the adventitia, were removed and discarded. The remaining tissue was minced and processed as previously defined for VSMC isolation.21 Cells were seeded into 24-well plates containing round cover slips, centrifuged at 178g for 10 minutes, and cultured with Dulbecco modified Eagle medium (DMEM) with 20% fetal calf serum (FCS) overnight. Cells were fixed with cold methanol for immunohistologic analysis.
Cell proliferation and apoptosis
To determine cell proliferation, CD34+ cells were seeded at 104cells/well in 96-well plates. After 5 days, [3H]-thymidine (Amersham Biosciences, Bucks, United Kingdom) was added at 0.037 MBq/mL (1 μCi/mL) for the final 3 hours. Assays were analyzed using a Micro 96 harvester (Skatron Instruments, Lier, Norway) and liquid scintillation counter (1205 Betaplate; Wallac, Turku, Finland). Apoptosis was performed with an in situ cell death detection kit according to the manufacturer's instructions (Roche Applied Science, Philadelphia, PA). All analyses were performed in triplicate.
Statistical analysis
Data were expressed as means plus or minus SEM. Significance of the difference between 2 groups was determined by unpaired Student t test. P values less than .05 were considered statistically significant.
Results
Circulating CD34+ cells after wire-induced injury express PAR and TF
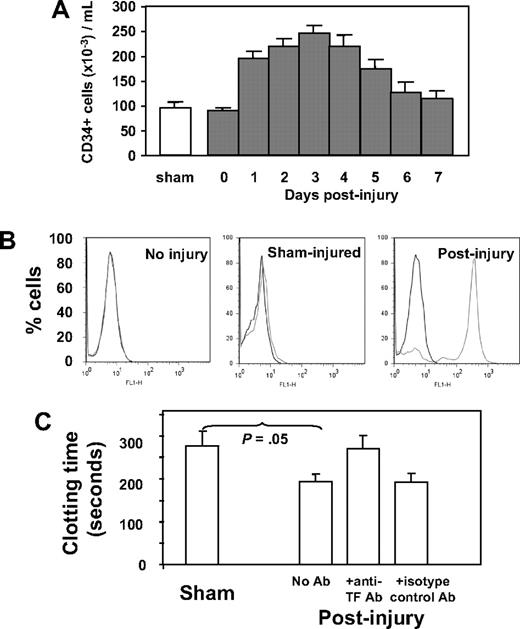
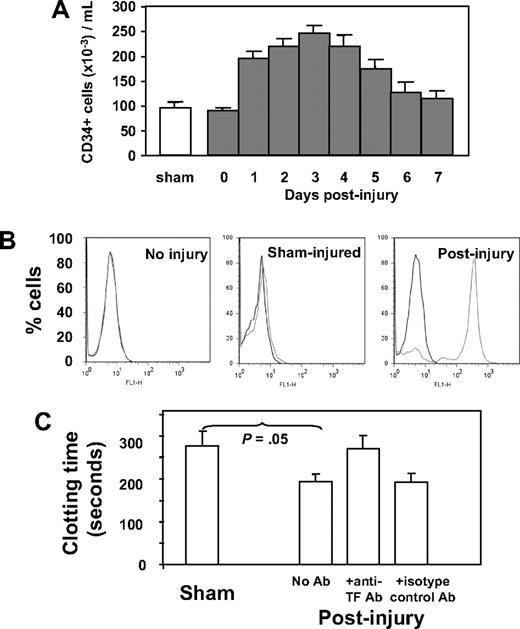
Because our previous work implicated CD34+ cells in thrombin-induced IH, we characterized circulating CD34+ cells after wire-induced injury. As shown in Figure 1A, the number of CD34+ cells rose after injury, peaking on day 3. Immunocytochemistry was used to determine the phenotypic changes in the population after injury, with a particular interest in expression of TF and PAR. These results are shown in Table 1. In control (sham-operated) mice, circulating CD34+ cells expressing TF or PARs (-1, -2, or -4), α-SMA, Flk-1, or CD31 were detectable but represented minority populations, whereas in wire-injured mice, the number of CD34+ cells expressing one or another of these markers rose significantly; TF-positive cells increased 5-fold to a peak of 85% of total CD34+ cells, PAR-expressing cells increased 10-fold (to 71%-87%), cells expressing α-SMA increased 7-fold (to 14%), those positive for Flk-1 increased 6-fold (to 90%), and cells expressing CD31 increased 7-fold (to 60%) after injury. The vast majority of these CD34+ cells did not express Sca-1, c-kit, or CD133 (Table 1).
Characteristics and phenotype of CD34+ cells after wire-induced injury. (A) Number of CD34+ cells isolated by bead selection from control (sham) or WT injured mice at various time points after injury, expressed as cells per milliliter of blood. Sham animals received a general anesthetic and skin incision but no wire injury. CD34+ cells from day-0–injured animals were isolated within 1 hour of injury. (N = 12 per group). (B) Flow cytometric analysis of TF expression on CD34+ cells from noninjured mice, sham-injured mice, or mice injured 2 days previously. (C) Clotting time of recalcified mouse plasma in the presence of CD34+ cells isolated from sham mice or 3 days after injury. An anti-TF antibody or isotype control was added to cells after injury as indicated. N = 3. Clotting time in the absence of cells was 1020 (± 109) seconds. Data are presented as means plus or minus standard error of mean (SEM).
Characteristics and phenotype of CD34+ cells after wire-induced injury. (A) Number of CD34+ cells isolated by bead selection from control (sham) or WT injured mice at various time points after injury, expressed as cells per milliliter of blood. Sham animals received a general anesthetic and skin incision but no wire injury. CD34+ cells from day-0–injured animals were isolated within 1 hour of injury. (N = 12 per group). (B) Flow cytometric analysis of TF expression on CD34+ cells from noninjured mice, sham-injured mice, or mice injured 2 days previously. (C) Clotting time of recalcified mouse plasma in the presence of CD34+ cells isolated from sham mice or 3 days after injury. An anti-TF antibody or isotype control was added to cells after injury as indicated. N = 3. Clotting time in the absence of cells was 1020 (± 109) seconds. Data are presented as means plus or minus standard error of mean (SEM).
The TF expressed by CD34+ cells was examined by flow cytometry. As shown in Figure 1B, cells from noninjured mice expressed no TF, and those from sham-injured had very low levels, but the majority isolated 2 days after injury had high levels of cell-surface TF. CD34+ cells were incubated in recalcified mouse plasma, and the time to generate a fibrin clot was measured (Figure 1C). Compared with those isolated from sham controls, cells from injured mice promoted accelerated clotting, which was inhibited by an inhibitory anti-mouse TF antibody. These results confirm that the TF expressed on circulating CD34+ cells after injury was capable of generating thrombin.
Thrombin acts directly on CD34+ cells to cause IH
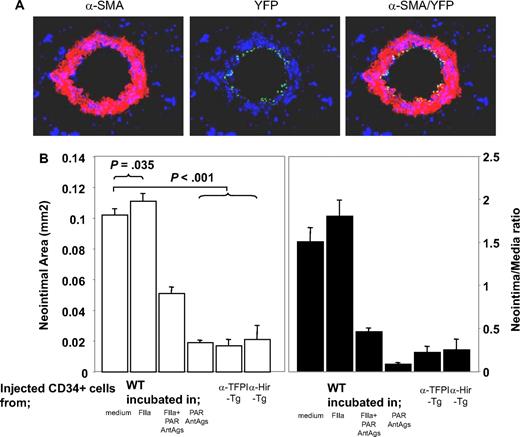
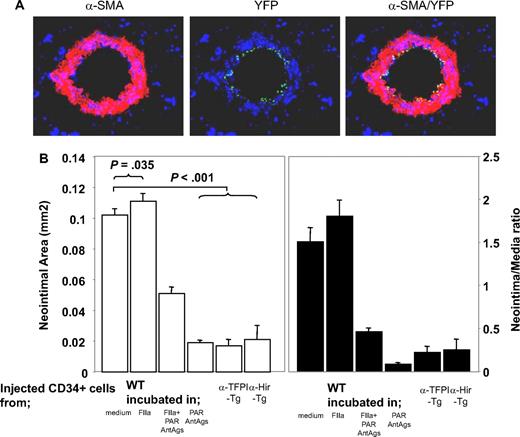
We previously showed that inhibition of TF or thrombin on the surface of α-SMA–expressing CD34+ cells was sufficient to inhibit IH, but only in the areas of vessel that were infiltrated by the cells. From this we hypothesized that IH was due to the local, direct actions of thrombin on CD34+ cells.21 To test this hypothesis, we isolated CD34+ cells from mice 2 days after injury and incubated them in vitro for 1 hour in the presence of either thrombin and/or PAR antagonists before injecting them back into a second mouse at the time of injury to compete with endogenous CD34+ cells for recruitment into the injured vessel wall. Experiments using CD34+ cells from mice with the enhanced yellow fluorescent protein targeted to the ROSA-26 locus showed that thrombin-incubated CD34+ cells were recruited into the injured vessel wall (Figure 2A). On examination of vessels 28 days after injury, the mice injected with control cells incubated in medium had a similar degree of neointima as WT controls (data not shown), whereas cells incubated with thrombin promoted the development of neointimal lesions that were slightly exaggerated, though this was statistically significant (Figure 2B). CD34+ cells incubated with PAR-1 and -4 antagonists inhibited IH to a degree that was similar to that seen after cells from α-TFPI–Tg and α-Hir–Tg mice were injected into WT mice. This effect was partially overcome by incubating the cells with thrombin prior to injection; looked at another way, the effect of thrombin preincubation was inhibited by coincubation with PAR-1 and -4 antagonists. Neointimal–media area ratios showed the same pattern of variance as neointimal area (Figure 2B). Thus, inhibition of thrombin signaling through PAR-1 and -4 on injected circulating CD34+ cells is sufficient to inhibit neointima formation, and this is as efficient as when anticoagulants are expressed on the surface of α-SMA–expressing cells. Therefore, IH is caused by the direct effect of thrombin on these cells.
Manipulated CD34+ cells influence development of IH. (A) 3-color immunohistologic analysis of carotid artery sections from injured WT mice taken on day 5 after injury. On day of injury, mice received 7.5 × 105 CD34+ cells from ROSA-EYFP mice—these were exposed to thrombin 50 nM for 1 hour immediately before injection. Sections were stained with anti–α-SMA (red) and DAPI (blue). Original images taken at ×100 magnification. (B) Neointimal area (left panel) and intima-media ratios (right panel) of vessels taken from WT animals 28 days after injury. All received 7.5 × 105 CD34+ cells on the day of injury from WT or Tg mice. The cells from WT mice were incubated in vitro for 1 hour, as indicated, prior to injection. Data derived from examination of 3 random sections from 5 different vessels. Data are presented as means plus or minus SEM.
Manipulated CD34+ cells influence development of IH. (A) 3-color immunohistologic analysis of carotid artery sections from injured WT mice taken on day 5 after injury. On day of injury, mice received 7.5 × 105 CD34+ cells from ROSA-EYFP mice—these were exposed to thrombin 50 nM for 1 hour immediately before injection. Sections were stained with anti–α-SMA (red) and DAPI (blue). Original images taken at ×100 magnification. (B) Neointimal area (left panel) and intima-media ratios (right panel) of vessels taken from WT animals 28 days after injury. All received 7.5 × 105 CD34+ cells on the day of injury from WT or Tg mice. The cells from WT mice were incubated in vitro for 1 hour, as indicated, prior to injection. Data derived from examination of 3 random sections from 5 different vessels. Data are presented as means plus or minus SEM.
Effect of thrombin on proliferation and apoptosis of CD34+ cells in vitro
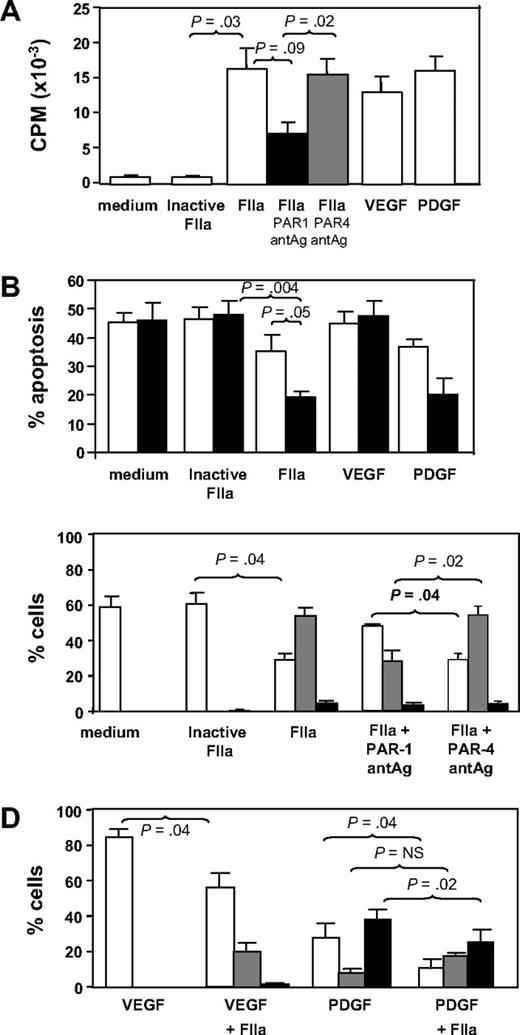
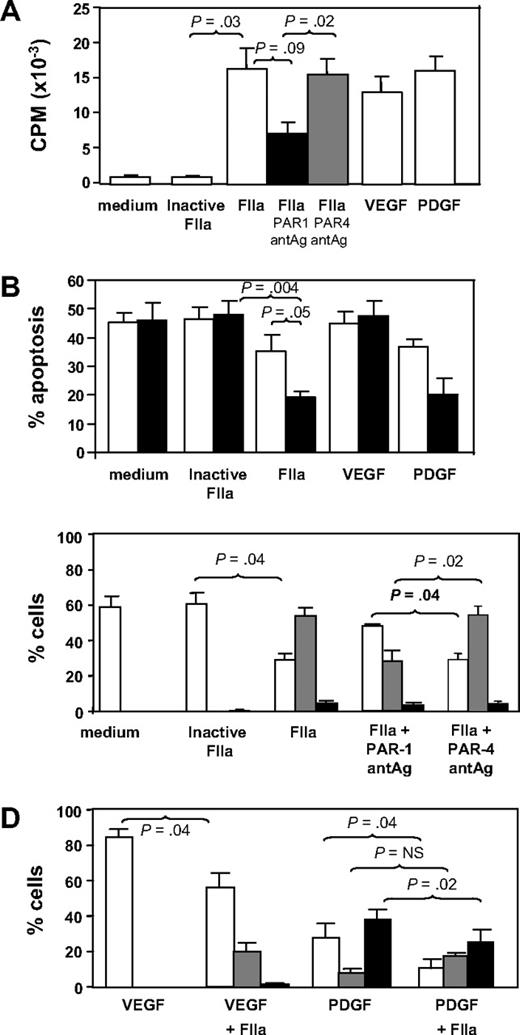
CD34+ cells isolated 2 days after injury were incubated in vitro for up to 5 days under various conditions. In comparison to control cells incubated in medium or active site-inactivated thrombin, thrombin promoted significant proliferation comparable in magnitude with that induced by the growth factors VEGF or PDGF (Figure 3A). The action of thrombin was partially inhibited by a PAR-1 but not a PAR-4 antagonist. Thrombin also inhibited the rate of apoptosis comparable with that seen when cells were incubated with PDGF (Figure 3B). These results indicate that thrombin, mainly through PAR-1, promotes the proliferation and survival of CD34+ cells in vitro.
In vitro incubation with thrombin. (A) Proliferation, expressed as mean counts per minute (CPM) plus or minus SEM assessed by incorporation of 3H-thymidine by triplicates. CD34+ cells were isolated from WT mice 2 days after wire-induced injury and incubated for 5 days either in medium alone or in medium containing active site-inhibited thrombin (FIIa), FIIa, FIIa with PAR-1 antagonist (antAg), FIIa with PAR-4 antagonist, VEGF, or PDGF before a 16-hour incubation with 3H-thymidine. (B) Proportion of cells undergoing apoptosis after incubation in vitro for 1 (□) or 5 days (■). Phenotype was determined by annexin V staining and is expressed as a proportion of the cells remaining. CD34+ cells were isolated from WT mice 2 days after wire-induced injury and incubated for 5 days either in medium alone, or in medium containing inactive FIIa, FIIa, VEGF, or PDGF. (C) CD34+ cells were isolated from WT mice 2 days after wire-induced injury and incubated for 5 days either in medium alone, medium containing inactive thrombin, thrombin, or thrombin with a PAR-1 or PAR-4 antagonist. Graphs show the proportion of cells expressing CD31 alone (□), both CD31 and α-SMA (▩), or α-SMA alone (■). Phenotype was determined by immunocytochemistry of 3 random fields as in Figure 4 and is expressed as a proportion of the cells remaining. (D) As in panel C, except cells incubated with VEGF, VEGF plus thrombin, PDGF, or PDGF plus FIIa. All in vitro experiments were repeated at least twice. Data are presented as means plus or minus SEM.
In vitro incubation with thrombin. (A) Proliferation, expressed as mean counts per minute (CPM) plus or minus SEM assessed by incorporation of 3H-thymidine by triplicates. CD34+ cells were isolated from WT mice 2 days after wire-induced injury and incubated for 5 days either in medium alone or in medium containing active site-inhibited thrombin (FIIa), FIIa, FIIa with PAR-1 antagonist (antAg), FIIa with PAR-4 antagonist, VEGF, or PDGF before a 16-hour incubation with 3H-thymidine. (B) Proportion of cells undergoing apoptosis after incubation in vitro for 1 (□) or 5 days (■). Phenotype was determined by annexin V staining and is expressed as a proportion of the cells remaining. CD34+ cells were isolated from WT mice 2 days after wire-induced injury and incubated for 5 days either in medium alone, or in medium containing inactive FIIa, FIIa, VEGF, or PDGF. (C) CD34+ cells were isolated from WT mice 2 days after wire-induced injury and incubated for 5 days either in medium alone, medium containing inactive thrombin, thrombin, or thrombin with a PAR-1 or PAR-4 antagonist. Graphs show the proportion of cells expressing CD31 alone (□), both CD31 and α-SMA (▩), or α-SMA alone (■). Phenotype was determined by immunocytochemistry of 3 random fields as in Figure 4 and is expressed as a proportion of the cells remaining. (D) As in panel C, except cells incubated with VEGF, VEGF plus thrombin, PDGF, or PDGF plus FIIa. All in vitro experiments were repeated at least twice. Data are presented as means plus or minus SEM.
Effect of thrombin on phenotype of CD34+ cells in vitro
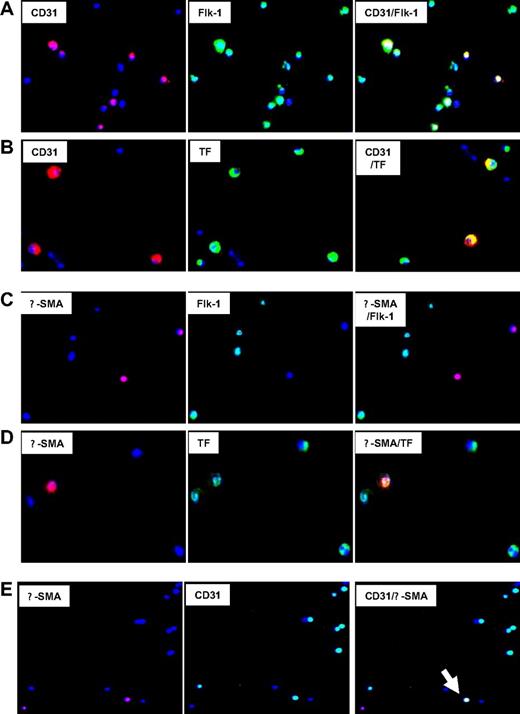
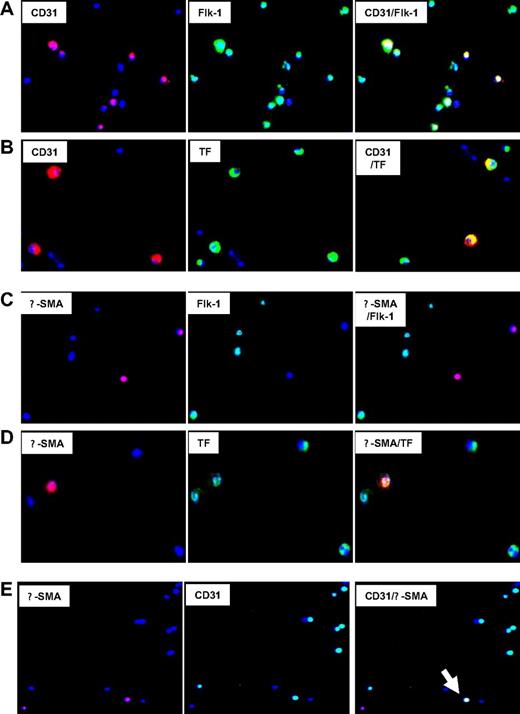
First, the coexpression of phenotypic markers on cells isolated 2 days after injury was studied by 2-color immunocytochemistry. Approximately 55% of CD34+ coexpressed CD31+ with Flk-1, consistent with an EC progenitor cell phenotype, though a significant proportion of Flk-1 cells were negative for CD31 (Figure 4A). All these cells coexpressed TF (Figure 4B), PAR-1, PAR-2, and CXCR4, but some were PAR-4− (data not shown). The subgroup of α-SMA–expressing cells, representing approximately 8% to 10% of CD34+ cells (Table 1), coexpressed SM22 (data not shown), which is compatible with a VSMC progenitor cell phenotype. All expressed TF (Figure 4D), PAR-1, PAR-2, PAR-4, and CXCR4 (data not shown). Most were Flk-1− and CD31− (Figure 4C), but there was a distinct subgroup, representing approximately 3% of circulating CD34+ cells, that had a mixed phenotype, coexpressing CD31 and Flk-1 with α-SMA and SM22 (Figure 4E). Therefore, circulating CD34+ cells after injury contained at least 4 different subpopulations of VP cells, including early Flk-1+CD31− cells, EC and VSMC progenitors and, most important, a minority population with a mixed phenotype, expressing markers of both ECs and VSMCs.
Immunocytochemical analysis of phenotype of CD34+ cells after injury. Immunocytochemical analysis of CD34+ cells isolated on day 2. All cells stained with DAPI (blue). Original images taken at 100× magnification—some of the images chosen have been electronically magnified (1-3×) in the process of selecting appropriate fields. (A) Cells stained for CD31 (red) and Flk-1 (green). Dual exposure image is on the right and illustrates that all CD31-expressing cells coexpress Flk-1 (yellow), but not all Flk-1+ cells express CD31 (green). (B) Cells stained for CD31 (red) and TF (green). Dual exposure image is on the right and shows that all CD31-expressing cells stain for TF (yellow). (C) Cells stained for α-SMA (red) and Flk-1 (green). Dual exposure image is on right of the panel, and illustrates that in this field, none of the α-SMA–expressing cells stain for Flk-1. (D) Cells stained for α-SMA (red) and TF (green). Dual exposure image is on right of the panel, and shows that the single α-SMA–expressing cell in this field stains for TF (yellow). (E) Cells stained for α-SMA (red) and CD31 (green). Dual exposure image is on right of the panel, and shows a single cell in this field that stains for both markers (yellow). Representative of 2 independent assessments.
Immunocytochemical analysis of phenotype of CD34+ cells after injury. Immunocytochemical analysis of CD34+ cells isolated on day 2. All cells stained with DAPI (blue). Original images taken at 100× magnification—some of the images chosen have been electronically magnified (1-3×) in the process of selecting appropriate fields. (A) Cells stained for CD31 (red) and Flk-1 (green). Dual exposure image is on the right and illustrates that all CD31-expressing cells coexpress Flk-1 (yellow), but not all Flk-1+ cells express CD31 (green). (B) Cells stained for CD31 (red) and TF (green). Dual exposure image is on the right and shows that all CD31-expressing cells stain for TF (yellow). (C) Cells stained for α-SMA (red) and Flk-1 (green). Dual exposure image is on right of the panel, and illustrates that in this field, none of the α-SMA–expressing cells stain for Flk-1. (D) Cells stained for α-SMA (red) and TF (green). Dual exposure image is on right of the panel, and shows that the single α-SMA–expressing cell in this field stains for TF (yellow). (E) Cells stained for α-SMA (red) and CD31 (green). Dual exposure image is on right of the panel, and shows a single cell in this field that stains for both markers (yellow). Representative of 2 independent assessments.
The effect of thrombin on these subpopulations is shown in Figure 3C. In control cultures (medium only), all α-SMA+ cells had disappeared by 5 days, and most cells (approximately 60%) were CD31+. In contrast, after 5 days of incubation with thrombin (but not active site-blocked thrombin), most of cells in culture (approximately 60%) had the mixed phenotype, coexpressing α-SMA with CD31. These thrombin-induced changes were mediated, at least partly, through PAR-1, as they were inhibited by coincubation with a PAR-1 antagonist, but not a PAR-4 antagonist (Figure 3C). CXCR4 expression appeared not to vary on each of the subpopulations after thrombin (data not shown).
Finally, we investigated how thrombin affected the differentiation of EC or VSMC progenitors induced by VEGF or PDGF, respectively. As anticipated, VEGF promoted the outgrowth of CD31+ progenitors so that by 5 days, approximately 80% of the remaining cells were CD31+α-SMA− (Figure 3D). Addition of thrombin promoted the outgrowth of a sizeable minority population of mixed phenotype cells. Conversely, after PDGF treatment, the largest population 5 days later expressed α-SMA without CD31, and thrombin tended to enhance the proportion of mixed phenotype cells at the expense of the others (Figure 3D).
Taken altogether, these data support the hypothesis that thrombin, predominantly through PAR-1, promotes the outgrowth of the mixed-phenotype population of progenitors, even in the presence of growth factors that would otherwise promote EC or VSMC progenitor cell differentiation.
Phenotype of neointimal cells after wire-induced injury in WT mice
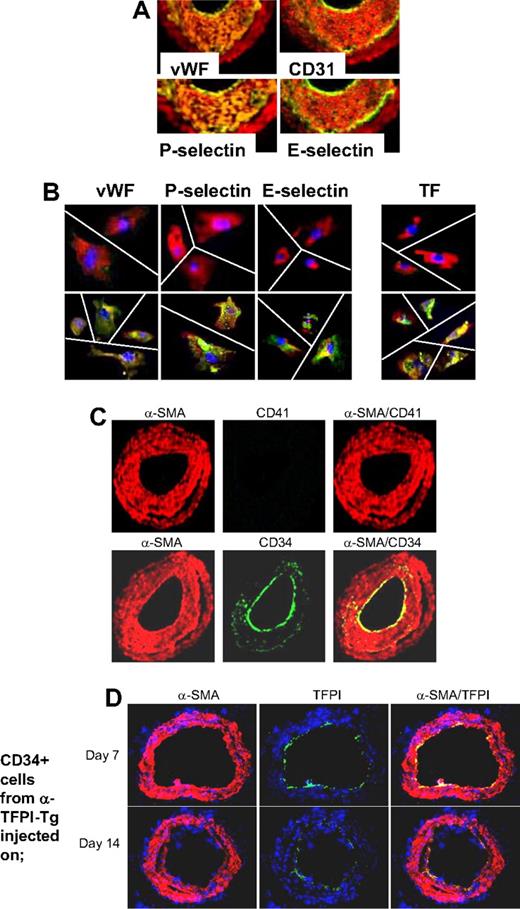
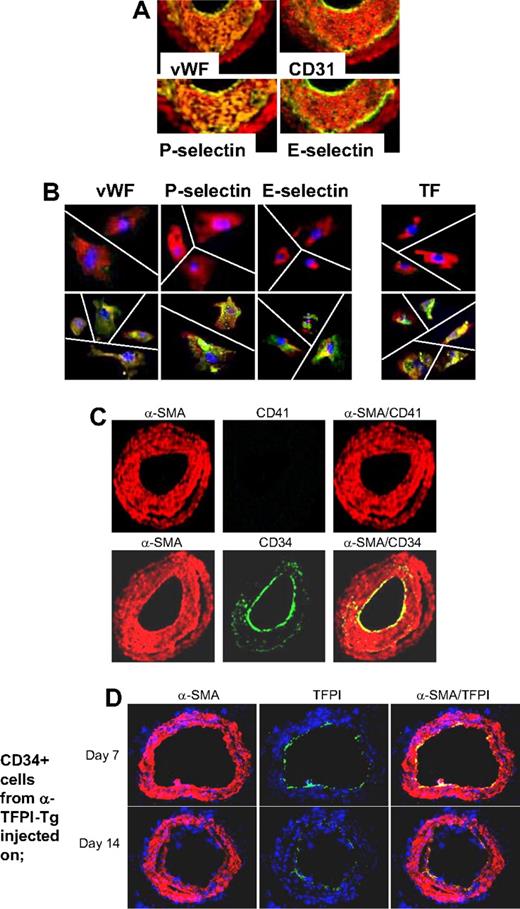
Based on these findings, we hypothesized that if IH was due entirely to thrombin-induced proliferation of recruited CD34+ cells, the neointima should contain a significant number of mixed phenotype cells coexpressing CD31 and α-SMA, similar to those seen in vitro after incubation with thrombin. WT arteries were examined by immunohistology 28 days after injury. Multiple proteins, not usually expressed by medial VSMCs but characteristic of ECs, were detectable throughout the neointima, including von Willebrand factor (VWF), CD31, P-selectin, and also E-selectin, all with a pattern of staining that overlapped that of α-SMA (Figure 5A), so that no distinct α-SMA− endothelium was seen. Immunocytochemistry of neointimal cells isolated from injured WT arteries confirmed that the pattern of staining was caused by individual cells coexpressing α-SMA alongside the EC markers (Figure 5B). Compatible with our previous study, and the in vitro findings, these cells also expressed TF. In contrast to the findings we reported during the acute injury phase,21 no CD41 (Figure 5C) or CD68 (data not shown) was detectable at this time, establishing that these patterns of staining did not represent platelet or macrophage infiltration into the damaged vessels. Therefore, these results are consistent with the hypothesis that IH is due to thrombin-induced proliferation of recruited VP cells.
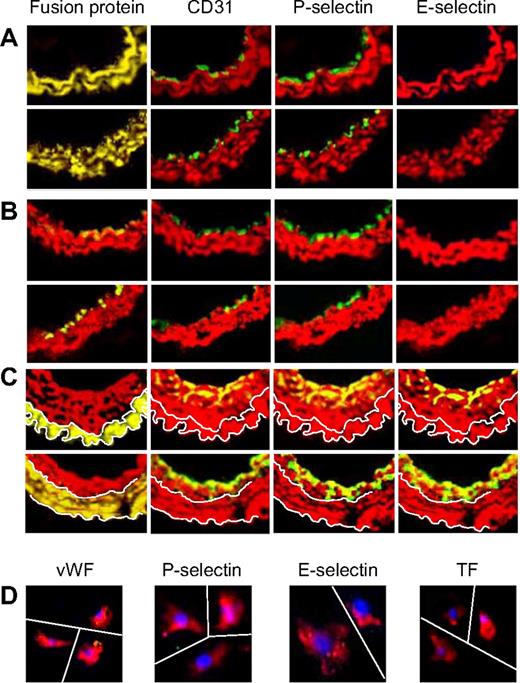
Phenotype of neointimal cells. (A) 2-color immunohistologic analysis of carotid artery sections from WT arteries taken on day 28 after injury. Consecutive sections were stained with anti–α-SMA (red) and either anti-VWF, anti-CD31, anti–P-selectin, or anti–E-selectin (green). Yellow indicates colocalization. Original images taken at ×100 magnification. (B) 3-color immunohistologic analysis of single cells isolated from intima of control uninjured (top row) and day 28–injured arteries (bottom row) from WT mice. Cells were prepared after first scraping off and discarding the luminal layer. All sections were stained with DAPI (blue), anti–α-SMA (red), and (green) either anti-VWF, anti–P-selectin, or anti–E-selectin. Yellow indicates colocalization. Original images taken at ×400 magnification and then enlarged 5× digitally. As indicated by the white lines, images of individual cells have been cut and spliced into a single frame in Adobe Photoshop (San Jose, CA). (C) 2-color immunohistologic analysis of carotid artery sections from WT arteries taken on day 28 after injury. Dual exposure image is on the right. Consecutive sections were stained with anti–α-SMA (red) and (green) either anti-CD41 (top row) or anti-CD34 (bottom row). Yellow indicates colocalization. Original images taken at ×100 magnification. (D) 3-color immunohistologic analysis of carotid artery sections from WT arteries taken on day 21 after injury. Injured mice were injected with 7.5 × 105 CD34+ cells from α-TFPI–Tg mice on day 7 (top row) or day 14 (bottom row). All sections were stained with DAPI (blue), anti–α-SMA (red) and anti-TFPI (green). (A-D) Representative of experiments performed on at least 2 independent cohorts of animals.
Phenotype of neointimal cells. (A) 2-color immunohistologic analysis of carotid artery sections from WT arteries taken on day 28 after injury. Consecutive sections were stained with anti–α-SMA (red) and either anti-VWF, anti-CD31, anti–P-selectin, or anti–E-selectin (green). Yellow indicates colocalization. Original images taken at ×100 magnification. (B) 3-color immunohistologic analysis of single cells isolated from intima of control uninjured (top row) and day 28–injured arteries (bottom row) from WT mice. Cells were prepared after first scraping off and discarding the luminal layer. All sections were stained with DAPI (blue), anti–α-SMA (red), and (green) either anti-VWF, anti–P-selectin, or anti–E-selectin. Yellow indicates colocalization. Original images taken at ×400 magnification and then enlarged 5× digitally. As indicated by the white lines, images of individual cells have been cut and spliced into a single frame in Adobe Photoshop (San Jose, CA). (C) 2-color immunohistologic analysis of carotid artery sections from WT arteries taken on day 28 after injury. Dual exposure image is on the right. Consecutive sections were stained with anti–α-SMA (red) and (green) either anti-CD41 (top row) or anti-CD34 (bottom row). Yellow indicates colocalization. Original images taken at ×100 magnification. (D) 3-color immunohistologic analysis of carotid artery sections from WT arteries taken on day 21 after injury. Injured mice were injected with 7.5 × 105 CD34+ cells from α-TFPI–Tg mice on day 7 (top row) or day 14 (bottom row). All sections were stained with DAPI (blue), anti–α-SMA (red) and anti-TFPI (green). (A-D) Representative of experiments performed on at least 2 independent cohorts of animals.
The staining for CD34 was much brighter on the luminal aspect of the neointima and only weakly expressed beneath this layer (Figure 5C). Since, by other criteria, these luminal cells appeared to have the mixed phenotype, we hypothesized that this pattern of staining might arise if progenitor cells were being constantly recruited to the luminal aspect of the expanding neointima, before down-regulating CD34 expression. Consistent with this, luminal TFPI fusion protein expression was detectable on day 21 when CD34+ cells from α-TFPI–Tg mice were injected into WT mice that were injured 1 or 2 weeks previously (Figure 5D), indicating that these cells were still recruited to the injured vessel at this time.
Regenerative repair associated with apparently normal differentiation of ECs and VSMCs when thrombin or thrombin receptors are inhibited on CD34+ cells
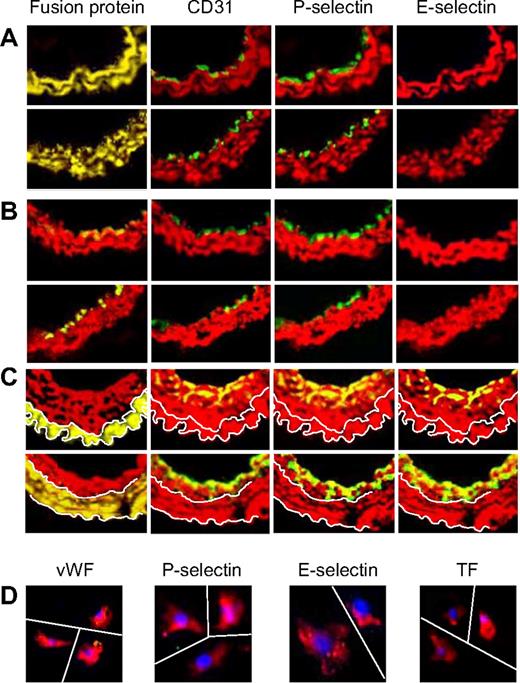
We next explored the phenotype of the neointima when the generation of thrombin or the actions of thrombin were inhibited on CD34+ cells. In sections of vessels from the 2 parental strains of α-Tg mice, no mixed-phenotype cells could be detected in any sections examined by immunohistology (Figure 6A). Instead, a distinct luminal layer of quiescent ECs developed by day 28; these cells did not express α-SMA, TF, or E-selectin. WT mice reconstituted with BM from either α-Tg mouse showed the same pattern of staining (Figure 6B), with a layer of neointimal VSMCs (expressing the Tg fusion protein) visible beneath a quiescent endothelium. In contrast, the neointima of Tg mice reconstituted with WT BM stained with a WT pattern (Figure 6C), though in these mice the areas of neointima that stained for EC markers were restricted to the most luminal aspects. Single cells isolated from injured α-TFPI–Tg arteries expressed α-SMA but neither TF nor EC markers (Figure 6D).
Phenotype of neointimal cells affected by fusion proteins. 2-color immunohistologic analysis of carotid artery sections from α-TFPI–Tg or α-Hir–Tg mice (A), WT mice reconstituted with BM from α-TFPI–Tg or α-Hir–Tg (B) mice, or α-TFPI–Tg or α-Hir–Tg (C) mice reconstituted with BM from WT mice, all taken on day 28 after injury. Consecutive sections were stained with anti–α-SMA (red) and either antifusion protein, anti-CD31, anti–P-selectin, or anti–E-selectin (green). Yellow indicates colocalization. Original images taken at ×100 magnification. White lines in panel C trace out the boundaries of medial fusion protein staining; the same lines have been superimposed on the other images to indicate the boundary between medial and neointimal α-SMA staining. Representative images from experiments performed more than 3 times. (D) 3-color immunohistologic analysis of single cells isolated from intima of α-TFPI–Tg (bottom row) mice. Cells were prepared after first scraping off and discarding the luminal layer. All sections were stained with DAPI (blue), anti–α-SMA (red), and (green) either anti-VWF, anti–P-selectin, or anti–E-selectin. Yellow indicates colocalization. Original images taken at ×400 magnification and then enlarged ×5 digitally. As indicated by the white lines, images of individual cells have been cut and spliced into a single frame in Adobe Photoshop. Representative of experiments performed on at least 2 independent cohorts of animals.
Phenotype of neointimal cells affected by fusion proteins. 2-color immunohistologic analysis of carotid artery sections from α-TFPI–Tg or α-Hir–Tg mice (A), WT mice reconstituted with BM from α-TFPI–Tg or α-Hir–Tg (B) mice, or α-TFPI–Tg or α-Hir–Tg (C) mice reconstituted with BM from WT mice, all taken on day 28 after injury. Consecutive sections were stained with anti–α-SMA (red) and either antifusion protein, anti-CD31, anti–P-selectin, or anti–E-selectin (green). Yellow indicates colocalization. Original images taken at ×100 magnification. White lines in panel C trace out the boundaries of medial fusion protein staining; the same lines have been superimposed on the other images to indicate the boundary between medial and neointimal α-SMA staining. Representative images from experiments performed more than 3 times. (D) 3-color immunohistologic analysis of single cells isolated from intima of α-TFPI–Tg (bottom row) mice. Cells were prepared after first scraping off and discarding the luminal layer. All sections were stained with DAPI (blue), anti–α-SMA (red), and (green) either anti-VWF, anti–P-selectin, or anti–E-selectin. Yellow indicates colocalization. Original images taken at ×400 magnification and then enlarged ×5 digitally. As indicated by the white lines, images of individual cells have been cut and spliced into a single frame in Adobe Photoshop. Representative of experiments performed on at least 2 independent cohorts of animals.
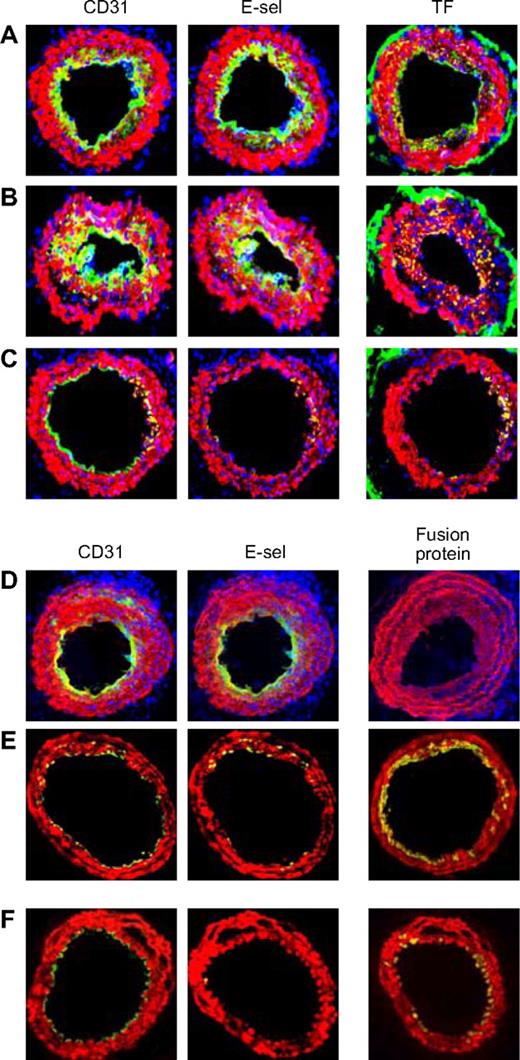
The arteries from mice that were administered in vitro–manipulated CD34+ cells following wire injury were examined. Neointima from mice given cells incubated in medium (Figure 7A) or thrombin (Figure 7B) showed the same pattern of staining as in WT mice. However, those given CD34+ cells incubated with PAR antagonists (Figure 7C) contained few mixed-phenotype neointimal cells and developed, in the main, a quiescent endothelium. Similarly, compared with mice given control CD34+ cells from WT mice (Figure 7D), those given cells from either Tg animal (Figure 7E,F) showed few mixed-phenotype cells and developed mainly E-selectin− ECs.
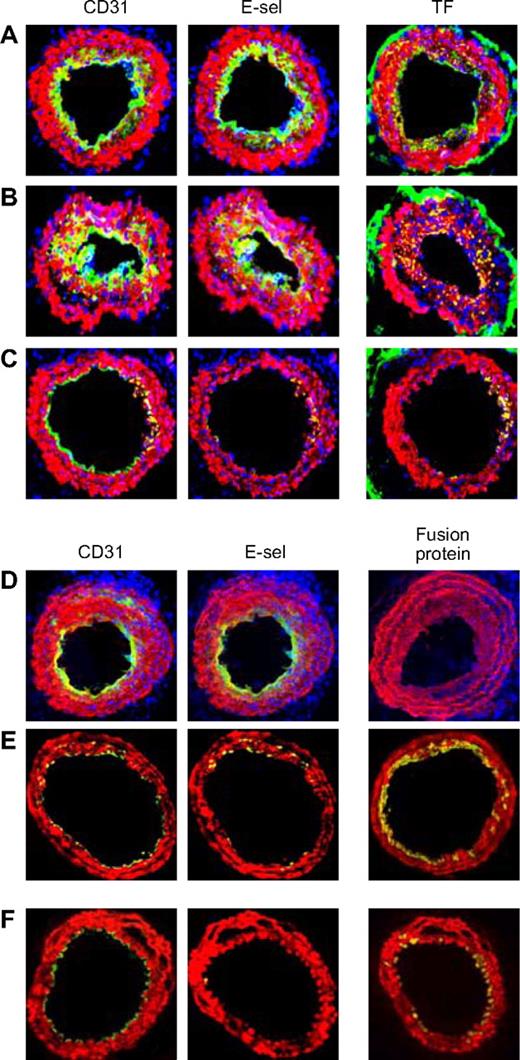
Effect of injected CD34+ cells on neointimal phenotype. CD34+ cells from WT mice were purified 2 days after wire injury, and incubated in vitro for 1 hour in either medium only (A) or with added thrombin (B) or PAR-1 and PAR-4 antagonists (C) before 7.5 × 105 cells were injected back into a second WT mouse immediately following wire injury. Alternatively, CD34+ cells were purified from WT (D), α-TFPI–Tg (E), or α-Hir–Tg mice (F) and injected immediately into a second injured mouse. (A-D) 3-color immunohistologic analysis of frozen sections from day 28 after injury. Sections were stained with DAPI (blue). (E,F) 2-color immunohistologic analysis. All sections stained with anti–α-SMA (red) and anti-CD31, anti–E-selectin, and either anti-TF or antifusion protein (green) as indicated. Yellow indicates colocalization. Original images taken at ×100 magnification. Repeated twice.
Effect of injected CD34+ cells on neointimal phenotype. CD34+ cells from WT mice were purified 2 days after wire injury, and incubated in vitro for 1 hour in either medium only (A) or with added thrombin (B) or PAR-1 and PAR-4 antagonists (C) before 7.5 × 105 cells were injected back into a second WT mouse immediately following wire injury. Alternatively, CD34+ cells were purified from WT (D), α-TFPI–Tg (E), or α-Hir–Tg mice (F) and injected immediately into a second injured mouse. (A-D) 3-color immunohistologic analysis of frozen sections from day 28 after injury. Sections were stained with DAPI (blue). (E,F) 2-color immunohistologic analysis. All sections stained with anti–α-SMA (red) and anti-CD31, anti–E-selectin, and either anti-TF or antifusion protein (green) as indicated. Yellow indicates colocalization. Original images taken at ×100 magnification. Repeated twice.
These data illustrate that inhibiting thrombin generation (with the TFPI fusion protein), the protease activity of thrombin (with the hirudin fusion protein), or blocking thrombin receptors on the surface of CD34+ cells prevents the appearance of mixed-phenotype cells in the neointima. Moreover, and importantly, they illustrate that these conditions appear to allow the same cells to respond appropriately to differentiation cues and mature into quiescent ECs and resting VSMCs, leading to regenerative repair of the damaged artery.
Discussion
The decision to focus on circulating CD34+ progenitors, which we defined here as c-kit−/sca-1− cells, was based on our previous study showing that CD34+ cells isolated from the peripheral blood of α-Tg mice inhibited IH in WT mice after wire-induced injury.21 These are a relevant population, as in humans, the number of circulating CD34+ cells correlates with occurrence of arteriosclerosis, for instance after stent implantation in patients with coronary artery disease.24 Other studies in mice have used BM-derived progenitors to address which subtype is important in IH, and these have shown that CD34+,25 c-kit+,25,26 and c-kit− fractions19 all appear capable of being recruited and/or differentiating into VSMCs.
Consistent with one other report,26 we found the numbers of circulating CD34+ cells increased significantly following endoluminal injury. The phenotype of cells was altered considerably by the injury such that by day 2, the majority (approximately 80%) expressed procoagulant TF, along with PAR-1, -2, and -4. Most of the TF-expressing cells expressed Flk-1 (VEGF-R2), an accepted marker of EC, hematopoietic, and VSMC lineages.15,27,28 Most Flk-1+ cells expressed CD31, a phenotype consistent with an EC progenitor population. However, 2 other CD34+ populations were represented: a CD31−/Flk-1−/α-SMA+/SM22α+ fraction, which we have called VSMC progenitors, and, importantly, a mixed-phenotype minority population that expressed both VSMC and EC markers.
Cells with a similar mixed phenotype have been identified in studies using murine embryonic progenitors, where they have been defined as an “intermediate” progenitor population, found in culture conditions that favor the outgrowth of VSMC progenitors. Under these conditions, they are hypothesized to represent progenitors in the process of up-regulating VSMC proteins while down-regulating Flk-1 expression.15
Our primary hypothesis was that thrombin promoted IH primarily by a direct action on CD34+ progenitor cells. Consistent with this, thrombin, predominantly through PAR-1, promoted survival and proliferation of these cells in vitro, using basal culture conditions favoring apoptosis. When CD34+ cells were incubated for 60 minutes with thrombin before injection back into a second injured WT animal, these were recruited into the vessel wall and promoted exaggerated IH, whereas cells incubated with PAR antagonists inhibited IH to a degree that was similar to when cells from the Tg animals were used.
By showing that circulating CD34+ cells are capable of generating thrombin, have thrombin receptors, and proliferate to thrombin in vitro, and by demonstrating that IH is inhibited when PAR-1 and -4 are antagonized on these cells, we conclude that IH in this model is due to the direct effects of thrombin on CD34+ cells.
Thrombin also promoted the outgrowth of the minority mixed-phenotype progenitor cells over a 5-day culture period. These effects were still evident when CD34+ cells were incubated with VEGF to promote EC progenitor differentiation or PDGF to promote VSMC progenitor differentiation, indicating that the thrombin effect was not completely inhibited by these growth factors and suggesting that thrombin might antagonize the influence of factors favoring maturation and differentiation of ECs and VSMCs.
We reasoned therefore that a good test of our primary hypothesis was to show that the mixed-phenotype intermediate cells were present in the neointima of WT animals but not in the Tg mice or after antagonism of PAR on CD34+ cells. Our immunohistologic analyses confirmed that this was the case, with many cells in WT neointima coexpressing α-SMA and a range of EC markers, including E-selectin.
Other groups have reported results that might appear to contradict ours. For instance, thrombin has been shown to enhance the differentiation and maturation of murine EC progenitors from BM cells, an effect mediated through PAR-1.29 Smadja et al, using human cells, also reported enhanced angiopoietin-2, SDF-1, and CXCR4 expression, and proliferation and migration by late EC progenitors derived from cord (but not adult) blood after PAR-1 activation.30-32 However, these results are not necessarily contradictory to our findings, because these other studies were performed on BM-derived rather than circulating cells, involved relatively prolonged in vitro culture, and used conditions designed specifically to promote EC progenitor development, so that the effects of thrombin on other progenitor cell populations would have been missed.
It has been recognized for some time that compared with medial cells, neointimal VSMCs have a “modulated” phenotype, characterized by increased proliferation and synthetic functions.33 Neointimal staining for EC markers after vascular injury has been documented before, but interpreted in some studies as indicating either significant neointimal platelet infiltration34 or secretion of soluble VWF into the extracellular space.35 Coexpression of P-selectin with α-SMA by some neointimal cells was described in one recent study following wire-induced injury.36 These mixed-phenotype cells, which had constitutive NFκB activity and showed enhanced binding of leukocytes in vitro, could be found on the luminal aspect of injured vessels, where they formed a pseudoendothelium. Our data are compatible with these findings and indicate that individual neointimal cells express numerous other markers found on ECs. In our model, these cells made up a significant proportion of the neointima on day 28, and most of the sections we examined from WT animals had a luminal aspect lined by cells expressing α-SMA. In contrast, in the Tg mice and in WT animals treated with Tg or PAR-antagonized CD34+ cells, a distinct quiescent endothelium developed, beneath which was a layer of mature VSMCs, visible in the Tg mice as these cells expressed anticoagulant fusion protein. These results indicate that in these mice, EC and VSMC differentiation and maturation appeared normal, and suggest that rendering CD34+ cells unresponsive to thrombin is sufficient to promote complete regenerative repair.
Our interpretation of these results is that mixed-phenotype cells accumulate in the vessel wall because thrombin, probably generated on the cell surface of recruited TF-expressing VPs, promotes their outgrowth, even in the face of differentiation cues from growth factors such as VEGF and PDGF. The observations in α-Tg mice reconstituted with WT BM, in which BM-derived VSMCs next to the media stained negative for EC markers, are consistent with this; these cells, next to the relatively anticoagulant environment beside the Tg medial VSMCs, appear to have developed into more mature VSMCs. However, another possibility, which we have not addressed, is that thrombin may promote the differential recruitment of various subtypes of CD34+ progenitors.
Two recent studies have highlighted the critical role that platelets play in the recruitment of VPs to injured vessels. Zernecke et al showed that SDF-1, released from apoptotic medial SMC and localizing on the surface of adherent platelets, was critical in recruiting c-kit−/sca-1+/PDGFR-β+ early progenitors,19 an effect that was dependent on both CXCR4 and P-selectin expression by the progenitors and platelets, respectively. More recently, Massberg et al demonstrated a similar mechanism involving recruitment of either BM-derived CD34+ or c-kit−/sca-1+ VPs, but crucially, this group showed that the SDF-1 detected early after endoluminal damage was derived from the platelets themselves; they found SDF-1 in α-granules and documented release after GPIIb-dependent aggregation.25 Both studies highlight that platelet adherence to areas of EC denudement is necessary for efficient recruitment of BM-derived progenitors in the early phase following injury.
Because thrombin is a potent platelet activator (through PAR-4, not PAR-1, on murine platelets37 ), it is valid to ask whether reduced platelet activation at the time of the injury may have contributed to the attentuated IH seen in α-Tg mice. Although we have not addressed this directly, 2 observations suggest that it was not a prominent mechanism. First, we previously showed a significant degree of platelet and fibrin deposition in the injured vessels of α-Tg mice, similar to that seen in WT mice.21 Second, WT mice reconstituted with BM, or injected with CD34+ cells from α-Tg mice, have an acute injury, including platelet deposition, that is similar to that in WT mice, but these animals manifest regenerative repair rather than progressive neointima formation.
Our data also indicate that injected CD34+ cells continue to be recruited beyond the acute phase, in the absence of significant platelet or fibrin deposition. Although we cannot rule out the possibility that platelets are involved, another possibility is that the activated P-selectin+ luminal cells making up the pseudoendothelium are important at this time.36
These data provide significant new insights into the understanding of the pathophysiology of repair and regeneration after endoluminal injury and provide evidence for the critical roles that TF, thrombin, and PAR signaling play in determining the function and differentiation of circulating VPs after endoluminal injury. While these results may have implications for how coagulation proteases contribute to vasculogenesis,38 they also establish the foundations for future research into the impact of inflammatory stimuli on progenitor cell function and the utility of a therapeutic strategy based on highly specific manipulation of different progenitor cell populations to inhibit progressive vascular remodeling after injury or during inflammatory disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
This research was sponsored by the Medical Research Council United Kingdom.
Authorship
Contribution: D.C. did most of the experimental work; J.M.A. was responsible for animal husbandry; L.M.S. did additional experimental work; J.H.M. provided intellectual and technical input and helped write the manuscript; R.I.L. initiated the work and helped write the manuscript; and A.D. was responsible for day-to-day supervision and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Present address for R.I.L.: Kings College London, Guys Campus, London UK SE1.
Correspondence: Anthony Dorling, Department of Immunology, Imperial College London, Hammersmith Hospital, Du Cane Road, London W12 0NN, United Kingdom; e-mail: a.dorling@imperial.ac.uk.