Abstract
Although genetic induction of factor VIII (FVIII) expression in platelets can restore hemostasis in hemophilia A mice, this approach has not been studied in the clinical setting of preexisting FVIII inhibitory antibodies to determine whether such antibodies would affect therapeutic engraftment. We generated a line of transgenic mice (2bF8) that express FVIII only in platelets using the platelet-specific αIIb promoter and bred this 2bF8 transgene into a FVIIInull background. Bone marrow (BM) from heterozygous 2bF8 transgenic (2bF8tg+/−) mice was transplanted into immunized FVIIInull mice after lethal or sublethal irradiation. After BM reconstitution, 85% of recipients survived tail clipping when the 1100-cGy (myeloablative) regimen was used, 85.7% of recipients survived when 660-cGy (nonmyeloablative) regimens were used, and 60% of recipients survived when the recipients were conditioned with 440 cGy. Our further studies showed that transplantation with 1% to 5% 2bF8tg+/− BM cells still improved hemostasis in hemophilia A mice with inhibitors. These results demonstrate that the presence of FVIII-specific immunity in recipients does not negate engraftment of 2bF8 genetically modified hematopoietic stem cells, and transplantation of these hematopoietic stem cells can efficiently restore hemostasis to hemophilic mice with preexisting inhibitory antibodies under either myeloablative or nonmyeloablative regimens.
Introduction
Hemophilia A is a severe congenital bleeding disorder caused by a deficiency of clotting factor VIII (FVIII).1 Currently, hemophilia is treated with protein replacement therapy using either plasma-derived or recombinant FVIII.2 Although FVIII replacement markedly improves the life expectancy of patients with hemophilia, up to 30% of patients with severe hemophilia A develop antibodies after FVIII replacement therapy.3-7 These antibodies cause the clinical failure of treatment in response to routine replacement therapy for bleeding episodes and therefore are referred to as FVIII inhibitors.8-10 Clinically, inhibitor titers greater than 5 Bethesda units (BU)/mL are considered untreatable using routine FVIII replacement.11 Induction of immune tolerance to the FVIII protein is a treatment option for the eradication of anti-FVIII inhibitors, but it is very expensive and may not always be effective.12-14 Infusion of NovoSeven (FVIIa) may also restore hemostasis in patients with inhibitors, but it is more costly because of its short half life and may result in thrombotic complications.15-17
Gene therapy could be an alternative treatment for hemophilia A. There has been substantial progress in gene therapy of hemophilia A in preclinical trials.18-29 Gene therapy of hemophilia A with preexisting FVIII immunity is especially challenging because circulating inhibitory antibodies in plasma may inactivate functional FVIII if it is constitutively secreted into plasma. Therefore, developing a mechanism for secure cellular delivery to sequester and protect FVIII from inhibitor inactivation is a more promising approach than plasma delivery of FVIII to the site of injury when inhibitory antibodies are present in the plasma. The novel gene therapy approach we investigated is based on the hypothesis that targeting FVIII expression to megakaryocytes will result in storage of FVIII together with its carrier protein, von Willebrand factor,30-32 in α-granules in platelets. This FVIII will be released on platelet activation in the immediate vicinity of sites where FVIII is needed and therefore minimizes its exposure to inhibitory antibodies.
Our previous studies have demonstrated that genetic induction of FVIII expression in platelets can restore hemostasis in hemophilia A mice.33,34 We have developed a vector (2bF8) that targets human B-domain deleted FVIII (hBDDFVIII) expression to platelets under control of the platelet-specific glycoprotein IIb gene promoter (αIIb promoter). Using the 2bF8 construct, we can correct the murine hemophilia A phenotype, even in the presence of high titer inhibitory antibodies in a transgenic mouse model. This approach has not previously been studied in the clinical setting of preexisting FVIII inhibitory antibodies to determine whether such antibodies would affect therapeutic bone marrow (BM) engraftment.
In the current study, we explored (1) whether the presence of FVIII immunity in recipients would alter the engraftment of 2bF8 genetically modified hematopoietic stem cells (HSCs); (2) whether syngeneic transplantation of these HSCs would restore hemostasis to hemophilia A with preexisting inhibitory antibodies; (3) whether nonmyeloablative conditioning regimens can be successfully used in this platelet-dependent FVIII gene therapy; and (4) what percent of platelets might need to contain FVIII to rescue the hemorrhagic phenotype in hemophilia A with inhibitors. This platelet-dependent approach may be promising for gene therapy of hemophilia with preexisting inhibitory antibodies.
Methods
Mice
FVIIInull mice,35 which were a kind gift from H. Kazazian (University of Pennsylvania School of Medicine), and heterozygous transgenic mice (2bF8tg+/−) with platelet-specific expression of hBDDFVIII, which were generated by our laboratory,33 were used in this study. Both FVIIInull mice and 2bF8 transgenic mice used in this study were in a C57BL/6 × 129S background. Genotyping was determined by PCR as previously reported.33,35 For the inhibitor model development, 6- to 10-week-old FVIIInull or 2bF8tg+/− mice were immunized by weekly injections of recombinant human B-domain deleted FVIII (rhBDDFVIII, ReFacto, Wyeth Pharmaceuticals, Collegeville, PA) at 50 U/kg via the retro-orbital venous plexus. One week after the fourth immunization, blood samples were collected for Bethesda assay to determine the titer of inhibitors. Animal studies were performed according to a protocol approved by the Animal Care and Use Committee of the Medical College of Wisconsin.
Bone marrow transplantation
Immunized FVIIInull recipient mice were conditioned for cellular transplantation with a lethal irradiation dose of 1100 cGy (myeloablative regimen) or various sublethal doses (nonmyeloablative regimens with 660, 440, or 220 cGy) total body irradiation (TBI) using a cesium irradiator. BM cells were collected from femurs and tibias of 2bf8tg+/− donor mice with or without FVIII-specific immunity. BM mononuclear cells were isolated using Fico/Lite-LM (mouse) (Atlanta Biological, Lawrenceville, GA) as previously described, and transplanted into irradiated immunized FVIIInull recipients at a cell dose of 10 × 106 to 12 × 106 in 300 μL phosphate-buffered saline by retro-orbital plexus venous injection at 24 hours after irradiation. To produce mice with chimeric BM, different proportions of BM mononuclear cells from 2bF8tg+/− (20%, 10%, 5%, and 1%) and FVIIInull (80%, 90%, 95%, and 99%, respectively) were mixed and transplanted into lethally irradiated immunized FVIIInull mice at the same total cell dose described herein. Recipients were analyzed beginning 3 weeks after BM transplantation.
Blood collection
For plasma isolation, mice were anesthetized using isoflurane inhalation, and 100 μL of blood was collected by tail vein or retro-orbital plexus bleeds into a microtube containing 0.1 vol of 0.1 M sodium citrate. Cells were removed by centrifugation at 900g for 20 minutes, and the platelet poor plasma was used for Bethesda assays to determine inhibitor titers. Bethesda assays were performed as described in “Inhibitor assay.”
For platelet isolation, mice were anesthetized by isoflurane inhalation, and 200 μL of blood was collected by tail-vein or retro-orbital plexus bleeds into a microtube containing 1.3 mL Tyrode buffer containing 0.01 M sodium citrate and 50 ng/mL of prostaglandin E1 (Sigma-Aldrich, St Louis, MO). Platelets were recovered in the supernate after a soft spin at 200g for 3 minutes. Supernatant containing platelets, plasma, and buffer was transferred into a 15-mL polypropylene tube, washed with 8 mL Tyrode buffer with prostaglandin E1, and centrifuged at 900g for 20 minutes. The platelet pellet was resuspended in 1 mL Tyrode buffer, counted with an Animal Blood Counter (Heska, Loveland, CO), centrifuged at 900g for 20 minutes, and the platelet pellets were recovered for platelet lysate FVIII activity assays to determine the levels of platelet-FVIII.
The blood cells remaining after platelet and plasma isolation were lysed with red blood cell lysing buffer (10 mM Tris-Cl, 0.025 M MgCl2, 0.32 M sucrose, and 1% Triton X-100), and white blood cell (WBC) DNA was purified for PCR and quantitative real-time PCR.
PCR and quantitative real-time PCR
DNA was purified from peripheral WBC using QIAamp DNA Blood Mini Kit (QIAGEN, Valencia, CA). Because the 2bF8 transgene insertion site in chromosome 18 is known,33 transgene genotype status can be determined by multiplex PCR using 2 alternative sense primers with a common antisense primer adjacent to the insertion site (M18-a4743: 5′-ACT GGA GAA TTG GCA GGA GAT-3′). A 0.292-kb PCR product indicates amplification from sense primer pCIs 3371-3392 (5′-GAA TCG ATA GCG ATA AGG ATC C-3′), which lies within the transgene sequence, whereas a 0.207-kb product indicates amplification of wild-type sequence across the transgene insertion site from sense primer M18-s4536 (5′-GGT CGT GAG CTA TCA ATG TGA G-3′). DNA from heterozygous animals yields both PCR products. A total of 100 ng of WBC-derived genomic DNA was amplified using PCR GoTaq Mixture (Promega, Madison, WI). DNA from a known 2bF8tg+/− mouse served as a positive control and water was used as a negative control.
For quantitative real-time PCR, 100 ng of peripheral WBC-derived genomic DNA was analyzed for quantitation of the 2bF8 transgene sequence, with normalization to Apo B using Platinum Quantitative PCR SuperMix-UDG (Invitrogen, Carlsbad, CA). Triplicates were performed for all real-time PCR reactions using an iCycler real-time PCR thermocycler (Bio-Rad, Munich, Germany). The primers for 2bF8 transgene were 5′-ATC GCT AAG CCA AGG CCA CCC T-3′ (sense) and 5′-GCT CCC TCA GAA GCT TTC CAG TAG GA-3′ (antisense). The primers for Apo B were 5′-CAC GTG GGC TCC AGC ATT-3′ (sense) and 5′-ACA CCA GTC ATT TCT GCC TTT G-3′ (antisense). DNA from 2bF8tg+/−, FVIIInull, and wild-type mice were used as controls.
FVIII activity assay
Our previous studies have demonstrated that functional FVIII:C is detected in platelets but not in plasma in 2bF8 transgenic mice.33 Therefore, platelet lysate FVIII activity assay was used to determine the expression of platelet-derived FVIII in immunized FVIIInull mice that received BM mononuclear cells from 2bF8 transgenic mice. Platelet pellets were lysed in 200 μL of 0.5% CHAPS (the zwitterionic detergent, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, MP Biomedicals, Santa Ana, CA) by vortex until suspended, incubated on ice for 10 minutes, and spun down at full speed at 4°C for 10 minutes. Dilutions of the supernatant were then assayed for FVIII:C using a modified FVIII chromogenic assay that we have developed using the Coatest SP4 FVIII Kit (DiaPharma, Franklin, OH) as previously described.33,36 A standard curve was constructed by plotting known amounts of rhBDDFVIII (ReFacto) in lysis buffer against Vmax (mOD/min) at 405 nm minus 490 nm. The Vmax of each reaction was converted to units of FVIII:C (U/mL) using the instrument manufacturer's software program, SOFTmax, version 2.34 (Molecular Devices, Menlo Park, CA), the data were averaged, and the mU of FVIII:C per 108 platelets was calculated.
Inhibitor assay
The titer of FVIII-neutralizing inhibitors in BM transplantation recipients at different time points was determined by a modified Bethesda assay as described in our previous report.33 Sequential dilutions of mouse plasma were incubated with an equal volume of 1 U/mL rhBDDFVIII at 37°C for 2 hours, and residual FVIII:C was subsequently analyzed by chromogenic assay as described in our previous report.33 Bethesda units were defined by dilution of the blood plasma until 50% of the initial FVIII activity was neutralized.37
Phenotype correction analysis
Phenotypic correction of hemophilia A in recipients was assessed by tail clip survival test as previously described.33,34 After at least 1-month BM reconstitution, the tails of anesthetized mice were clipped at a diameter of 1.59 mm, without subsequent cauterization. Mice surviving beyond 24 hours were considered to have achieved phenotypic correction.
Statistical analysis
Real-time PCR results and FVIII:C results are presented as mean plus or minus SD, and the significance of differences was evaluated by 2-tailed Student t test. The significance of different survival rates between different groups of recipients was evaluated by Fisher exact test. A value of P less than .05 was considered statistically significant.
Results
Induction of an anti-FVIII immune response in FVIIInull mice
To mimic clinical hemophilia A patients who develop inhibitors after protein infusion in a mouse model, we induced FVIIInull mice to produce FVIII inhibitory antibodies by infusion of rhBDDFVIII at 50 U/kg by intravenous injection. All FVIIInull mice developed inhibitory antibodies if they were older than 6 weeks when the immunization was initiated. FVIIInull mice could be tolerized to rhBDDFVIII if they were younger than 6 weeks old when the first immunization was introduced (data not shown).
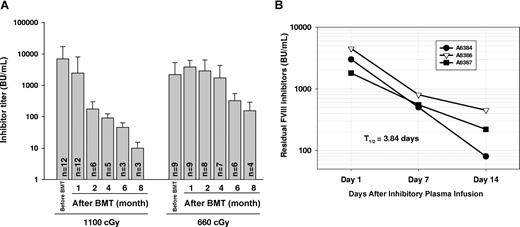
The levels of anti-FVIII inhibitory antibodies in plasma tended to decline after lethal irradiation, but inhibitors could persist in immunized recipients up to 8 months after BM transplantation (Figure 1A). When reduction of the titer over time was calculated as a half-life (t1/2), the t1/2 of inhibitory antibody titer in this group of recipients was 36.01 plus or minus 7.56 days, which was shorter (P < .05) than the half-life in the group of recipients conditioned with 660 cGy (t1/2 = 50.78 ± 15.01 days). This calculation reflects both the continued level of antibody production as well as the rate of clearance. In contrast, the clearance t1/2 was only 3.84 plus or minus 1.02 days when plasma from highly immunized FVIIInull mice was infused into FVIIInull mice (Figure 1B). These results indicate that inhibitory antibodies continue to be produced in recipients even after lethal irradiation.
Immune response in immunized FVIIInull mice. (A) FVIIInull mice received 4 weekly intravenous injections of recombinant human B-domain deleted FVIII (rhBDDFVIII) to a final plasma level of 1 U/mL. One week after the last immunization, mice received whole body irradiation at either 1100 cGy or 660 cGy followed by BM transplantation from 2bF8tg+/− mice. Plasma was collected and inhibiter titer was determined by Bethesda assay at various time points. Bars represent mean plus or minus SD. Data shown are summarized from 7 BM transplantation trials. (B) Inhibitory plasma from immunized FVIIInull mice was infused into FVIIInull mice, and then plasma was collected at days 1, 7, and 14. The residual inhibitor titer was determined by Bethesda assay to define the half-life (t1/2) of inhibitory antibody clearance (in the absence of additional antibody production). These results demonstrate that inhibitory antibodies continue to be produced in recipients even after lethal irradiation.
Immune response in immunized FVIIInull mice. (A) FVIIInull mice received 4 weekly intravenous injections of recombinant human B-domain deleted FVIII (rhBDDFVIII) to a final plasma level of 1 U/mL. One week after the last immunization, mice received whole body irradiation at either 1100 cGy or 660 cGy followed by BM transplantation from 2bF8tg+/− mice. Plasma was collected and inhibiter titer was determined by Bethesda assay at various time points. Bars represent mean plus or minus SD. Data shown are summarized from 7 BM transplantation trials. (B) Inhibitory plasma from immunized FVIIInull mice was infused into FVIIInull mice, and then plasma was collected at days 1, 7, and 14. The residual inhibitor titer was determined by Bethesda assay to define the half-life (t1/2) of inhibitory antibody clearance (in the absence of additional antibody production). These results demonstrate that inhibitory antibodies continue to be produced in recipients even after lethal irradiation.
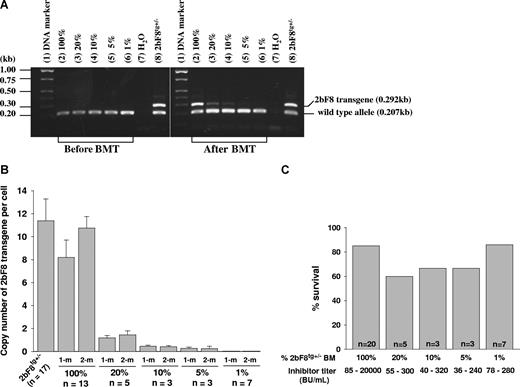
PCR and real-time PCR analysis of 2bF8 transgene in BM transplant recipients
Recipients were analyzed beginning at 3 weeks after transplantation. DNA was purified from peripheral WBCs, and the presence of the 2bF8 transgene was confirmed by PCR amplification of 2bF8 genomic DNA in recipients conditioned with 440 cGy or greater TBI, but not in mice conditioned with 220 cGy (Figure 2A).
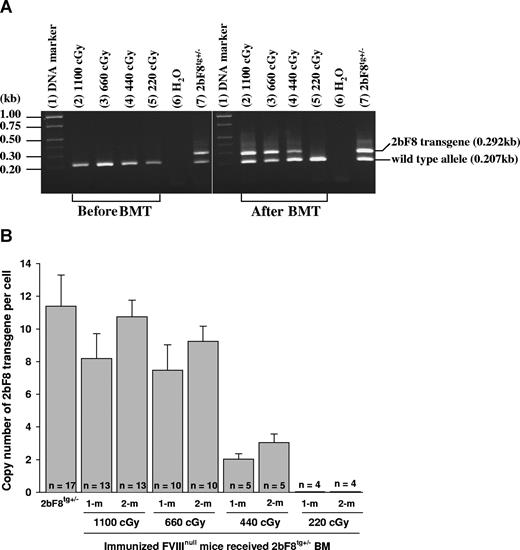
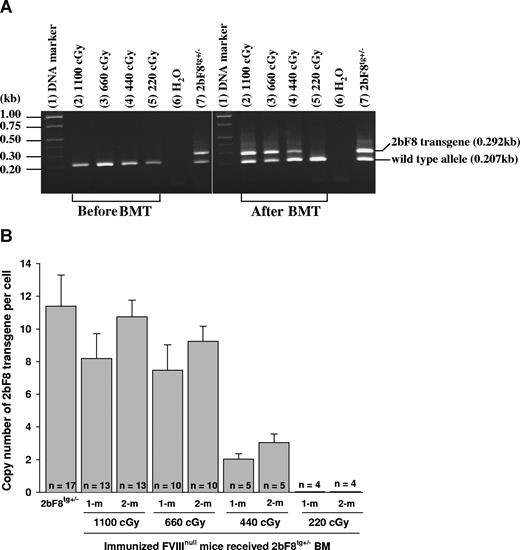
2bF8 transgene analysis. (A) PCR detection of 2bF8 transgene. DNA was purified from peripheral white blood cells. A common antisense primer and a sense transgene-specific primer were used to amplify a 0.292-kb fragment from the 2bF8 transgene. The same common antisense primer and a wild-type allele-specific sense primer were used to amplify a 0.207-kb fragment from the wild-type allele. (Left panel) Results from recipients before BM transplantation. (Right panel) Results from recipients at least 3 weeks after BM transplantation. Shown is one representative experiment that was performed 3 times. (B) Real-time PCR determined the average copy number of the 2bF8 transgene per cell in BMT recipients. DNA was purified from peripheral white blood cells, and 100 ng of DNA was analyzed for the 2bF8 transgene, with normalization to the Apo B. Bars represent mean plus or minus SD. Data shown are summarized from 10 BM transplantation trials. These results demonstrate viable engraftment of 2bF8 genetically modified HSC in immunized recipients.
2bF8 transgene analysis. (A) PCR detection of 2bF8 transgene. DNA was purified from peripheral white blood cells. A common antisense primer and a sense transgene-specific primer were used to amplify a 0.292-kb fragment from the 2bF8 transgene. The same common antisense primer and a wild-type allele-specific sense primer were used to amplify a 0.207-kb fragment from the wild-type allele. (Left panel) Results from recipients before BM transplantation. (Right panel) Results from recipients at least 3 weeks after BM transplantation. Shown is one representative experiment that was performed 3 times. (B) Real-time PCR determined the average copy number of the 2bF8 transgene per cell in BMT recipients. DNA was purified from peripheral white blood cells, and 100 ng of DNA was analyzed for the 2bF8 transgene, with normalization to the Apo B. Bars represent mean plus or minus SD. Data shown are summarized from 10 BM transplantation trials. These results demonstrate viable engraftment of 2bF8 genetically modified HSC in immunized recipients.
The copy number of 2bF8 transgene in recipients was determined by quantitative real-time PCR using DNA derived from peripheral WBC. The percentage of 2bF8 transgenic WBC engraftment was calculated from the mean copy number of 2bF8 transgene per cell in recipients that received BM from 2bF8tg+/− donor divided by the copy number per cell in 2bF8tg+/− donors. At 1-month and 2-month BM reconstitution, there were 72% and 94% engrafted 2bF8 transgenic WBCs in recipients when lethal irradiation (1100 cGy) was used, 66% and 81% in the 660 cGy group, and 18% and 27% in 440 cGy irradiated mice, but transgene was undetectable in recipients conditioned with 220-cGy irradiation at both time points (Figure 2B).
These results demonstrated viable engraftment of 2bF8 genetically modified HSC in the immunized recipients when 440 cGy or greater TBI was used.
Platelet lysate FVIII:C assay to determine the level of platelet FVIII in recipients
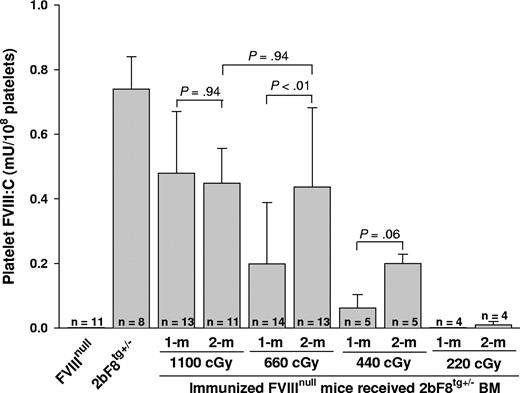
To address whether expression of functional platelet-derived FVIII was obtained in immunized recipients that were conditioned with various regimens before receiving BM from 2bF8tg+/− mice, FVIII chromogenic assays were performed on platelet lysates. Functional FVIII activity was detected in the platelet lysates from recipients, with a level of 0.48 plus or minus 0.19 mU/108 platelets when a myeloablative regimen (1100 cGy TBI) was used, 0.20 plus or minus 0.19 mU/108 platelets in the group receiving 660 cGy irradiation, and 0.06 plus or minus 0.04 mU/108 platelets in 440 cGy irradiated mice after 1-month BM reconstitution. The levels of platelet FVIII were 0.45 plus or minus 0.11, 0.44 plus or minus 0.25, and 0.20 plus or minus 0.03 mU/108 platelets after 2-month BM reconstitution in groups of mice conditioned with 1100, 660, and 440 cGy, respectively. The level of platelet-FVIII:C significantly increased between 1 and 2 months after BM transplantation under nonmyeloablative conditioning regimens (660 cGy) but remained stable in mice conditioned with the myeloablative regimen (1100 cGy). FVIII:C was under the detection limit of this assay33 in platelet lysates of recipients conditioned with 220 cGy (Figure 3). These results demonstrate that platelet-FVIII activity was present in recipients conditioned with 440 cGy or greater, confirming that FVIII sequestered in platelets is secured from antibody inactivation and remains functional in hemophilia A mice with preexisting FVIII-specific immunity. These results also demonstrate that the 2bF8 genetically modified megakaryocytic lineage is successfully engrafted into hemophilia A mice with preexisting immunity.
Quantitative evaluation of FVIII:C levels in recipients' platelets. Platelets were isolated from recipients after 4-week BM constitution and lysed in 0.5% CHAPS. The levels of platelet-FVIII activity were determined by chromogenic assay. Bars represent mean plus or minus SD. Statistical analysis by 2-tailed Student t test revealed a P value less than .01 between 1-month and 2-month FVIII levels when 660 cGy TBI was used. A value of P less than .05 was considered statistically significant. Data shown are summarized from 10 BM transplantation trials. This result demonstrates that 2bF8 genetically modified cells of the megakaryocytic lineage are successfully engrafted into hemophilic mice with preexisting immunity.
Quantitative evaluation of FVIII:C levels in recipients' platelets. Platelets were isolated from recipients after 4-week BM constitution and lysed in 0.5% CHAPS. The levels of platelet-FVIII activity were determined by chromogenic assay. Bars represent mean plus or minus SD. Statistical analysis by 2-tailed Student t test revealed a P value less than .01 between 1-month and 2-month FVIII levels when 660 cGy TBI was used. A value of P less than .05 was considered statistically significant. Data shown are summarized from 10 BM transplantation trials. This result demonstrates that 2bF8 genetically modified cells of the megakaryocytic lineage are successfully engrafted into hemophilic mice with preexisting immunity.
Tail clip survival challenge to assess phenotypic correction in recipients
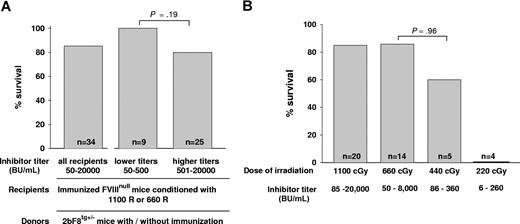
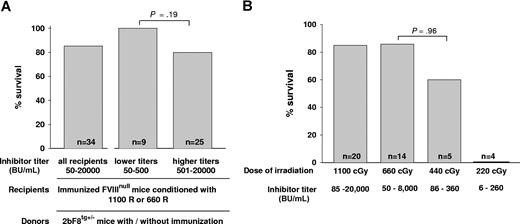
To assess the phenotypic correction of the FVIIInull coagulation defect, we determined the ability to clot and survive after inducing a minor wound by tail clipping. When we looked at the effect of inhibitor titer after 1-month BM reconstitution, we found that, when either 1100 cGy or 660 cGy regimens were used, all recipients survived tail clipping with inhibitor titers of 50 to 400 BU/mL (n = 9) and 80% of recipients survived tail clipping with titers of 401 to 20 000 BU/mL (n = 25). The survival rates were not significantly different between these 2 groups (P = .19; Figure 4A). We also investigated whether using donors with anti-FVIII specific immunity would affect the phenotypic correction of immunized recipients and found that the tail clip survival rate was not significantly different between recipients receiving BM from immunized or nonimmunized 2bF8 transgenic donors (data not shown), indicating that using donors with anti-FVIII immunity did not abrogate graft reconstitution.
Tail clip survival test assessing phenotypic correction of hemophilia A mice with preexisting immunity. (A) The effect of inhibitor titer on phenotypic correction. Immunized FVIIInull mice were conditioned with either 1100 cGy or 660 cGy TBI and received BM cells from 2bF8tg+/− Mice. After allowing 1-month BM reconstitution, tail clip survival tests were performed to assess phenotypic correction, and the percentage of animals that survived beyond 24 hours was determined. Data shown are summarized from 9 BM transplantation trials. (B) The effect of conditioning regimens on phenotypic correction. Immunized FVIIInull mice were conditioned with a myeloablative regimen (1100 cGy TBI) or various nonmyeloablative conditions (660, 440, or 220 cGy TBI) and received BM transplantation from 2bF8tg+/− mice. After 1-month BM reconstitution in the groups conditioned with 1100 cGy or 660 cGy and 2 months in the groups with 440 cGy or 220 cGy, phenotypic correction was assessed by tail clip survival test, and the percentage of animals that survived beyond 24 hours was determined. Data shown are summarized from 10 BM transplantation trials. These results demonstrate that transplantation of 2bF8 genetically modified HSCs can restore hemostasis to hemophilic mice with preexisting immunity.
Tail clip survival test assessing phenotypic correction of hemophilia A mice with preexisting immunity. (A) The effect of inhibitor titer on phenotypic correction. Immunized FVIIInull mice were conditioned with either 1100 cGy or 660 cGy TBI and received BM cells from 2bF8tg+/− Mice. After allowing 1-month BM reconstitution, tail clip survival tests were performed to assess phenotypic correction, and the percentage of animals that survived beyond 24 hours was determined. Data shown are summarized from 9 BM transplantation trials. (B) The effect of conditioning regimens on phenotypic correction. Immunized FVIIInull mice were conditioned with a myeloablative regimen (1100 cGy TBI) or various nonmyeloablative conditions (660, 440, or 220 cGy TBI) and received BM transplantation from 2bF8tg+/− mice. After 1-month BM reconstitution in the groups conditioned with 1100 cGy or 660 cGy and 2 months in the groups with 440 cGy or 220 cGy, phenotypic correction was assessed by tail clip survival test, and the percentage of animals that survived beyond 24 hours was determined. Data shown are summarized from 10 BM transplantation trials. These results demonstrate that transplantation of 2bF8 genetically modified HSCs can restore hemostasis to hemophilic mice with preexisting immunity.
Comparing conditioning regimens, in recipients conditioned with a myeloablative regimen of 1100 cGy that received BM transplantation from 2bF8tg+/− donors, the tail clip survival rate was 85% (n = 20). In the groups in which the 660-cGy nonmyeloablative conditioning regimen was used, 85.7% (n = 14) of recipients survived tail clipping. In the recipients conditioned with 440 cGy that received BM from 2bF8 donors, 60% (n = 5) survived. There was no statistical difference in the survival rates of the mice receiving 660 cGy vs those receiving 440 cGy (P = .96). In the group of mice in which 220 cGy was used, none (n = 4) survived tail clipping (Figure 4B), although the same amount of BM mononuclear cells from 2bF8tg+/− mice were transplanted. Mice were not selected based on their titers, but the groups irradiated with 440 cGy and 220 cGy might have lower titers because they were tested at 2 months (vs 1 month in the groups irradiated with 660 cGy and 1100 cGy).
Long-term engraftment was obtained in recipients with myeloablative or nonmyeloablative conditioning regimens
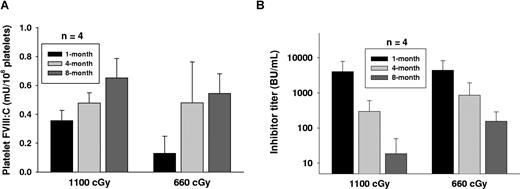
We have followed up monitoring the levels of inhibitor titer and platelet-FVIII activity in recipients conditioned with 1100 cGy or 660 cGy before BM transplantation throughout their entire lifetimes. Sustained platelet-FVIII expression was obtained in these mice (Figure 5A). It is notable that FVIII inhibiter titer showed a gradual decline with time (Figure 5B). Whereas inhibitor titers were maintained at a detectable level (≥ 1 BU/mL) in most recipients at 8 months after BM transplantation, in 1100 cGy or 660 cGy TBI groups when the inhibitor titer decreased to less than 50 BU/mL, the platelet-FVIII in recipients was similar to the levels in donors. Platelet-FVIII was detected for the remaining lifetime of the recipients under either myeloablative or nonmyeloablative regimens (some recipients survived longer than 12 months after BM transplantation; data not shown). These results demonstrate that long-term engraftment was obtained in recipients with preexisting immunity under both myeloablative and nonmyeloablative conditioning regimens.
Long-term engraftment was obtained in recipients. We have followed up monitoring the level of platelet-FVIII activity and the inhibitor titer in immunized recipients after BM transplantation. (A) Sustained platelet-FVIII expression in recipients. Platelets were collected from recipients at various time points. Platelets were lysed with 0.5% CHAPS and the platelet-FVIII:C was determined by chromogenic assay. (B) Inhibitor titer in recipients. Plasma was collected from recipients at various time points, and the inhibitor titer was determined by Bethesda assay. The result shows that preexisting FVIII immunity does not abrogate long-term reconstitution of megakaryocytes expressing FVIII in BM transplants.
Long-term engraftment was obtained in recipients. We have followed up monitoring the level of platelet-FVIII activity and the inhibitor titer in immunized recipients after BM transplantation. (A) Sustained platelet-FVIII expression in recipients. Platelets were collected from recipients at various time points. Platelets were lysed with 0.5% CHAPS and the platelet-FVIII:C was determined by chromogenic assay. (B) Inhibitor titer in recipients. Plasma was collected from recipients at various time points, and the inhibitor titer was determined by Bethesda assay. The result shows that preexisting FVIII immunity does not abrogate long-term reconstitution of megakaryocytes expressing FVIII in BM transplants.
The dosage effect of platelet-derived FVIII on phenotypic correction
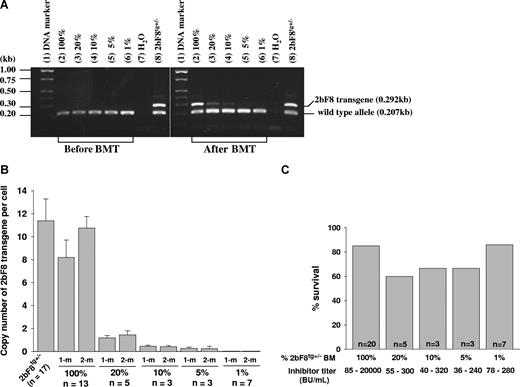
To address what fraction of platelets need to contain FVIII to restore hemostasis in hemophilia A mice with preexisting inhibitory antibodies, various proportions of BM cells from 2bF8tg+/− mice and FVIIInull mice were mixed and transplanted into lethally irradiated immunized FVIIInull mice. After BM reconstitution, recipients were analyzed by PCR, real-time PCR, platelet lysate FVIII:C assay, and Bethesda assay. Phenotypic correction was assessed by tail clip survival test. 2bF8 transgene was easily detected by PCR in mice that received mixed BM cells consisting of as little as 10% 2bF8tg+/− mixed with FVIIInull cell, but results were inconsistent in the 5% group and transgene was undetectable in the 1% group when 100 ng of DNA was used in PCR reactions (Figure 6A). Using real-time PCR, 2bF8 transgene was still detectable in the 5% group, but not in the 1% group (Figure 6B). The tail clip survival test demonstrated that 6 of 7 recipients with inhibitor titers of 78 to 280 BU/mL survived tail clipping even when only 1% 2bF8tg+/− BM cells were transplanted (Figure 6C), indicating that having 1% of platelets containing FVIII still results in improved hemostasis in the tail clip injury model in hemophilia A mice with preexisting anti-FVIII inhibitory antibodies.
The dosage effect of platelets expressing FVIII on phenotypic correction. Different proportions of mixed BM mononuclear cells from 2bF8tg+/− (20%, 10%, 5%, and 1% of 2bF8tg+/−) and FVIIInull (80%, 90%, 95%, and 99% of FVIIInull) were prepared and transplanted into lethally irradiated immunized FVIIInull recipients. (A) PCR detection of 2bF8 transgene. DNA was purified from peripheral white blood cells. A 0.292-kb fragment from 2bF8 transgene and a 0.207-kb fragment from the wild-type allele were amplified by PCR. (Left panel) PCR results from recipients before BM transplantation. (Right panel) Results after BM transplantation. Shown is one representative experiment that was performed 4 times. (B) Quantitative real-time PCR determined the average copy number of 2bF8 transgene per cell in BMT recipients. (C) Tail clip survival test assessed phenotypic correction in recipients. There is no significant difference between those groups. Data shown are summarized from 2 BM transplantation trials. These data show that having even a small proportion of platelets that contain FVIII still improves hemostasis in hemophilic mice with preexisting immunity.
The dosage effect of platelets expressing FVIII on phenotypic correction. Different proportions of mixed BM mononuclear cells from 2bF8tg+/− (20%, 10%, 5%, and 1% of 2bF8tg+/−) and FVIIInull (80%, 90%, 95%, and 99% of FVIIInull) were prepared and transplanted into lethally irradiated immunized FVIIInull recipients. (A) PCR detection of 2bF8 transgene. DNA was purified from peripheral white blood cells. A 0.292-kb fragment from 2bF8 transgene and a 0.207-kb fragment from the wild-type allele were amplified by PCR. (Left panel) PCR results from recipients before BM transplantation. (Right panel) Results after BM transplantation. Shown is one representative experiment that was performed 4 times. (B) Quantitative real-time PCR determined the average copy number of 2bF8 transgene per cell in BMT recipients. (C) Tail clip survival test assessed phenotypic correction in recipients. There is no significant difference between those groups. Data shown are summarized from 2 BM transplantation trials. These data show that having even a small proportion of platelets that contain FVIII still improves hemostasis in hemophilic mice with preexisting immunity.
Discussion
Development of inhibitory antibodies to FVIII is a serious complication of hemophilia treatment.6-8 The clinical hallmark of inhibitor development in hemophilia A patients is failure to respond to routine replacement therapy for bleeding episodes.9-11 The novel approach that we developed is to sequester FVIII within quiescent platelets using a gene transfer–based strategy, with the expectation that these platelets will deliver FVIII after platelet activation at sites of vessel injury, avoiding inhibitory antibody inactivation and achieving hemostasis. Because we have demonstrated efficacy of this approach in the presence of inhibitory antibodies to FVIII,33 we need to address whether bone marrow reconstitution can take place in the presence of preexisting inhibitory antibodies in recipients. Our current studies have demonstrated that syngeneic transplantation of HSCs that are genetically modified to express FVIII in platelets can efficiently restore hemostasis to hemophilic mice with preexisting inhibitory antibodies under myeloablative, and, more importantly, for potential human gene therapy, using nonmyeloablative conditioning regimens.
Our studies showed that the presence of anti-FVIII inhibitory antibodies in recipients was not prohibitive to engraftment of 2bF8 genetically modified HSCs. Cellular and humoral responses can be significant obstacles to gene therapy of hemophilia A in the clinical setting of preexisting FVIII inhibitory antibodies. FVIII inhibitory antibodies are T cell–dependent humoral immune responses.38 Activation of CD4+ or CD8+ T cells could lead to antibody formation or cytotoxic T lymphocyte responses against transgene-expressing cells. It has been reported that humoral immunity is considered the dominant barrier for allogeneic BM engraftment in presensitized mice.39,40 Doering et al41 recently reported that HSCs encoding porcine FVIII under control of a constitutive promoter successfully engrafted into lethally irradiated hemophilia A mice with preexisting antihuman FVIII antibodies but only achieved partial success when sublethal irradiation was used. They demonstrated that antibodies by themselves are not sufficient to prevent the engraftment of donor HSC encoding a porcine FVIII in myeloablated mice, but lower levels of donor cell engraftment and FVIII expression were observed in nonmyeloablated animals, probably because of host-derived effector cell-mediated, and possibly antibody-assisted, extinction of porcine FVIII-expressing cells. Our previous studies have demonstrated that targeting FVIII expression under control of the platelet-specific αIIb promoter results in FVIII storage together with von Willebrand factor in α-granules in platelets with undetectable FVIII in plasma.33 Thus, this platelet-sequestered FVIII may avoid being inactivated by anti-FVIII inhibitory antibodies as well as avoiding recognition and elimination by presensitized cytotoxic T lymphocytes. In our setting, preexisting antihuman FVIII inhibitory antibodies did not prevent the engraftment of donor BM cells expressing platelet-derived human FVIII under either myeloablative or nonmyeloablative conditioning regimens.
BM transplantation after conditioning with lethal TBI is associated with considerable toxicity and probably would not be justified for use in hemophilia patients. To reduce the toxicity of irradiation, nonmyeloablative conditioning regimens with reduced doses of irradiation were used in the current studies. When immunized recipients conditioned with 660 cGy TBI received BM transplants from 2bF8 transgenic mice, the recipients displayed with lower levels of platelet-FVIII activity at 1 month, but similar levels at 2 months after BM transplantation compared with animals conditioned with a myeloablative regimen. These platelet-FVIII levels may be affected by 2 factors. First, donor cell reconstitution increased with time. This is supported by the real-time PCR results. Tomita et al also demonstrated that donor reconstitution increased between 2 and 12 weeks after BMT and remained constant thereafter in mice receiving BM cells after sublethal irradiation.42 Second, if any trace amount of high-titer inhibitory antibodies was carried over when platelet pellets were prepared, FVIII:C in platelet lysates might be partially inactivated, resulting in an artifactually low measurement. There is also evidence to support the second factor. One recipient (data not shown) that had an inhibitor titer of 10 000 BU/mL after being irradiated with 660 cGy and receiving BM cells from 2bF8 mice survived tail clipping, but FVIII:C was not detected in its platelet lysates for the first 2 months. When its inhibitor titer dropped to 460 BU/mL at 8 months after BMT, the measured level of platelet-FVIII had increased to 0.42 mU/108 platelets. We have found that extremely high-titer inhibitory antibodies in vivo tend to inhibit FVIII:C in our platelet lysate FVIII:C assay in vitro, even though we wash the platelets with a large volume of buffer before lysis. After BM transplantation, there was no significant difference in tail clip survival rate between the BM recipients irradiated with 1100 cGy (85%) and 660 cGy (85.7%). All recipients survived tail clipping if the titer of inhibitors was lower than 500 BU/mL in both groups, indicating that syngeneic transplantation of 2bF8 genetically modified BM cells can successfully correct the hemorrhagic phenotype in hemophilia A mice with preexisting immunity under either lethal or sublethal irradiation conditioning.
TBI is needed to create a permissive environment for syngeneic HSC engraftment.42 Lower TBI doses are associated with less toxicity, but making enough space for a sufficient number of 2bF8 genetically modified HSC to engraft is important for hemorrhagic phenotype correction in this platelet-dependent gene therapy approach. If this approach were to be extended to human subjects, a nonmyeloablative regimen would have lower short-term risk because conditioning would not be associated with severe pancytopenia. The nonmyeloablative approach needs to be contrasted to the myeloablative regimen, which would maximize the percentage of transduced, FVIII-expressing megakaryocytes. Our studies demonstrated that 2bF8 transgenic BM cells engrafted into all immunized recipients when 440 cGy was used, with 60% of recipients obtaining phenotypic correction. None of the mice survived tail clipping in the group of recipients irradiated with 220 cGy, although the same doses of 2bF8 transgenic BM cells were transplanted, indicating that this dose of irradiation does not create enough space for 2bF8 BM cell engraftment to provide enough platelet-FVIII for phenotypic correction in hemophilic mice with inhibitors. Our further studies demonstrated that lethally irradiated immunized hemophilia A mice that received 1% to 5% 2bF8tg+/− BM cells mixed with 99% to 95% FVIIInull cells experienced improved hemostasis in the tail clip injury model, implying that having only 1% to 5% of platelets that contain FVIII can improve hemostasis in hemophilia A mice with preexisting inhibitory antibodies. This small amount of platelet-derived FVIII might not be sufficient for large vessel injury, such as a FeCl3 carotid artery injury model, as recently demonstrated by Gewirtz et al using a similar platelet-FVIII transgenic model.43 Further investigation of the clinical efficacy and safety of this platelet-derived FVIII in a large animal model will need to be pursued in the future.
Hematopoietic reconstitution has been divided into short-term (produced by committed progenitors) and long-term (produced by the pluripotent stem cells) phases.44 Long-term reconstitution of 2bF8 genetically modified pluripotent HSCs is essential for achieving sustained phenotypic correction in gene therapy for hemophilia A with or without preexisting inhibitory antibodies using our platelet-derived FVIII approach. Our results demonstrated that long-term engraftment of 2bf8 genetically modified HSCs was achieved in immunized recipients conditioned with either lethal (1100 cGy) or sublethal irradiation (≥ 440 cGy). Long-term 2bF8 engraftment was assessed by real-time PCR and platelet lysate FVIII:C assays for a follow-up period of 8 months after BMT.
Previous studies have demonstrated that memory B cells are easily depleted by irradiation,45-47 but plasma cells are notably radiation resistant.47,48 Recently, studies have shown that plasma cells can survive for more than 90 days in bone marrow and spleen in lymphocytic choriomeningitis virus-immune mice that were given 600 cGy of TBI.49 The immune response in platelet-derived FVIII gene therapy is not our primary focus in the current study, but some experiments have been necessary to understand our results. Whereas the in vivo half-life of inhibitory antibody measured by our inhibitory plasma infusion studies is only 3.8 days, the endogenously produced inhibitory antibodies in BM transplantation recipients persisted for more than 8 months (with a half-reduction of 36 days), indicating continued production of inhibitory antibodies despite their exposure to lethal irradiation. Whether platelet-derived FVIII is immunogenic in the context of a preexisting immune response triggered by exogenous FVIII protein infusion is the subject of further investigation by our group.
Thus, in the presence of preexisting FVIII immunity, HSCs that are genetically modified to express FVIII in platelets were successfully transplanted into hemophilic mice under myeloablative and various levels of nonmyeloablative conditioning. Our results show that transplant of HSCs, which are genetically modified to express FVIII only in platelets, into immunized FVIIInull recipients is effective in correcting the hemophilia phenotype in hemophilia A mice with preexisting FVIII immunity. Expression of platelet FVIII was successfully transferred to recipients, even when both donors and recipients had been immunized to induce an anti-FVIII response. This scenario closely mimics the situation that would be encountered by a hemophilia A patient with inhibitors in which stem cells would be harvested for in vitro introduction of a corrected FVIII gene, followed by reintroduction into the patient in an autologous transplant. The presence of an immune response in both the graft donor and the recipient was not prohibitive to successful engraftment. Hence, the strategy that combines sublethal conditioning with ex vivo genetic modification of HSCs is a promising approach for gene therapy of hemophilia A patients with inhibitory antibodies as well as nonhemophilic patients with acquired inhibitory antibodies, as they can also have life-threatening clinical bleeding, which is difficult to treat by conventional therapy.50 Such an approach for hemophilia gene therapy will need further assessment of safety, efficacy, and potential for insertional mutagenesis51 in a large animal model.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by American Heart Association National Center Scientist Development Award (0730183N, Q.S.), National Hemophilia Foundation Career Development Award (Q.S.), National Institutes of Health (grants HL 44 612 and HL 33 721, R.R.M.), the Goerlich Foundation (R.R.M.), HL 68 138 (D.A.W.), and the American Heart Association Midwest Affiliate (Grant in Aid 0755827Z, D.A.W.).
National Institutes of Health
Authorship
Contribution: Q.S. designed and performed research, analyzed data, and wrote the manuscript; S.A.F. performed research, analyzed data, and made comments on manuscript; D.A.W. assisted with the platelet-specific αIIb promoter construct; E.L.K., P.A.M., and N.M. performed research; H.W. generated transgenic mice; R.R.M. helped in the design of the project, provided research support, critiqued results, and made critical comments on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qizhen Shi, Department of Pediatrics, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226; e-mail: qizhen.shi@bcw.edu.