Interleukin-15 (IL-15) is essential for natural killer (NK) cell differentiation. In this study, we assessed whether the receptor tyrosine kinase Axl and its ligand, Gas6, are involved in IL-15–mediated human NK differentiation from CD34+ hematopoietic progenitor cells (HPCs). Blocking the Axl-Gas6 interaction with a soluble Axl fusion protein (Axl-Fc) or the vitamin K inhibitor warfarin significantly diminished the absolute number and percentage of CD3−CD56+ NK cells derived from human CD34+ HPCs cultured in the presence of IL-15, probably resulting in part from reduced phosphorylation of STAT5. In addition, CD3−CD56+ NK cells derived from culture of CD34+ HPCs with IL-15 and Axl-Fc had a significantly diminished capacity to express interferon-γ or its master regulator, T-BET. Culture of CD34+ HPCs in the presence of c-Kit ligand and Axl-Fc resulted in a significant decrease in the frequency of NK precursor cells responding to IL-15, probably the result of reduced c-Kit phosphorylation. Collectively, our data suggest that the Axl/Gas6 pathway contributes to normal human NK-cell development, at least in part via its regulatory effects on both the IL-15 and c-Kit signaling pathways in CD34+ HPCs, and to functional NK-cell maturation via an effect on the master regulatory transcription factor T-BET.
Introduction
Natural killer (NK) cells belong to the innate immune system and play an important role in host defense against microorganisms and malignancy1 in the absence of prior sensitization.2 NK cells are also involved in regulating the adaptive immune responses, including T- helper cell differentiation.3,4 NK cells are primarily derived from CD34+ hematopoietic stem cells (HSCs) residing in the bone marrow. HSCs differentiate into common lymphoid progenitors, which in turn can develop into NK precursor cells and then mature into NK cells.5,6
Interleukin-15 (IL-15) is critical for the development of NK cells.7 Mice that are genetically deficient in IL-15 or its receptor (IL-15R) components lack NK cells.8,9 In addition, mice that are deficient in STAT5, a signaling intermediate in the IL-15R/IL-15 pathway, are also NK deficient.10 IL-15 is also able to induce NK-cell differentiation from human CD34+ hematopoietic progenitor cells (HPCs) in vitro.11,12 Recently, Horng et al showed that the IL-15R/15 signaling pathway was coupled with the NKG2D/DAP10 signaling pathway and that these 2 pathways trans-activate each other.13 The IL-15R/15 signaling pathway can functionally interact with signals from other NK-cell receptors, and this in turn can shape NK-cell function and development.1 It also suggests that there might be molecules, particularly receptors on NK precursor cells, mediating effects of IL-15 on human NK-cell development. However, little is known about molecules or the mechanisms by which they might interact with the IL-15R/15 signaling pathway.
Axl is a receptor tyrosine kinase member of the Axl family of proteins composed of Axl, Tyro3 (or Sky), and Mer14 and was originally discovered in human myeloid leukemia cells.15 Two natural and highly homologous ligands for the Axl family are growth arrest–specific gene 6 (Gas6)16 and protein S.17,18 Gas6 can bind to all 3 Axl family members,19 whereas protein S has been shown to bind to only Tyro3 and Mer.20 Both Gas6 and protein S need vitamin K–dependent γ-carboxylation for their binding to Axl family receptors on a cell surface.21 Thus, vitamin K inhibitors such as warfarin can block these Axl ligands from binding to their receptors.22 The Axl/Gas6 pathway is important in a variety of cellular responses, including cell survival and proliferation. The Axl/Gas6 pathway rescues serum-starved NIH3 fibroblasts from cell-cycle arrest and apoptosis, which is mediated through the phosphatidylinositol 3-kinase (PI3K) pathway.23
Caraux et al recently showed that the Axl family is crucial for NK-cell differentiation in mice.24 NK cells from mice lacking all 3 receptors had fewer NK cells and had diminished NK production of interferon-γ (IFN-γ) and NK cytotoxicity, compared with their wild-type littermates. NK cells from mice lacking only Axl also had impaired NK functions compared with wild-type mice, albeit less than mice lacking all 3 Axl family members. NK cells from those null mutant mice had defects in expression of inhibitory and activating Ly49 and other cell-surface molecules, and NK-cell development appeared to be blocked or delayed between stage II and stage III.25 However, the mechanism(s) responsible for these phenotypic and functional changes in NK-cell development were not explored.
IL-15R/15 and Axl/Gas6 pathways have partially overlapping intracellular signaling molecules that mediate their biologic functions.20 In addition, Budagian et al reported that IL-15Rα and Axl physically associate with each other and that IL-15 was able to transactivate the Axl signaling pathway.26 Furthermore, in their study, IL-15 failed to activate several of its downstream signaling pathways, such as PI3K, AKT, and ERK1/2, in the absence of Axl. Based on these data, we hypothesized that the Axl/Gas6 pathway is required for human NK-cell development driven by IL-15. Here we provide evidence and mechanistic insight to support this hypothesis.
Methods
Reagents
Recombinant human IL-12 and IL-15 proteins were purchased from R&D Systems (Minneapolis, MN). No exogenous ligands to Axl were used in any experiments. Human irrelevant control Fc and Axl-Fc chimeric proteins (R&D Systems) were applied in cultures at the final concentration of 1 μg/mL. Warfarin (Sigma-Aldrich, St Louis, MO) was used at the concentration of 100 μg/mL in dimethyl sulfoxide (DMSO). All antibodies used for flow cytometry were purchased from BD Biosciences (San Jose, CA) unless otherwise indicated.
Isolation of human CD34+ HPCs and in vitro culture to generate CD3−CD56+ NK cells
Human CD34+ HPCs were isolated from human leukopaks (American Red Cross, Columbus, OH) with approval from The Ohio State University Institutional Review Board. CD34+ cells were first enriched using a Rosette separation cocktail (StemCell Technologies, Vancouver, BC) following the manufacturer's instructions, and mononuclear cells were then obtained using Ficoll-Paque Plus (GE Healthcare, Little Chalfont, United Kingdom). In the case of bone marrow, mononuclear cells from human bone marrow were obtained from the Stem Cell Technologies. CD34+ HPCs were further enriched using human CD34+ Progenitors Isolation kit (Miltenyi Biotec, Auburn, CA; > 97% purity). Purified CD34+ progenitor cells were plated (5000-10 000 cells/well) on a 96-well plate with culture medium containing α-minimum essential medium (Lonza Baltimore, Baltimore, MD) and 20% fetal bovine serum (Invitrogen, Carlsbad, CA). After culturing for 3 weeks (changing one-half of the medium every 3 to 4 days and supplementing with the same concentration of cytokines and reagents indicated in the figures), cells were harvested, counted using trypan blue, and stained with anti-CD3 and anti-CD56 antibodies for analysis by the FACSCalibur flow cytometer (BD Biosciences). Absolute numbers of CD3−CD56+ NK cells were calculated by multiplying the number of total cells by the percentage of CD3−CD56+ NK cells among the total cells.
Isolation of CD56bright and CD56dim NK cells from human peripheral blood
Human primary NK cells were isolated from leukopaks (American Red Cross) with the approval from The Ohio State University Institutional Review Board. Human NK cells were enriched by using Rosette human NK enrich cocktail (StemCell Technologies) and Ficoll (GE Healthcare). CD3−CD56bright and CD3−CD56dim NK cells were separated using a FACSDiVa sorter (BD Biosciences) based on the expression density of CD56 on a cell surface as described previously.27
Measuring IFN-γ production by ELISA
After culture of human CD34+ cells for 21 days, cells were harvested and CD56+CD3− NK cells were isolated using CD56 microbeads (Miltenyi Biotec). Purity (> 97%) was confirmed by flow cytometry. CD56bright and CD56dim human mature NK cells were isolated by FACS sorting. Cells were plated in equivalent numbers (2500 cells/well) in a 96-well plate in RPMI 1640 (Invitrogen) containing 20% fetal bovine serum (Invitrogen). Cells were then treated with recombinant human IL-12 (10 ng/mL) and IL-15 (100 ng/mL) for 48 hours, and cell-free culture supernatant was collected and frozen at −80°C until use. IFN-γ concentration was measured by ELISA using anti–IFN-γ antibodies from Pierce Endogen (Rockford, IL).
51Cr-release cytotoxicity assay
Human CD34+ cells were cultured for 3 weeks and enriched as indicated for assessment of IFN-γ production. CD56bright and CD56dim human NK cells from peripheral blood were prepared as described in “Isolation of CD56bright and CD56dim NK cells from human peripheral blood.” NK cells generated from human CD34+ cells under each culture condition were plated in equivalent numbers and then cocultured for 4 hours at 37°C, 5% CO2 with 51Cr (PerkinElmer Life and Analytical Sciences, Boston, MA)–labeled NK-sensitive K-562 target cells (2500 cells/well) at an effector:target ratio of 2:1 in a V-bottom 96-well plate. For mature human CD56bright and CD56dim NK cells, the effector-to-target cell ratio was 3:1. Supernatants were collected, and the percentage of specific lysis was measured as previously described.12
Limiting dilution assay
NK precursor frequency was determined by limiting dilution assay as previously described.11,28 Briefly, isolated human CD34+ HPCs from peripheral blood were plated onto a 96-well plate with a serial dilution from row to row starting at a concentration of 6000 cells per well to 94 cells per well. CD34+ cells were cultured with c-Kit ligand (KL; 100 ng/mL; R&D Systems) in the presence of irrelevant Fc or Axl-Fc (each 1 μg/mL) for 7 days. Cells were next washed twice and cultured in IL-15 (100 ng/mL) alone for another 2 weeks. Separately, CD34+ cells from the same donor were cultured in the presence of only IL-15 for 2 weeks. After culture, cells were harvested and analyzed for expression of CD56 and CD3 by flow cytometry, and each well was scored positive (CD56+CD3−) or negative by comparison to staining with isotype control antibody. NK precursor frequency was calculated as the reciprocal of the number of cells that resulted in 37% negative wells as described previously.11,28
Reverse transcription–polymerase chain reaction
Expression of human Axl, Gas6, Sky (Tyro3), Mer, and protein S genes were examined by polymerase chain reaction (PCR). Total RNA was isolated from human CD34+ HPCs in peripheral blood after culturing in the presence of IL-15 for the indicated times. Human CD56bright and CD56dim NK cells were prepared from peripheral blood, and total RNA was also isolated. Complementary DNA was generated using Moloney murine leukemia virus reverse transcriptase (Invitrogen) and was subject to PCR with Taq DNA polymerase and gene-specific primers as described previously.29,–31 To quantify the level of IFN-γ or T-BET mRNA, real-time reverse transcription (RT)-PCR was performed using the Taqman system (Applied Biosystems, Foster City, CA) along with 18S rRNA as a normalization control as previously described.32
Measurement of STAT5 and c-Kit phosphorylation
CD34+ cells isolated from human peripheral blood were cultured for 5 days in the presence of KL (100 ng/mL) and Flt-3 ligand (FL) (100 ng/mL; Amgen, Thousand Oaks, CA) to increase responsiveness of CD34+ cells to IL-15.11 Cells were washed twice and then treated with IL-15 (100 ng/mL) in the presence of irrelevant control Fc or Axl-Fc for 30 minutes, followed by lysis and Western blotting to detect phosho-STAT5 protein using an enhanced chemiluminescence system (GE Healthcare). For intracellular phospho-STAT5 protein staining, cells prepared as described above were fixed with 2% (vol/vol) formaldehyde for 10 minutes at 37°C, permeabilized by 90% (vol/vol) methanol for 30 minutes on ice, and incubated with anti–phospho-STAT5 rabbit antibody (Cell Signaling Technology, Danvers, MA) for 30 minutes at room temperature. Cells were then incubated with goat anti–rabbit secondary antibody labeled with Alexa 488 (Invitrogen) for 30 minutes at room temperature. After washing twice, cells were analyzed with the FACSCalibur (BD Biosciences). For assessment of c-Kit phosphorylation, isolated CD34+ cells were treated with KL (100 ng/mL) in the presence of irrelevant Fc or Axl-Fc for the indicated times at 37°C and then assayed for phospho–c-Kit by Western blotting or intracellular staining using anti–phospho-c-Kit (Y719) rabbit antibody (Cell Signaling Technology) and goat anti–rabbit secondary antibody conjugated with Alexa 488 (Invitrogen).
Statistical analysis
Data from experimental groups were compared using the Student t test, with P values less than .05 considered significant.
Results
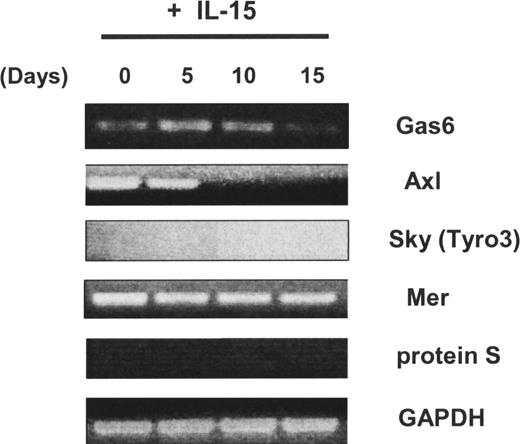
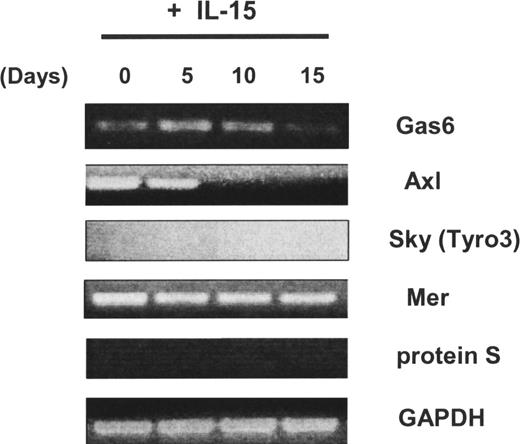
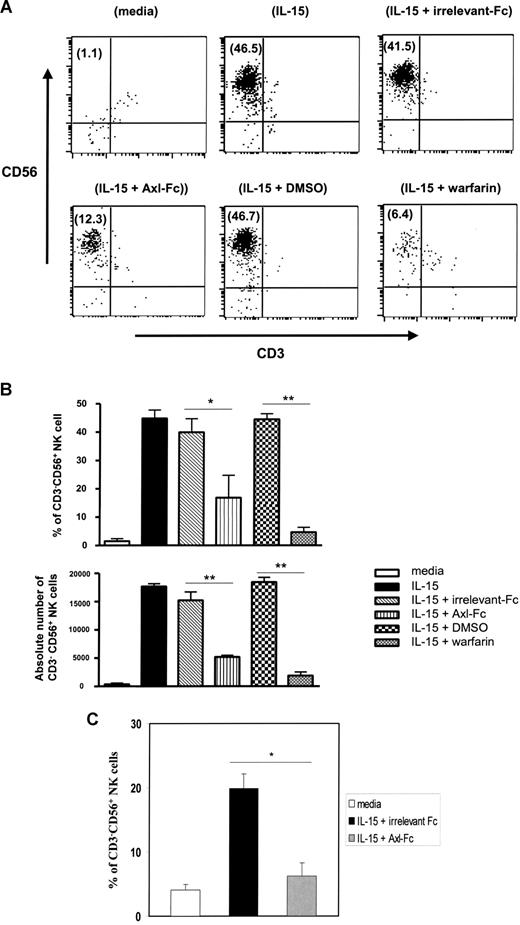
We first observed that the Axl and Mer receptor tyrosine kinases, as well as one of their ligands, Gas6, were expressed in freshly isolated human CD34+ HPCs, whereas neither Sky (Tyro3) nor protein S was detected (Figure 1). We previously demonstrated that highly enriched human CD34+ HPCs obtained from peripheral blood could differentiate into CD56bright NK cells in vitro in the presence of IL-15 without any stromal cell layer more than 21 days.11,12 Using this system, we noted that Axl was down-regulated after 10 days and its ligand Gas6 expression was down-regulated after 15 days (Figure 1). We next tested whether NK-cell differentiation from CD34+ HPCs was also codependent on the Axl/Gas6 pathway. When the soluble receptor Axl-Fc or the vitamin K inhibitor warfarin was added to initial cultures of CD34+ HPCs in the presence of IL-15, the percentage and absolute number of CD3−CD56+ NK cells obtained after 21-day culture were significantly lower compared with cells cultured with vehicle control (DMSO; 16.7% ± 7.9% for IL-15 + Axl-Fc vs 39.8% ± 8.2% for IL-15 + irrelevant Fc, P < .03; 4.5% ± 1.6% for IL-15 + warfarin vs 44.3% ± 1.9% for IL-15 + vehicle control; P < .001; Figure 2A,B). Similar results were obtained using CD34+ HPCs from human bone marrow (Figure 2C). Collectively, the data suggest that the Axl/Gas6 pathway is important for IL-15–driven human NK-cell differentiation from CD34+ HPCs.
Expression of Axl family members and their ligands in human CD34+ HPCs. Isolated CD34+cells from human peripheral blood were cultured in the presence of IL-15 for 0, 5, 10, or 15 days. Then nonquantitative assessment of Gas6, Axl, Sky (Tyro3), Mer, protein S, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression was performed by RT-PCR. These results are representative of 2 experiments.
Expression of Axl family members and their ligands in human CD34+ HPCs. Isolated CD34+cells from human peripheral blood were cultured in the presence of IL-15 for 0, 5, 10, or 15 days. Then nonquantitative assessment of Gas6, Axl, Sky (Tyro3), Mer, protein S, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression was performed by RT-PCR. These results are representative of 2 experiments.
Blockade of Axl/Gas6 pathway inhibits IL-15–induced in vitro differentiation of human CD34+ HPCs into NK cells. (A) Representative flow cytometry plot showing differentiation of NK cells (CD3−CD56+) from blood CD34+ HPCs. Value in parentheses indicates a percentage of CD3−CD56+ NK cells (upper left quadrant) detected among the total cells after a 3-week culture under the experimental conditions indicated. (B) Top graph shows a percentage of differentiated CD3−CD56+ NK cells among the total cells after culture for 3 weeks (triplicate wells). Bottom graph shows absolute numbers of NK cells (calculated by multiplying total number of cells counted in each well times the percentage of NK cells noted by flow cytometric analysis). Results for panel B are summarized from at least 3 experiments; error bar represents SD. *P < .03; **P < .001. (C) CD34+ cells isolated from human bone marrow were cultured with IL-15 in the presence of irrelevant Fc or Axl-Fc for 3 weeks, and the resultant percentage of CD3−CD56+ NK cells among the total cells is shown. The results show the mean plus or minus SD and are from 3 separate donors, each in triplicate wells. *P < .02.
Blockade of Axl/Gas6 pathway inhibits IL-15–induced in vitro differentiation of human CD34+ HPCs into NK cells. (A) Representative flow cytometry plot showing differentiation of NK cells (CD3−CD56+) from blood CD34+ HPCs. Value in parentheses indicates a percentage of CD3−CD56+ NK cells (upper left quadrant) detected among the total cells after a 3-week culture under the experimental conditions indicated. (B) Top graph shows a percentage of differentiated CD3−CD56+ NK cells among the total cells after culture for 3 weeks (triplicate wells). Bottom graph shows absolute numbers of NK cells (calculated by multiplying total number of cells counted in each well times the percentage of NK cells noted by flow cytometric analysis). Results for panel B are summarized from at least 3 experiments; error bar represents SD. *P < .03; **P < .001. (C) CD34+ cells isolated from human bone marrow were cultured with IL-15 in the presence of irrelevant Fc or Axl-Fc for 3 weeks, and the resultant percentage of CD3−CD56+ NK cells among the total cells is shown. The results show the mean plus or minus SD and are from 3 separate donors, each in triplicate wells. *P < .02.
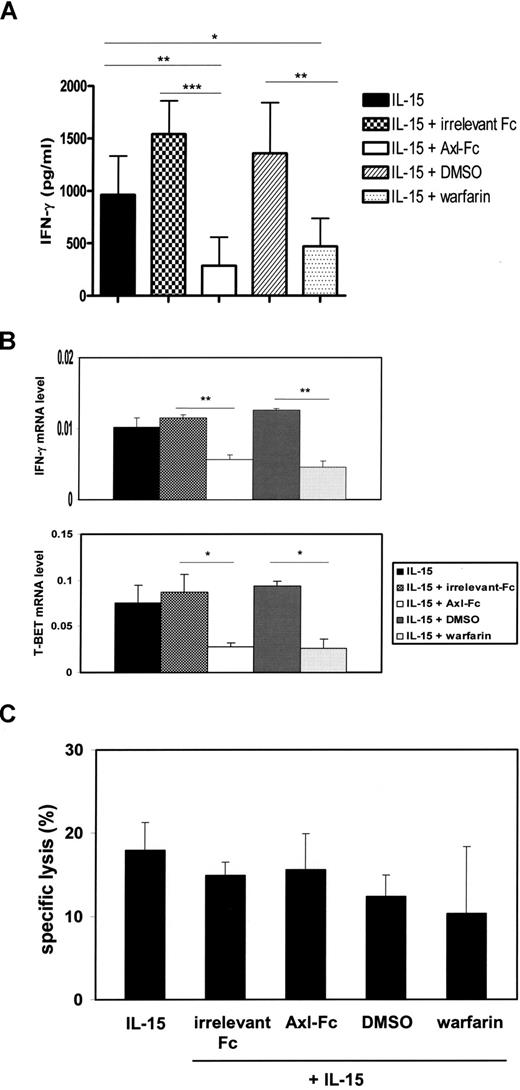
CD3−CD56+ NK cells were still detectable in the cultures of IL-15 and Axl-Fc or warfarin, albeit at much lower frequencies compared with control cultures. We purified these NK cells to examine their functional competency. NK cells differentiated in the presence of IL-15 plus Axl-Fc or IL-15 plus warfarin showed impaired IFN-γ production at both the protein and mRNA level on a per-cell basis in the presence of IL-12 and IL-15 (285.8 ± 272.0 pg/mL for IL-15 + Axl-Fc vs 1540.5 ± 318.5 pg/mL for IL-15 + irrelevant Fc; P < .001; 469.7 ± 268.2 pg/mL for IL-15 + warfarin vs 1357.1 ± 484.2 pg/mL for IL-15 + DMSO; P < .01; Figure 3A). T-BET, a master regulator of IFN-γ production,33 was also significantly reduced in differentiated NK cells that had been derived from CD34+ HPCs in the presence of Axl-Fc or warfarin (Figure 3B). However, NK cells cultured in a similar fashion had no significant reduction in NK cytotoxic activity against NK-sensitive K-562 target cells compared on a per-cell basis with the cells that had been cultured with IL-15 and vehicle controls (Figure 3C). These results suggest that the Axl/Gas6 pathway is important for differentiation and some functional maturation of developing human NK cells.
The Axl/Gas6 pathway is important for generation of functionally competent NK cells from human CD34+ HPCs. (A) CD34+ HPCs from human peripheral blood were cultured for 21 days under different experimental conditions as indicated. On day 21, equal numbers of CD3−CD56+ NK cells were plated into each well, followed by stimulation with IL-12 and IL-15 for 48 hours. Supernatants were harvested, and the concentration of IFN-γ was measured by ELISA. Results shown are the compilation of data from 2 donors, and each experiment was performed in triplicate wells. Error bar represents SD. *P < .05; **P < .01; ***P < .001. (B) On day 21, NK cells that were derived from CD34+ HPCs under different experimental conditions as described in panel A were plated in equal numbers into a 96-well culture plate. After being treated with IL-12 and IL-15 for 6 hours, cells were lysed, total RNA was isolated, and IFN-γ or T-BET mRNA was quantified by Taqman real-time RT-PCR. 18S rRNA was used for normalization. Error bar represents SD. These results are representative of 3 experiments. *P < .05; **P < .01. (C) After a 21-day culture of CD34+ cells under the indicated experimental conditions, the same number of differentiated CD3−CD56+ NK cells were plated in each well and mixed with 51Cr-labeled NK-sensitive K-562 target cells at a 2:1 ratio of effector to target cells (triplicate wells). Results are representative of 3 experiments and show mean plus or minus SD. No significant differences in cytotoxicity were noted.
The Axl/Gas6 pathway is important for generation of functionally competent NK cells from human CD34+ HPCs. (A) CD34+ HPCs from human peripheral blood were cultured for 21 days under different experimental conditions as indicated. On day 21, equal numbers of CD3−CD56+ NK cells were plated into each well, followed by stimulation with IL-12 and IL-15 for 48 hours. Supernatants were harvested, and the concentration of IFN-γ was measured by ELISA. Results shown are the compilation of data from 2 donors, and each experiment was performed in triplicate wells. Error bar represents SD. *P < .05; **P < .01; ***P < .001. (B) On day 21, NK cells that were derived from CD34+ HPCs under different experimental conditions as described in panel A were plated in equal numbers into a 96-well culture plate. After being treated with IL-12 and IL-15 for 6 hours, cells were lysed, total RNA was isolated, and IFN-γ or T-BET mRNA was quantified by Taqman real-time RT-PCR. 18S rRNA was used for normalization. Error bar represents SD. These results are representative of 3 experiments. *P < .05; **P < .01. (C) After a 21-day culture of CD34+ cells under the indicated experimental conditions, the same number of differentiated CD3−CD56+ NK cells were plated in each well and mixed with 51Cr-labeled NK-sensitive K-562 target cells at a 2:1 ratio of effector to target cells (triplicate wells). Results are representative of 3 experiments and show mean plus or minus SD. No significant differences in cytotoxicity were noted.
As only IL-15 was necessary for driving NK-cell differentiation from CD34+ HPCs in our in vitro culture system, we hypothesized that blockade of the Axl/Gas6 pathway by Axl-Fc (or warfarin) may inhibit IL-15–induced signaling in human CD34+ HPCs. To test this hypothesis, CD34+ cells from human peripheral blood were first cultured for 5 days in the presence of KL and FL to up-regulate IL-15R expression.11 Cells were then washed and subsequently treated with IL-15 in the presence of Axl-Fc or irrelevant control Fc. Axl-Fc significantly diminished phosphorylation of STAT5 induced by IL-15 (Figure 4A). This was confirmed in a separate yet identical experiment by staining for intracellular phospho-STAT5 protein in the cells while gating on CD34+ HPCs (mean fluorescence intensity [MFI] of 65 for IL-15 + Axl-Fc vs MFI of 102 for IL-15 + irrelevant Fc; Figure 4B). For our entire series of donors, this decrease is statistically significant (P < .05). This result demonstrates that the Axl/Gas6 pathway is required for optimal IL-15–mediated signaling to promote NK differentiation from human CD34+ HPCs.
The Axl/Gas6 pathway is required for normal IL-15 signaling in human CD34+ HPCs. (A) CD34+ HPCs were isolated from human peripheral blood and treated with KL and FL for 5 days. Cells were washed, rested, and then treated with either irrelevant Fc or Axl-Fc in the presence of IL-15 for 30 minutes. Harvested cells were subject to Western blot to detect phospho-STAT5, and actin was used as a loading control. The figure is the representative of 2 similar experiments. (B) In a separate experiment, CD34+ cells were prepared and treated as described in panel A. Assessment of intracellular STAT5 phosphorylation using flow cytometry was performed while gating on CD34+ cells. Shown is 1 of 2 similar experiments. Dotted line represent media only; filled histogram, IL-15 + irrelevant Fc; open histogram, IL-15 + Axl-Fc. Dotted line and open histogram were indistinguishably overlapped.
The Axl/Gas6 pathway is required for normal IL-15 signaling in human CD34+ HPCs. (A) CD34+ HPCs were isolated from human peripheral blood and treated with KL and FL for 5 days. Cells were washed, rested, and then treated with either irrelevant Fc or Axl-Fc in the presence of IL-15 for 30 minutes. Harvested cells were subject to Western blot to detect phospho-STAT5, and actin was used as a loading control. The figure is the representative of 2 similar experiments. (B) In a separate experiment, CD34+ cells were prepared and treated as described in panel A. Assessment of intracellular STAT5 phosphorylation using flow cytometry was performed while gating on CD34+ cells. Shown is 1 of 2 similar experiments. Dotted line represent media only; filled histogram, IL-15 + irrelevant Fc; open histogram, IL-15 + Axl-Fc. Dotted line and open histogram were indistinguishably overlapped.
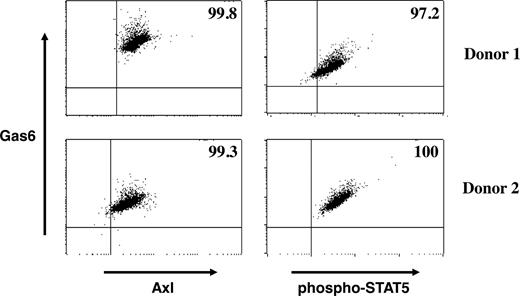
The results presented in Figure 4 suggest that both IL-15R and Axl are expressed on the same CD34+ HPCs during early NK-cell development. Culture experiments with purified CD34+ HPCs and soluble Axl-Fc or warfarin suggest that Gas6 is produced by CD34+ HPCs. To examine whether Gas6 is produced and secreted from the same CD34+ HPCs expressing IL-15R and Axl, we performed intracellular staining for Gas6, Axl, and phospho-STAT5 in CD34+ HPCs cultured with IL-15. As shown in Figure 5, nearly all CD34+ HPCs expressed Axl and coexpressed Gas6, suggesting that Axl can bind Gas6 in an autocrine fashion in human CD34+ HPCs. Furthermore, CD34+ cells coexpressing Gas6 and Axl also express phospho-STAT5 in the presence of IL-15, suggesting that coordination between IL-15Rβγ and Axl signaling pathways may occur in the same cell.
Evidence for an autocrine loop in the Axl/Gas6 pathway. CD34+ cells isolated from blood of 2 different donors were cultured for 3 days in the presence of IL-15. Harvested cells were fixed, permeabilized, and stained with antibodies against Gas6, Axl, and phospho-STAT5. Gating was on CD34+ cells, and numbers in the upper right quadrant indicate percentage of double-positive cell populations (Gas6+Axl+ or Gas6+ phospho-STAT5+).
Evidence for an autocrine loop in the Axl/Gas6 pathway. CD34+ cells isolated from blood of 2 different donors were cultured for 3 days in the presence of IL-15. Harvested cells were fixed, permeabilized, and stained with antibodies against Gas6, Axl, and phospho-STAT5. Gating was on CD34+ cells, and numbers in the upper right quadrant indicate percentage of double-positive cell populations (Gas6+Axl+ or Gas6+ phospho-STAT5+).
Receptor tyrosine kinases c-Kit and Flt-3, and their respective ligands, KL and FL, contribute to NK-cell development, probably by increasing NK-cell precursor frequency among CD34+ HPCs.11 We assessed whether blocking the Axl/Gas6 pathway could reduce the frequency of NK-cell precursors among CD34+ HPCs. Culturing human CD34+ HPCs in the presence of KL for 7 days before the addition of IL-15 increased NK precursor frequency as reported previously11 (Figure 6A). Coculture of CD34+ HPCs with KL and Axl-Fc before exposure to IL-15 significantly diminished NK-cell precursor frequency compared with culture of CD34+ HPCs with KL and irrelevant Fc (0.07% ± 0.03% for KL + Axl-Fc vs 0.20% ± 0.10% for KL + irrelevant Fc; P < .05). This suggests that blocking the Axl/Gas6 pathway may exert its inhibitory effect on NK-cell differentiation, at least in part, through reducing the frequency of NK precursor cells among the total CD34+ HPCs. These data also suggest that the Axl/Gas6 pathway is important for biologic functions of c-Kit as it relates to NK-cell development. To test whether blocking the Axl/Gas6 pathway inhibits c-Kit signaling in CD34+ cells, we treated isolated CD34+ cells with KL in the presence of Axl-Fc or irrelevant Fc. Axl-Fc diminished phosphorylation of c-Kit induced by KL in human CD34+ HPCs, which was shown by intracellular staining for phospho-c-Kit (MFI 124 for KL + irrelevant Fc vs 71 for KL + Axl-Fc) and in a separate experiment by Western blot (Figure 6B).
Blocking the Axl/Gas6 pathway reduces NK precursor frequency and inhibits c-Kit phosphorylation. (A) Isolated CD34+ HPCs from human peripheral blood were plated using serial dilution (from 6000 to 94 cells/well × 12 wells/condition). CD34+ HPCs were next cultured in 1 of 3 conditions and then directly stained with anti-CD3 and anti-CD56 antibodies, and analyzed by flow cytometry to quantify CD3−CD56+ NK cells: (1) IL-15 for 2 weeks (IL-15); (2) KL plus irrelevant Fc for 7 days followed by culture in IL-15 alone for 2 weeks (KL + irrelevant Fc → IL-15); or (3) KL plus Axl-Fc for 7 days, followed by culture in IL-15 alone for 2 weeks (KL + Axl-Fc → IL-15). CD56 expression was analyzed by flow cytometry, and the NK precursor frequency was determined as described in “Limiting dilution assay.” Results are from 3 donors. Error bar represents SEM. *P < .05. (B) CD34+ cells from human peripheral blood were plated, rested overnight, and treated with media only, KL plus irrelevant Fc, or KL + Axl-Fc for the indicated times. Cells were then harvested, and the level of phospho–c-Kit was measured by Western blot (top). In a separate experiment, intracellular staining for phospho–c-Kit that was gated on CD34+ cells was performed (bottom). Dotted line represents media only; thick line, KL + irrelevant Fc; thin line, KL + Axl-Fc. Results are representative of 4 separate experiments with similar results.
Blocking the Axl/Gas6 pathway reduces NK precursor frequency and inhibits c-Kit phosphorylation. (A) Isolated CD34+ HPCs from human peripheral blood were plated using serial dilution (from 6000 to 94 cells/well × 12 wells/condition). CD34+ HPCs were next cultured in 1 of 3 conditions and then directly stained with anti-CD3 and anti-CD56 antibodies, and analyzed by flow cytometry to quantify CD3−CD56+ NK cells: (1) IL-15 for 2 weeks (IL-15); (2) KL plus irrelevant Fc for 7 days followed by culture in IL-15 alone for 2 weeks (KL + irrelevant Fc → IL-15); or (3) KL plus Axl-Fc for 7 days, followed by culture in IL-15 alone for 2 weeks (KL + Axl-Fc → IL-15). CD56 expression was analyzed by flow cytometry, and the NK precursor frequency was determined as described in “Limiting dilution assay.” Results are from 3 donors. Error bar represents SEM. *P < .05. (B) CD34+ cells from human peripheral blood were plated, rested overnight, and treated with media only, KL plus irrelevant Fc, or KL + Axl-Fc for the indicated times. Cells were then harvested, and the level of phospho–c-Kit was measured by Western blot (top). In a separate experiment, intracellular staining for phospho–c-Kit that was gated on CD34+ cells was performed (bottom). Dotted line represents media only; thick line, KL + irrelevant Fc; thin line, KL + Axl-Fc. Results are representative of 4 separate experiments with similar results.
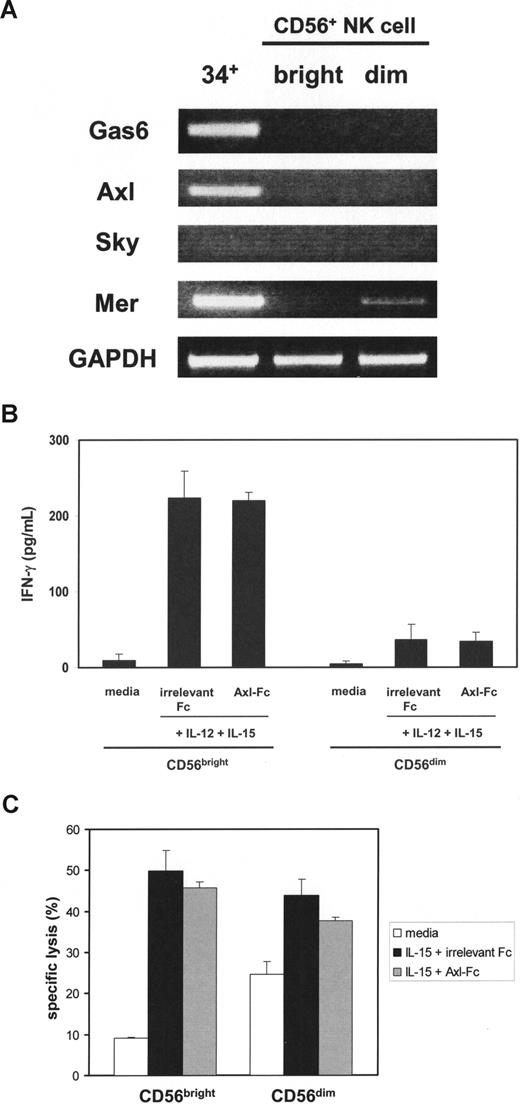
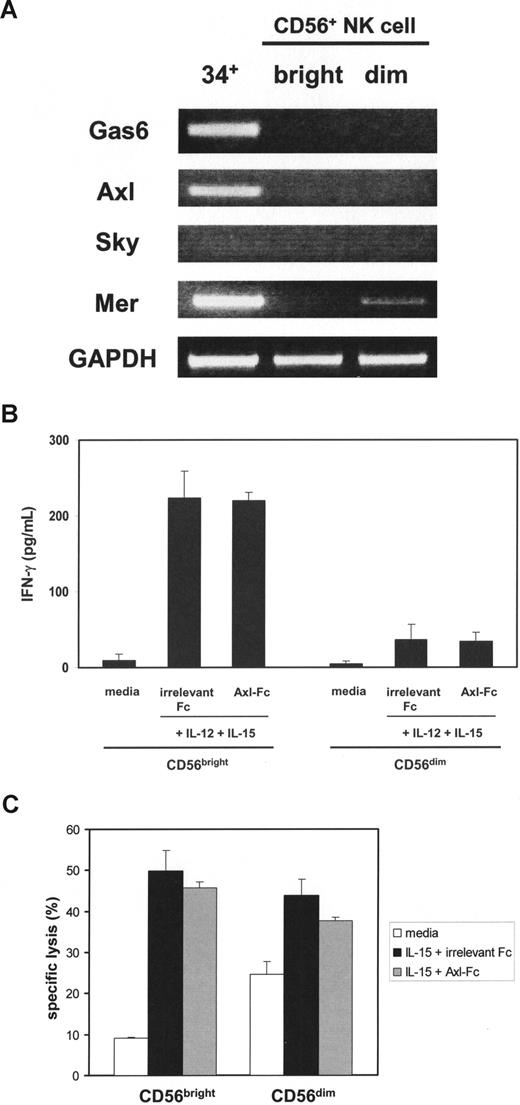
We also examined whether the Axl/Gas6 pathway is operational in mature NK cells. In contrast to CD34+ HPCs, neither Gas6 nor Axl mRNA was present in fresh CD56bright or CD56dim NK cells isolated from human peripheral blood (Figure 7A). Only a faint band representing another Axl family member Mer mRNA was expressed in CD56dim NK cells. The presence of Axl-Fc did not inhibit IFN-γ production or cytotoxicity in CD56bright or CD56dim NK cells on stimulation with IL-15 and IL-12 or IL-15 alone (Figure 7B,C). These data are consistent with the disappearance of Gas6 and Axl by day 15 of CD34+ HPCs in the presence of IL-15 (Figure 1) and suggest that the Axl/Gas6 pathway does not play a role in regulating functional activities of mature NK cells.
Assessment of the Axl/Gas6 pathway in purified mature NK cells. (A) After total RNA was isolated from sorted fresh human CD34+ HPCs (34+), CD56bright (bright), or CD56dim (dim) NK cells, RT-PCR was performed to detect Gas6, Axl, Sky, and Mer mRNA. GAPDH was used as a loading control. The figure represents 1 of 2 experiments performed with separate donors. (B) Purified CD56bright and CD56dim NK cells were sorted from human peripheral blood, plated with the same cell numbers, and stimulated with IL-12 and IL-15 in the presence of irrelevant Fc or Axl-Fc for 24 hours. Supernatants were collected and IFN-γ production was measured by ELISA. Results illustrate the mean plus or minus SD from triplicate wells and represent 1 of 3 similar experiments showing no significant difference in the presence or absence of Axl-Fc for CD56bright or CD56dim NK cells. (C) CD56bright and CD56dim NK cells were prepared as described in panel B and cultured with IL-15 in the presence of irrelevant Fc or Axl-Fc for 24 hours and then mixed with 51Cr-labeled K-562 target cells at an effector:target ratio of 3:1, again without significant difference. These results are representative of 3 similar experiments.
Assessment of the Axl/Gas6 pathway in purified mature NK cells. (A) After total RNA was isolated from sorted fresh human CD34+ HPCs (34+), CD56bright (bright), or CD56dim (dim) NK cells, RT-PCR was performed to detect Gas6, Axl, Sky, and Mer mRNA. GAPDH was used as a loading control. The figure represents 1 of 2 experiments performed with separate donors. (B) Purified CD56bright and CD56dim NK cells were sorted from human peripheral blood, plated with the same cell numbers, and stimulated with IL-12 and IL-15 in the presence of irrelevant Fc or Axl-Fc for 24 hours. Supernatants were collected and IFN-γ production was measured by ELISA. Results illustrate the mean plus or minus SD from triplicate wells and represent 1 of 3 similar experiments showing no significant difference in the presence or absence of Axl-Fc for CD56bright or CD56dim NK cells. (C) CD56bright and CD56dim NK cells were prepared as described in panel B and cultured with IL-15 in the presence of irrelevant Fc or Axl-Fc for 24 hours and then mixed with 51Cr-labeled K-562 target cells at an effector:target ratio of 3:1, again without significant difference. These results are representative of 3 similar experiments.
Discussion
In the present study, we have shown that interruption of the Axl/Gas6 pathway alters the in vitro differentiation of CD34+ HPCs to CD56bright NK cells. Specifically, blocking the binding of endogenous Gas6 to Axl by either Axl-Fc or warfarin resulted in a remarkable reduction in the number of NK cells generated from human CD34+ HPCs in the presence of IL-15. This also resulted in impaired IFN-γ production by differentiated CD56bright NK cells on a per cell basis, suggesting that the Axl/Gas6 pathway is also required for functional maturation of NK cells, as shown similarly in mice.24 Whereas our data show that signaling via the Axl/Gas6 pathway is important for NK-cell development, the data also suggest that the pathway is not absolutely required for NK-cell development. Indeed, this is consistent with the genetic disruption studies performed in mouse,24 and is consistent with other pathways involved in NK development, such as c-Kit/KL34 and Flt-3/FL.35 The absence of any one of these pathways drastically reduces NK-cell number and function but does not completely eliminate NK cells.
We also sought to determine the mechanism responsible for the observations noted in our study. Like other lymphocytes, NK cells originate from a CD34+ HSC that differentiates to an NK-cell precursor that has commitment to the NK-cell lineage.11,36 Both human11,12 and mouse37 studies have shown that the type 3 receptor tyrosine kinases Flt-3 and c-Kit and their respective ligands FL and KL are required for normal NK development by producing adequate numbers of NK-cell precursors during differentiation. Here, we show that blocking the Axl/Gas6 pathway by Axl-Fc impedes phosphorylation of c-Kit expressed on human CD34+ HPCs in the presence of KL, thereby reducing the number of NK precursors. This suggests that Axl and its endogenously produced ligand, Gas6, are critical cofactors for normal c-Kit activation early in the process of NK-cell differentiation. Like c-Kit, Axl is a receptor tyrosine kinase and shows 61% homology to c-Kit within its C-terminus cytoplasmic domain, suggesting that these 2 receptors could share some downstream signaling inter-mediaries allowing for some cross-talk with each other. On the other hand, Axl's contribution to normal phosphorylation of c-Kit might relate to its ability to antagonize a phosphatase that down-regulates activated c-Kit. Similar studies with Flt-3 are underway.
Once an NK precursor cell is generated from CD34+ HPCs in the presence of KL and/or FL, IL-15 then drives differentiation to a mature NK cell.11,12,36 A recent report documented a physical and physiologic interaction between the Axl/Gas6 pathway and IL-15 receptor α-subunit in mouse fibroblasts and dendritic cells (DCs).26 Here, we explored whether the Axl/Gas6 pathway is required for IL-15–induced signaling via the IL-15Rβγ heterodimer expressed on CD34+ human NK precursor cells. We demonstrated that, when endogenous Gas6 is blocked from binding to Axl by Axl-Fc, CD34+ HPCs undergo less phosphorylation of STAT5 in the presence of IL-15, and as a consequence, fewer CD56bright NK cells are produced. These data suggest that coordinated, cooperative interaction between the Axl/Gas6 pathway and the IL-15 signaling pathway is essential for normal human NK-cell differentiation, and provide at least a partial understanding as to how this occurs mechanistically.
Our data also show that the Axl/Gas6 pathway is important for at least one aspect of human NK-cell functional maturation, ie, IFN-γ production, and that this probably results, at least in part, from an underproduction of T-BET, the master regulator of IFN-γ production. IL-15 induces T-BET expression,8,38,39 but it is unclear at this time whether this defect involves STAT5 pathway or another signaling intermediary that is modulated by the absence of an activated Axl. However, the Axl-mediated effect probably occurs between stages III and IV of human NK-cell development as T-BET is not constitutively expressed at stage III but is fully present in stage IV.40 Importantly, in addition to its effect on IFN-γ gene expression, the absence of T-BET has adverse consequences on other aspects of NK-cell maturation.39 Thus, the effect that blocking Axl activation has on IL-15–mediated human NK differentiation from an NK precursor may occur in part via T-BET as well as other STAT5-mediated downstream events.
In our study, blockade of Axl/Gas6 pathway by Axl-Fc or warfarin produced NK cells that were functionally defective in production of IFN-γ, but not in natural cytotoxicity. A previous study by Caraux et al demonstrated that mice lacking Axl and its family members (Tyro3 and Mer) possessed NK cells that were also functionally defective in both production of IFN-γ and cytotoxicity.24 Although we do not have experimental data to explain this discrepancy at this time, one explanation might be attributed to the nature of in vitro–generated NK cells from human CD34+ HPCs. When CD34+ HPCs from human peripheral blood were cultured in the presence of IL-15, they could differentiate into only CD56bright NK cells.40,41 CD56bright NK cells produce larger amount of cytokines, including IFN-γ, than CD56dim NK cells do. On the other hand, CD56dim NK cells are more naturally cytotoxic against NK-sensitive target cells.2 Caraux et al24 used peripheral NK cells from mice genetically deficient in Axl and other Axl family member proteins. Although mouse NK-cell populations that are equivalent to human CD56bright and CD56dim, respectively, have not been reported yet, under in vivo biologic conditions, the Axl/Gas6 pathway might be important for NK-cell cytotoxicity and exert its effect through CD56dim-like NK-cell population. This hypothesis can only be tested when we better understand how to generate CD56dim NK cells from human CD34+ HPCs.
We also show that Axl, Gas6, and STAT5 can all originate from the same CD34+ HPCs. This is in contrast to the mouse study where a 3T3 fibroblast feeder cell line expressing Gas6 was used to analyze NK-cell differentiation from NK precursor cells in culture, although they did not show whether Gas6 was also produced from precursor cells.24 Thus, one way that IL-15 may drive in vitro human NK-cell differentiation from an NK precursor in the absence of a stromal cell feeder layer may involve Gas6 protein secreted from CD34+ HPCs that in turn binds Axl in an autocrine fashion followed by the promotion of both c-Kit and STAT5 phosphorylation. The situation may be a bit more redundant in vivo as both bone marrow stromal cells and DCs from secondary lymphoid tissues express Gas6,6,42,43 and both appear important for human NK-cell development. CD34+ HPCs may therefore respond to Gas6 protein not only from CD34+ HPCs themselves in an autocrine fashion but also from other neighboring cell types along with IL-15 in a paracrine fashion. It is interesting to note that in Sertoli cells, whose phagocytic activity is essential for spermatogenesis,44 the levels of Gas6 and protein S cycle as a function of stage during sperm development.45 Cyclic variation in the intensity of the Axl/Gas6 signaling was also observed in DCs and macrophages during inflammation.46
Although the Axl/Gas6 pathway plays a role during human NK-cell development, it does not have such a role in mature NK cells that lack expression of both molecules. However, CD56dim NK cells did show modest expression of another Axl family member, Mer. Thus it is possible, if not probable, that in a biologic context, these NK cells could cross-talk with neighboring cells expressing ligands, Gas6, or protein S. Future studies will explore this relationship.
In conclusion, we have demonstrated that the Axl/Gas6 pathway can contribute to NK-cell differentiation in humans. It appears that the Axl/Gas6 pathway is involved in multiple aspects of this process. By cooperative interaction with c-Kit/KL pathway, a seemingly autocrine Axl/Gas6 pathway participates in the generation of an NK precursor cell from CD34+ HPCs. Early on, as the NK precursor cell differentiates into a mature NK cell, the Axl/Gas6 pathway positively regulates IL-15/STAT5 signaling and T-BET expression. Continued investigation of the Axl/Gas6 pathway will probably reveal its importance in additional NK-cell functions and homeostasis through its regulation of signaling from a variety of cytokines.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank The Ohio State University Comprehensive Cancer Center Flow Cytometry Shared Resource for analysis.
This work was supported by National Cancer Institute grants CA068458 and CA95426 (M.A.C.) and the Up on the Roof postdoctoral fellowship from The Ohio State University Comprehensive Cancer Center (I.-K.P.).
National Institutes of Health
Authorship
Contribution: I.-K.P. designed and performed research, analyzed and interpreted data, and wrote the paper; C.G. performed research; T.L.H. collected data; J.Y. analyzed and interpreted data; R.T. analyzed and interpreted data; and M.A.C. designed research, analyzed and interpreted data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael A. Caligiuri, Comprehensive Cancer Center, Ohio State University, 320 West 10th Avenue, Columbus, OH 43210; e-mail: Michael.caligiuri@osumc.edu.