Abstract
Abundant bone marrow angiogenesis is present in almost all myeloma patients requiring therapy and correlated to treatment response and survival. We assessed the expression of 402 angiogenesis-associated genes by Affymetrix DNA microarrays in 466 samples, including CD138-purified myeloma cells (MMCs) from 300 previously untreated patients, in vivo microcirculation by dynamic contrast-enhanced magnetic resonance imaging, and in vitro angiogenesis (AngioKit-assay). Normal bone marrow plasma cells (BMPCs) express a median of 39 proangiogenic (eg, VEGFA, ADM, IGF-1) and 28 antiangiogenic genes (eg, TIMP1, TIMP2). Supernatants of BMPCs unlike those of memory B cells induce angiogenesis in vitro. MMCs do not show a significantly higher median number of expressed proangiogenic (45) or antiangiogenic (31) genes, but 97% of MMC samples aberrantly express at least one of the angiogenic factors HGF, IL-15, ANG, APRIL, CTGF, or TGFA. Supernatants of MMCs and human myeloma cell lines induce significantly higher in vitro angiogenesis compared with BMPCs. In conclusion, BMPCs express a surplus of proangiogenic over antiangiogenic genes transmitting to the ability to induce in vitro angiogenesis. Aberrant expression of proangiogenic and down-regulation of antiangiogenic genes by MMCs further increases the angiogenic stimulus, together leading to bone marrow angiogenesis at various degrees in all myeloma patients.
Introduction
Multiple myeloma (MM) is an incurable malignant disease of clonal plasma cells that accumulate in the bone marrow (BM), causing clinical signs and symptoms related to the displacement of normal hematopoiesis, formation of osteolytic bone lesions, and production of monoclonal protein.1
In the bone marrow microenvironment (BMME) affected by MM, substantial BM neovascularization (“angiogenesis”) is present: compared with healthy persons, a higher microvessel density (MVD),2 endothelial activation,3 capillary permeability,4 and increased perfusion4 can be detected. BM angiogenesis parallels disease activity, is returned to the normal state after successful treatment,5,6 and correlates with event-free survival (EFS) and overall survival (OS).7-10 Several proangiogenic cytokines (eg, VEGFA, FGF2, and HGF) are present in higher concentrations in myelomatous BM and peripheral blood sera6,11-16 while decreasing after successful treatment.6,13,14
In analogy to the “angiogenic switch” model for solid tumors by Folkman et al,17 the induction of angiogenesis in MM is considered to be related to malignant plasma cells progressively inducing a change in the balance between proangiogenic and antiangiogenic cytokines within the BMME. This change is attributed to malignant plasma cells obtaining the capability of aberrantly producing proangiogenic and concomitantly down-regulating antiangiogenic factors, either directly or by influencing the BMME. Here, it is a matter of debate whether these expression changes in malignant plasma cells take place at the stage of monoclonal gammopathy of unknown significance (MGUS) or MM. BM angiogenesis has been described to either correlate with the accumulation of MM cells (MMCs; tumor load), or their proliferation. MMCs are thought to benefit in turn from BM angiogenesis by improved oxygen and nutrient supply and likewise antiapoptotic and tumor-promoting effects mediated by endothelial-derived cytokines and myeloma-endothelial adhesion events.18
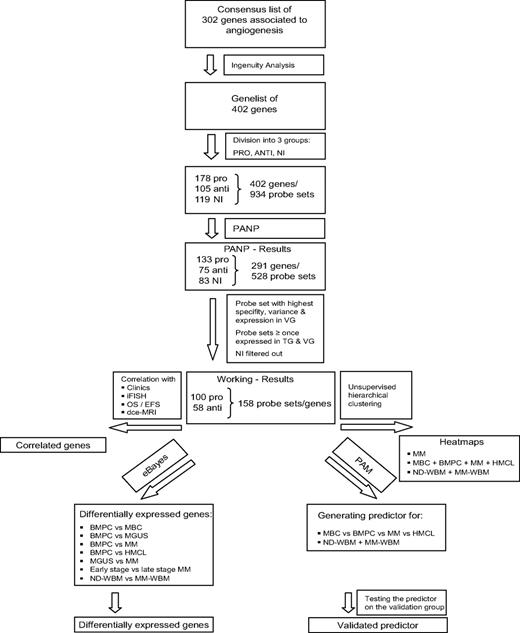
To assess a comprehensive set of “angiogenesis-associated” genes, we combined literature review and association of further related genes by Ingenuity Pathway Analysis (Figure 1). We subsequently assess presence and differential expression of these 402 genes in 466 gene expression profiles, including normal bone marrow plasma cells (BMPCs), primary MMCs, and human myeloma cell lines (HMCLs), BMME from normal healthy donors (NDs) and myeloma patients as well as the association with clinical parameters, genetic abnormalities, and survival.
We report here, for the first time, that already normal BMPCs express several proangiogenic genes, including VEGFA, IGF-1, and ADM and their culture supernatants (n = 11) significantly induce in vitro angiogenesis. Interestingly, this angiogenesis induction cannot be seen as a general property of cells of B-cellular lineage, as memory B-cell (MBC) supernatants do not induce angiogenesis in vitro (n = 6). Expectedly, malignant plasma cells show a various pattern of aberrant expression of several proangiogenic factors, and culture supernatants of primary MMCs (n = 20) and HMCLs (n = 10) induce in vitro angiogenesis (tubule formation). None of these factors, however, is expressed in all of the myeloma patient samples, and no significant correlation with in vivo surrogates of perfusion and MVD as determined by dynamic contrast-enhanced magnetic resonance imaging (dce-MRI, n = 64) could be found. Nevertheless, if the 6 most frequently aberrantly expressed factors are considered (HGF, IL-15, APRIL (TNFSF13), ANG, TGFA, CTGF), in 2 cohorts of patients 89% (n = 65) and 97% (n = 235) of MMC samples show an aberrant expression of at least one of these factors.
These results shed a different light on our understanding of the mechanism of angiogenesis induction in MM and might change the current paradigm of myeloma pathophysiology in a way that several of the “malignant” properties of MMCs might be attributed to primary plasma cell functions.
Methods
Patients and healthy donors
Patients presenting with previously untreated MM (n = 300) or MGUS (n = 23) at the University Hospitals of Heidelberg and Montpellier and 14 healthy ND have been included after written informed consent was obtained in accordance with the Declaration of Helsinki in the study approved by the institutional review boards of the Medical Faculty of the Ruprecht-Karls-University Heidelberg (Heidelberg, Germany), and the Centre Hospitalier Universitaire Montpellier (Montpellier, France), for the respective patients. Patients were diagnosed and staged and their response to treatment was assessed according to standard criteria.19-22 A total of 207 patients underwent frontline high-dose chemotherapy (HDT) with 200 mg/m2 melphalan and autologous stem cell transplantation (ASCT) according or in analogy to the GMMG-HD3 trial.23 Data were validated by an independent cohort of 345 patients treated within the total therapy 2 protocol.24 For clinical parameters, see supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Samples
For an overview, see Table S2. Bone marrow plasma cells were purified using CD138 microbeads (Miltenyi Biotec), and purity was assessed by flow cytometry (FACSCalibur; BD Biosciences). Aliquots of unpurified (whole) bone marrow (WBM) of patients (n = 57) and healthy donors (n = 7) were obtained after NH4 lysis as published.25 BMPCs for supernatant generation were subsequently FACSAria (BD Biosciences) sorted to purity more than 90% and peripheral CD27+ MBCs generated as published.26
The HMCLs XG-1, XG-2, XG-3, XG-4, XG-5, XG-6, XG-7, XG-10, XG-11, XG-12, XG-13, XG-14, XG-16, XG-19, and XG-20 were generated at Institut National de la Santé et de la Recherche Médicale U847 as published.27-29 U266, RPMI-8226, LP-1, OPM-2, SKMM-2, AMO-1, JJN-3, NCI-929, KMS-12-BM, KMS-11, KMS-12-PE, KMS-18, MM1S, JIM3, KARPAS 620, L363, and ANBL6 (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany, and ATCC) were cultured as recommended.
iFISH
Interphase fluorescence in situ hybridization (iFISH) analysis was performed on CD138-purified plasma cells as described30,31 using probes for chromosomes 1q21, 4p16, 6q21, 8p21, 9q34, 11q13, 11q23, 13q14.3, 15q22, 17p13, 19q13, 22q11, and translocations t(4;14)(p16.3;q32.3), t(11;14)(q13;q32.3) (Poseidon Probes, Kreatech Diagnostics). Ploidy status and clonal/subclonal aberrations (ie, present in ≥ 60% vs 20%-59% of assessed MMCs) were defined as published.30 A modified copy number score30 (excluding gains of 1q21) was used to assess ploidy state.
Gene expression analysis
Gene expression profiling (GEP) was performed as previously published.31 In brief, after RNA extraction, labeled cRNA was generated using the small sample labeling protocol vII (Affymetrix, Santa Clara, CA) and hybridized to U133 A + B GeneChip microarray (Affymetrix) for the training group (TG) and U133 2.0 plus arrays for the validation group (VG), according to the manufacturer's instructions. When different probe sets were available for the same gene, we chose the most specific probe set showing the maximal variance and the highest signal. Expression data for MMC samples are deposited in ArrayExpress under the accession numbers E-MTAB-81 and E-GEOD-2658.
To validate the Affymetrix gene expression data, expression of VEGFA (Hs00173626_m1), TGFA (Hs00608187_m1), CTGF (Hs00170014_m1), and ADM (Hs00181605_m1; all Applied Biosystems) was assessed by quantitative real-time polymerase chain reaction (RT-PCR) using the ABI Prism 7700 Sequence Detection System and the ΔΔCt method.32
Intracellular staining for VEGF
Intracellular vascular endothelial growth factor (VEGF) expression (clone 23410; R&D Systems) of 10 HMCLs, primary samples of 3 MM and one MGUS patient was measured by flow cytometry using a fixation and permeabilization kit (eBioscience). Overlays were established using the Infinicyt Software (Cytognos).
Protein detection by ELISA
Levels of VEGF, HGF, interleukin-15 (IL-15), TGFA, and IGF-1 were measured in culture supernatants of HMCLs (n = 10), primary MMCs (n = 2), and BM sera of myeloma patients (n = 10) and NDs (n = 3) according to the manufacturer's instructions (RayBio for VEGF, HGF and IL-15; R&D Systems for TGFA and IGF-1). Culture supernatants were obtained by growing 106 cells per mL for 24 hours in serum-free RPMI 1640 without addition of IL-6 (R&D Systems).
In vivo assessment of angiogenesis by dynamic contrast-enhanced magnetic resonance imaging
The entire spine of MM (n = 57) and MGUS patients (n = 7) was examined on a 1.5-Tesla-Tomograph (Symphony; Siemens) from the 1st cervical vertebra to the sacrum with a sagittal STIR and a sagittal T1-weighted SE as published.33 Two model variables are used to describe the tissue-specific information of the signal intensity-time curves: amplitude A (arbitrary units) is proportional to the relative signal enhancement as a surrogate for MVD and perfusion, the exchange rate constant kep (minutes) reflects the contrast agent transit between the extravascular and intravascular compartment.
In vitro assessment of angiogenesis
The angiogenic potential of 20 primary MMCs, 11 BMPCs, 6 MBC samples, and 10 HMCLs was investigated in the AngioKit assay (TCS Cellworks) according to the manufacturer's instructions. Culture supernatants were obtained as described for the enzyme-linked immunosorbent assays (ELISAs). Equal volumes of cell culture supernatants were added to the supplied growth medium. RPMI 1640, VEGF (2 ng/mL), and suramin (20 μM) served as medium, positive, and negative controls, respectively. All experiments were performed in triplicate, except for BMPCs and MBCs, because of limitations in achievable sample size (“Results”). After 11 days, cells were analyzed using a combined CD31 ELISA/CD31 tubule staining kit (TCS Cellworks). Tubular density was monitored using an Olympus IX-70 microscope (Olympus) at 40× magnification.
Consensus list of proangiogenic and antiangiogenic genes
A consensus list of 302 genes associated with angiogenesis has been obtained by review of Medline and the Cytokines & Cells Online Pathfinder Encyclopaedia (www.copewithcytokines.de). Subsequently, genes were analyzed using Ingenuity Pathway Analysis (Ingenuity Systems) and 100 genes added. These 402 genes were divided into 3 groups: proangiogenic, antiangiogenic, and “no information,” although some limitations apply to a gene expression–based analysis, as especially angio-inhibitory molecules are generated in vivo by cleavage of proteins by various proteases.
Statistical analysis
Gene expression data were normalized to GC-robust multi-array average (GC-RMA).34 To assess presence or absence of gene expression independently of Affymetrix-mismatch probe sets, the “Presence-Absence calls with Negative Probe sets (PANP)” algorithm35 was used. “Aberrant expression” of a gene within the MMC samples compared with BMPCs is defined as “presence” within the MMC samples, but not at least once in BMPCs within TG and VG. Differential gene expression was assessed using empirical Bayes statistics in linear models for microarray data.36 P values were adjusted for multiple testing controlling the false discovery rate as defined by Benjamini and Hochberg at a level of 5%.37 Expression profiles of 466 samples (13 MBCs, 14 BMPCs, 23 MGUS, 300 MM, 52 HMCLs [the same 20 HMCLs on different microarrays in TG and VG as well as AMO-1, JJN-3, NCI-929, KMS-12-BM, KMS-11, KMS-12-PE, KMS-18, MM1S, JIM3, KARPAS 620, L363 and ANBL6 in VG only], and 64 WBM) divided in TG (n = 113, MM n = 65) and VG (n = 353, MM n = 235) were analyzed. To assess the association of expressed angiogenic genes (signature) with EFS23 and OS23 for patients undergoing HDT and ASCT (Heidelberg/Montpellier group: 48 TG, 159 VG; Arkansas group: 345), Goeman global test38 was applied. Findings were validated using an independent set of 345 patients from the Arkansas group. Association of chromosomal aberrations and clinical parameters with gene expression was calculated using the 2-sample t-statistic. Differences in clinical parameters between defined groups were investigated by analysis of variance. Correlation was measured using the Spearman correlation coefficient (rs). Correlation with categorical variables was measured using the Kendall tau coefficient (τ). For assessing the relationship between categorical variables, Fisher exact test was used. The gene expression-based proliferation index is calculated as previously published.31
In all statistical tests, an effect was considered statistically significant if the P value of its corresponding statistical test was not greater than 5%. All statistical computations were performed using R,39 version 2.8.1; Bioconductor,40 version 2.3; and the Affymetrix Annotation Release 27. Results of the TG are shown in the supplemental data.
Results
Expression of angiogenesis-related genes
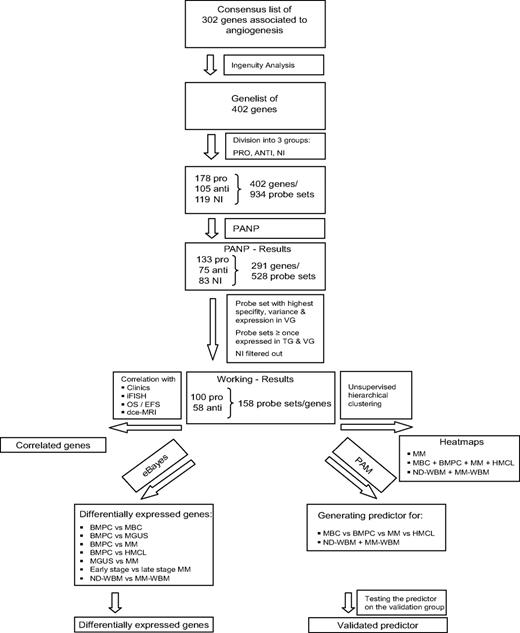
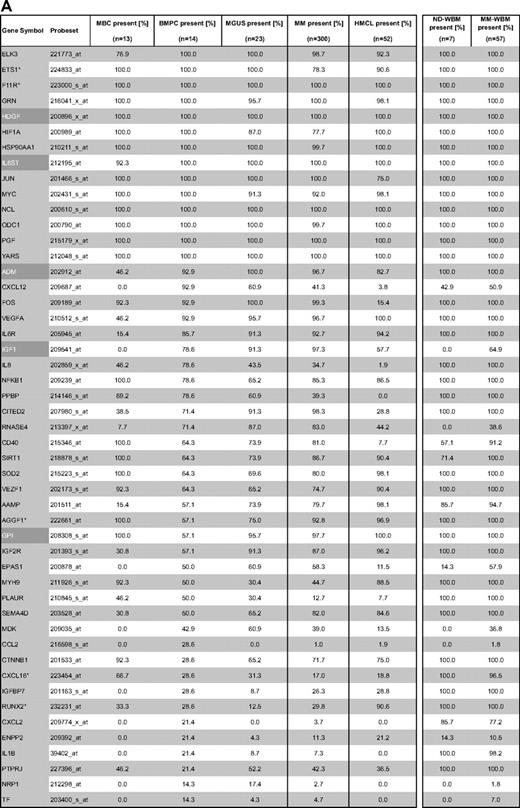
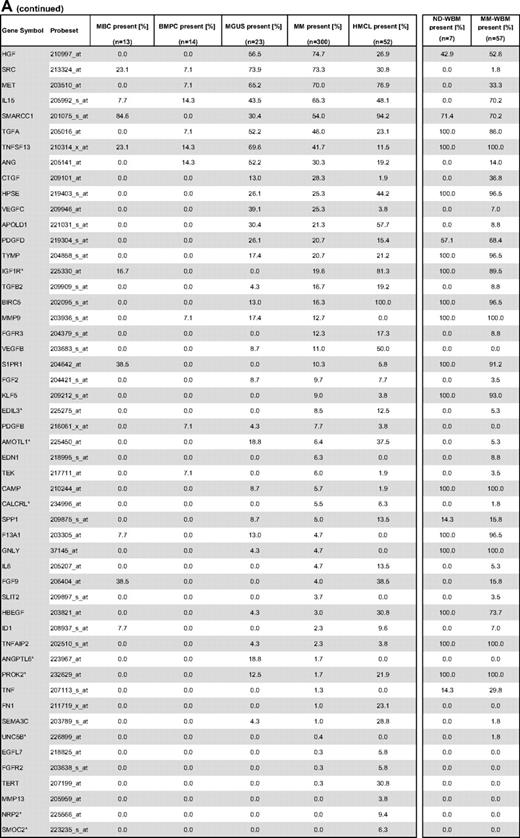
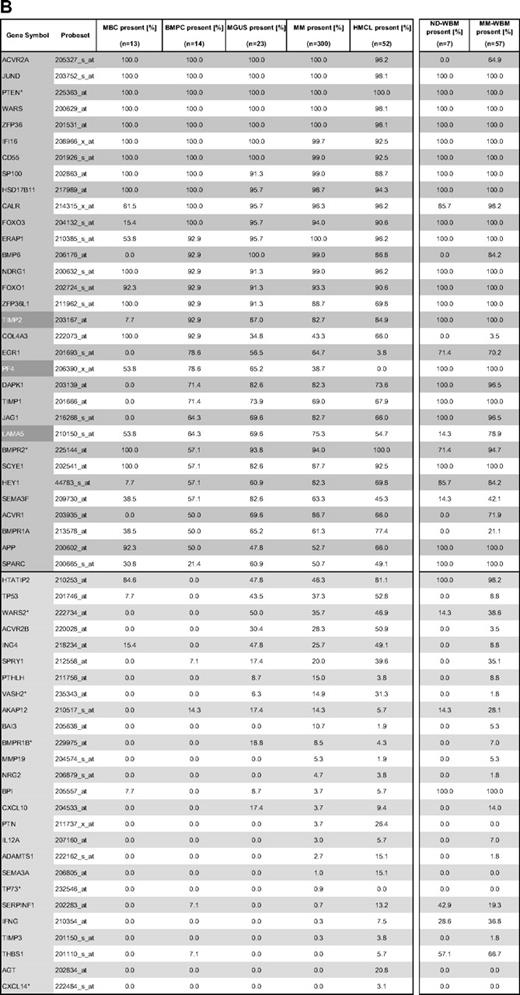
Gene expression of angiogenesis-related genes was evaluated using U133 A + B and U133 2.0 plus Affymetrix microarrays. Of the 402 genes initially included (Figure 1, selection strategy), 283 genes could be exploratively attributed using Medline review to be either proangiogenic (178 genes) or antiangiogenic (105 genes). Of these, 158 genes were expressed at least once in TG and VG, that is, 100 proangiogenic and 58 antiangiogenic genes, shown in Table 1. Genes not fulfilling these criteria are depicted in supplemental Table 3.
Genes and probe sets included in the respective parts of the analysis. Shown is our strategy for selecting angiogenesis-related genes. On the initial set of 402 genes after review of Medline and the Cytokines & Cells Online Pathfinder Encyclopaedia as well as Ingenuity Pathway Analysis, PANP-derived judgment of expression (“presence” vs “absence”) was assessed, leading to 291 genes being present at least once. Of these, 83 genes with no exploratively attributable information (NI) on proangiogenic or antiangiogenic activity were excluded. For further analyses, the 100 proangiogenic and 58 antiangiogenic genes present at least once in the training (TG) and validation group (VG) were retained.
Genes and probe sets included in the respective parts of the analysis. Shown is our strategy for selecting angiogenesis-related genes. On the initial set of 402 genes after review of Medline and the Cytokines & Cells Online Pathfinder Encyclopaedia as well as Ingenuity Pathway Analysis, PANP-derived judgment of expression (“presence” vs “absence”) was assessed, leading to 291 genes being present at least once. Of these, 83 genes with no exploratively attributable information (NI) on proangiogenic or antiangiogenic activity were excluded. For further analyses, the 100 proangiogenic and 58 antiangiogenic genes present at least once in the training (TG) and validation group (VG) were retained.
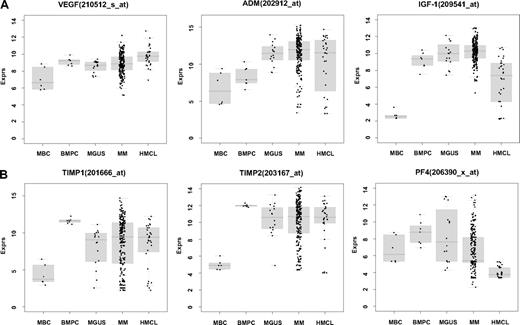
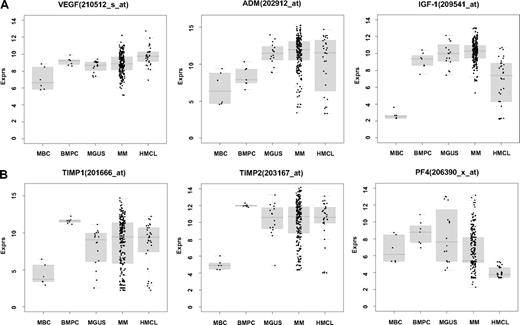
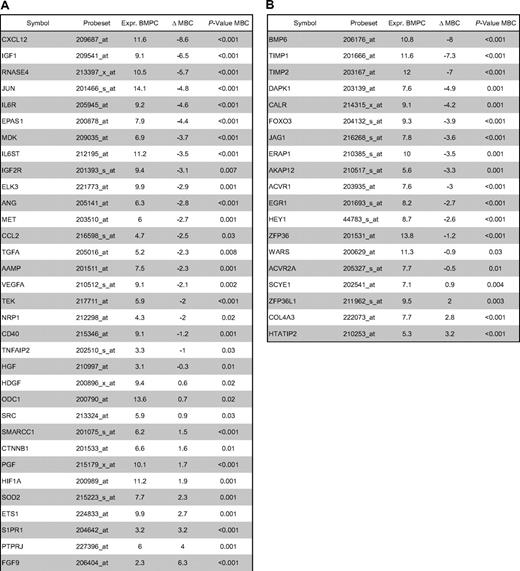
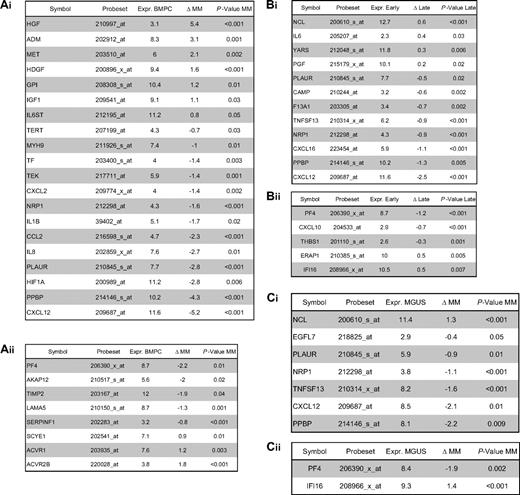
Using PANP-derived judgment of expression (“presence” vs “absence”), we found BMPCs to express 49 proangiogenic and 32 antiangiogenic genes with a median of 39 proangiogenic and 28 antiangiogenic genes in the VG, respectively (Table 1). MBCs express 47 proangiogenic and 30 antiangiogenic genes with a median of 32 proangiogenic and 19 antiangiogenic genes in the VG, respectively (Table 1). Of the proangiogenic BMPC genes, 21 genes are expressed significantly lower in MBCs, including major angiogenic factors, such as VEGFA, IGF-1, and ANG. Twelve genes show a significantly higher expression in MBCs, eg, HDGF and PGF; 4 antiangiogenic genes are up-regulated, 15 genes are significantly down-regulated in MBCs versus BMPCs (eg, BMP6, TIMP1, TIMP2; Figure 2; Table 2).
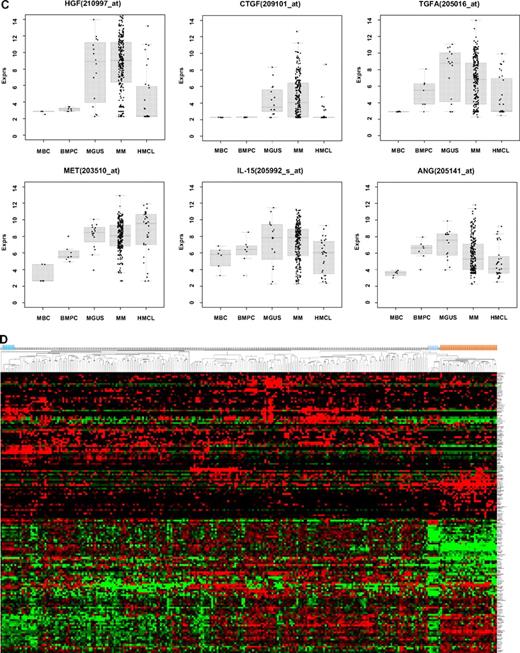
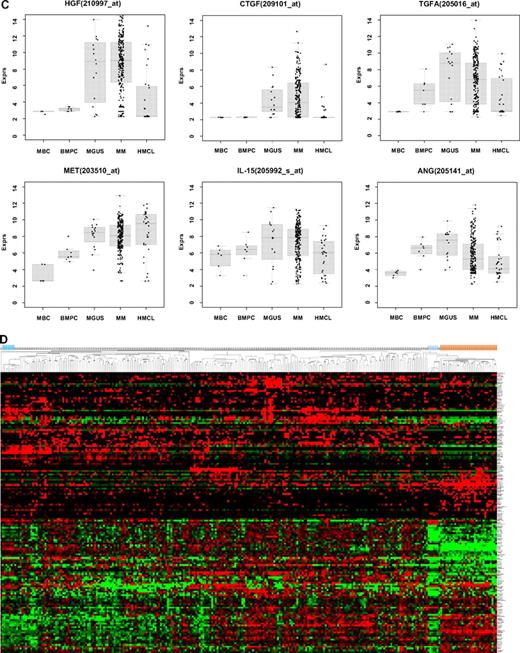
Expression of proangiogenic and antiangiogenic genes. Expression of (A) the proangiogenic genes VEGFA, ADM, and IGF-1, (B) the antiangiogenic genes TIMP1, TIMP2, and PF4, and (C) the aberrantly expressed genes HGF, CTGF, and TGFA as well as MET, IL-15, and ANG within the validation group. supplemental Figure 1 contains information on the training group. (D) The unsupervised hierarchical clustering shows BMPCs (depicted in blue) clustering together in a sub-branch within the MMCs (depicted in white). All HMCLs (depicted in orange) clustering together with the MBCs (depicted in light blue) in a separate branch each. supplemental Figure 1D contains information on the training group.
Expression of proangiogenic and antiangiogenic genes. Expression of (A) the proangiogenic genes VEGFA, ADM, and IGF-1, (B) the antiangiogenic genes TIMP1, TIMP2, and PF4, and (C) the aberrantly expressed genes HGF, CTGF, and TGFA as well as MET, IL-15, and ANG within the validation group. supplemental Figure 1 contains information on the training group. (D) The unsupervised hierarchical clustering shows BMPCs (depicted in blue) clustering together in a sub-branch within the MMCs (depicted in white). All HMCLs (depicted in orange) clustering together with the MBCs (depicted in light blue) in a separate branch each. supplemental Figure 1D contains information on the training group.
Compared with normal BMPCs, MMCs maintain expression of proangiogenic BMPC genes but show an aberrant expression of 51 proangiogenic and 26 antiangiogenic genes (Table 1; Figure 2A,B). The most frequently aberrantly expressed genes comprise HGF in 74.7%, HGF-receptor MET in 70%, IL-15 in 65.3%, TGFA in 46%, ANG in 30.3%, and CTGF in 28.3% of MMC samples (Figure 2C; Table 1). Of the proangiogenic BMPC genes, 7 show a significantly higher expression in MMCs, eg, HGF and ADM; 13 proangiogenic genes, however, are expressed significantly lower in MMCs (Figure 2; Table 3). Five antiangiogenic genes are significantly down-regulated in MMCs versus BMPCs (PF4, AKAP12, TIMP2, LAMA5, and SERPINF1), and 3 are up-regulated (Table 3).
Comparing MMCs of patients with early (MGUS and MMI) versus advanced-stage plasma cell dyscrasia (MMII and MMIII), we found 4 proangiogenic genes (including IL-6) to be significantly up-regulated and 8 down-regulated in the advanced stage. For the antiangiogenic genes, 2 genes (IFI16 and ERAP1) were significantly up-regulated and 3 (including PF4) down-regulated (Table 3). Comparing samples obtained from MGUS patients with MM samples, 9 genes are differentially expressed (Table 3); if this analysis is restricted to MGUS patients showing any clonal aberrations by iFISH (n = 5), no gene remains significant.
HMCLs maintain expression of aberrantly expressed MMC genes (Figure 2; Table 1) and show an additional aberrant expression of 3 proangiogenic and 3 antiangiogenic genes. No proangiogenic gene is aberrantly expressed or any antiangiogenic gene is lost in all HMCLs.
The unsupervised hierarchical clustering based on the proangiogenic and antiangiogenic genes shows BMPCs clustering together in a sub-branch within the MMCs of the VG. The 20 HMCLs cluster together with the MBCs, both appearing in a separate sub-branch (Figure 2D). A comparable picture was obtained with MMCs of the TG (supplemental Figure 1D).
A PAM-based predictor for MBCs, BMPCs, MMCs, and HMCLs of 133 genes calculated on the proangiogenic and antiangiogenic genes in the consensus list predicts group attribution with an estimated error rate of 3% (TG) and 3% (VG), respectively (supplemental Table 5A).
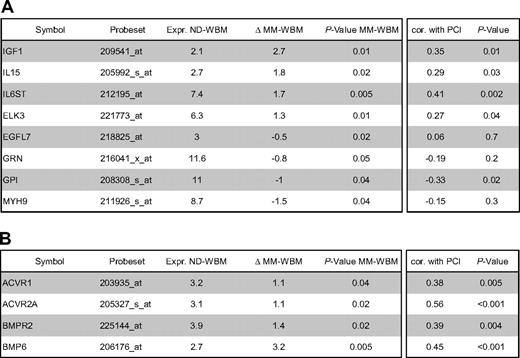
In the BMME of normal donors (ND-WBM) and myeloma patients (MM-WBM), 63 and 90 (of 100) proangiogenic as well as 34 and 53 (of 58) antiangiogenic genes are expressed. Twelve genes are differentially expressed between ND-WBM and MM-WBM (Table 4). In the unsupervised hierarchical clustering of the WBM samples, MM-WBM and ND-WBM separate (supplemental Figure 2).
A PAM-based predictor for ND-WBM and MM-WBM calculated on the 158 expressed proangiogenic and antiangiogenic genes and comprising 49 genes allows predicting the group attribution with an estimated error rate of 9% (supplemental Table 5B).
Validation of gene expression data
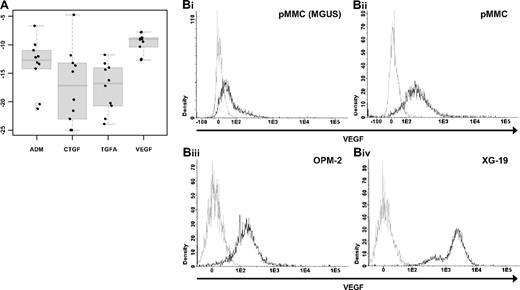
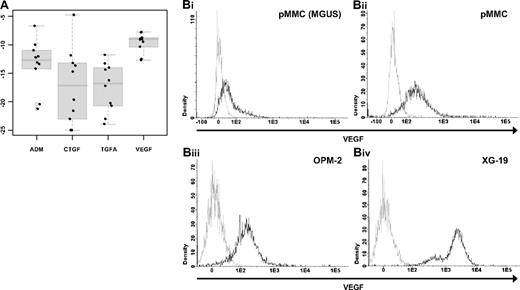
To validate gene expression data, quantitative real-time PCR, flow cytometry, and ELISAs were performed. Gene expression measured by quantitative RT-PCR verifies expression of VEGFA (rs = −0.45, P = .2), ADM (rs = −0.84, P = .004), CTGF (rs = −0.9, P = < .001), and TGFA (rs = −0.42, P = .2) in 10 HMCLs as detected by Affymetrix gene-chip (Figure 3A, supplemental Figure 3). An additional validation is given by the flow cytometric measurement of intracellular VEGF. VEGF expression can be detected in 10 of 10 HMCLs, 3 of 3 primary MMCs, and 1 of 1 MGUS cell samples. An exemplary primary MMC and MGUS sample as well as 2 HMCL samples are shown in Figure 3B.
Validation of gene expression by quantitative real-time PCR and flow cytometry. Expression of ADM, CTGF, TGFA, and VEGA as determined by (A) quantitative real-time PCR. Shown are −dCt values (reference gene 18S RNA). (B) Flow cytometric analysis of intracellular VEGF in (i) one MGUS sample (pMMC, MGUS), (ii) one exemplary primary myeloma cell sample (pMMC), as well as the 2 myeloma cell lines (iii) OPM-2 and (iv) XG-19.
Validation of gene expression by quantitative real-time PCR and flow cytometry. Expression of ADM, CTGF, TGFA, and VEGA as determined by (A) quantitative real-time PCR. Shown are −dCt values (reference gene 18S RNA). (B) Flow cytometric analysis of intracellular VEGF in (i) one MGUS sample (pMMC, MGUS), (ii) one exemplary primary myeloma cell sample (pMMC), as well as the 2 myeloma cell lines (iii) OPM-2 and (iv) XG-19.
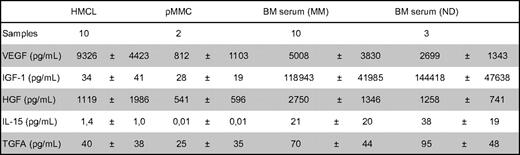
Secretion of VEGF, IGF-1, HGF, IL-15, and TGFA was measured by ELISA (Table 5). Of the proangiogenic factors already expressed by BMPCs, VEGF levels above the detection threshold of 20 pg/mL can be detected in all MMC and HMCL supernatants as well as all BM sera of myeloma patients and NDs. Measured values correlate well with VEGFA expression assessed by GEP for HMCLs (rs = 0.74, P < .01). IGF-1 levels above the mean detection threshold of 26 pg/mL (range given by the manufacturer, 7-56 pg/mL) can be found in 1 of 2 MMC and 4 of 10 HMCL supernatants as well as all BM sera. The values for BM sera are by several orders of magnitude higher compared with MMC or HMCL supernatants. Measured values correlate with IGF-1 expression assessed by GEP for HMCLs (rs = 0.64, P = .05). Of the aberrantly expressed factors, measured HGF levels are by orders of magnitude above the detection threshold within the BM sera of all samples. For HMCL supernatants, HGF secretion above the detection level of 8 pg/mL can be detected in all samples; however, 2 HMCL supernatants show a level around the detection threshold (8.1 and 8.4 pg/mL). Measured values correlate well with HGF expression assessed by GEP for HMCLs (rs = 0.89, P < .001). IL-15 levels above the median detection threshold of 3 pg/mL cannot be found in MMC or HMCL supernatants, whereas they are detectable in all BM sera, including normal BM. TGFA secretion above the median detection threshold of 2.27 pg/mL (range given by the manufacturer, 0.55-7 pg/mL) can be detected in 8 of 10 HMCL, 1 of 2 MMC supernatants, and all BM sera.
In vitro tubule formation by supernatants of memory B cells, normal and malignant plasma cells, and myeloma cell lines
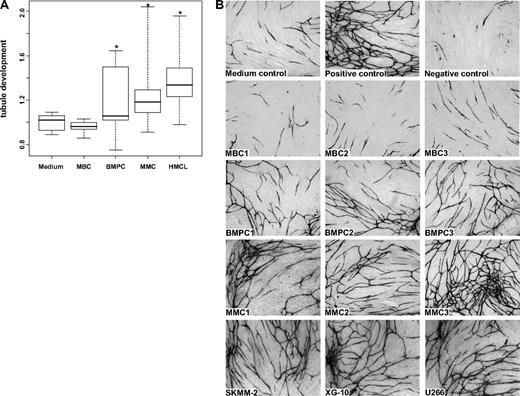
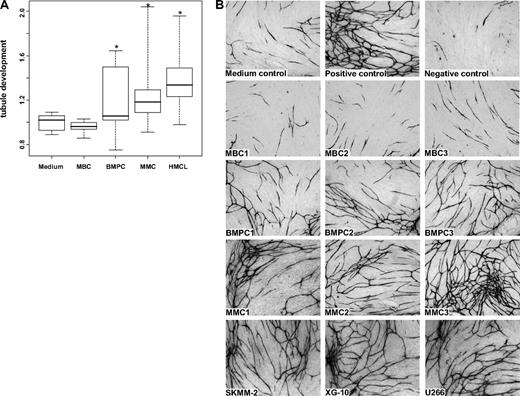
The angiogenic potential of supernatants of 6 MBC (but for 2 in a single measurement), 11 BMPC (but for 2 in a single measurement), 20 primary MMC (in triplicates), and 10 HMCL samples (in triplicates twice) was investigated in the AngioKit model. After 11 days, in vitro tubule formation was quantified using a CD31 ELISA and tubules were visualized by staining with an anti-CD31 (PECAM-1) antibody. Unlike those of MBCs, supernatants of BMPCs, MMCs, and HMCLs show a significant induction of tubule formation compared with medium control (P = .04, P < .001, and P < .001, respectively; Figure 4A). Three exemplary MBC, BMPC, and MMC samples as well as HMCLs, respectively, are shown in Figure 4B.
Induction of in vitro angiogenesis. Endothelial cell growth in the AngioKit model. (A) Box plot summarizing the ELISA results. Unlike those of MBC supernatants of BMPCs, pMMCs and HMCLs show a significant induction of tubule formation compared with medium control. *Significant difference compared with medium control (P < .05). (B) Immunostaining with monoclonal anti-human CD31 antibody: medium control (RPMI 1640), positive control (VEGF), negative control (suramin) as well as supernatants of memory B-cell samples (MBC1-3), normal bone marrow plasma cell samples (BMPC1-3), primary myeloma cell samples (pMMC1-3), and the myeloma cell lines SKMM-2, XG-10, and U266. Original magnifications ×40.
Induction of in vitro angiogenesis. Endothelial cell growth in the AngioKit model. (A) Box plot summarizing the ELISA results. Unlike those of MBC supernatants of BMPCs, pMMCs and HMCLs show a significant induction of tubule formation compared with medium control. *Significant difference compared with medium control (P < .05). (B) Immunostaining with monoclonal anti-human CD31 antibody: medium control (RPMI 1640), positive control (VEGF), negative control (suramin) as well as supernatants of memory B-cell samples (MBC1-3), normal bone marrow plasma cell samples (BMPC1-3), primary myeloma cell samples (pMMC1-3), and the myeloma cell lines SKMM-2, XG-10, and U266. Original magnifications ×40.
Correlation of angiogenic gene expression with biologic and clinical parameters
When considering only genes correlated significantly in TG and VG with a coefficient more than 0.4, the only chromosomal aberration correlating with one of the angiogenic genes is t(4;14) with FGFR3 expression (TG τ = 0.47, P = .002; VG τ = 0.73, P < .001). Only BIRC5 (survivin), a gene also associated with proliferation, correlates significantly with the plasma cell labeling index (n = 67, rs = 0.54, P = .001). By correlating expression of angiogenic genes with our gene expression-based proliferation index, of the genes not part of this index, one gene shows a significant positive correlation coefficient more than 0.4 (GPI, TG rs = 0.46, P = .001; VG rs = 0.51, P < .001), 3 a negative correlation (TERT, TG rs = −0.51, P < .001; VG rs = −0.43, P < .001; TEK, TG rs = −0.53, P < .001; VG rs = −0.56, P < .001; PDGFB, TG rs = −0.63, P < .001; VG rs = −0.47, P < .001), all being proangiogenic. Thus, no obvious connection could be found between MMC proliferation and angiogenic gene expression.
The dce-MRI surrogates for perfusion (A) and the exchange rate constant (kep) do not correlate significantly with any of the angiogenic genes. No correlation with the expression of (anti)angiogenic genes with those of D-type cyclins (CCND1, CCND2, CCND3) or clinical parameters (serum-β2-microglobulin (B2M), International Staging System stage, Salmon/Durie-stage, and serum albumin) could be found.
Prognostic value of angiogenic gene expression
Using Goeman global test, a significant association of the angiogenic gene expression (signature) could be found for EFS or OS within the VG and the Arkansas data (supplemental Figure 4). However, this signature is largely driven by expression of t(4;14) (eg, FGFR3) or proliferation-associated genes (eg, BIRC5). In a model including the presence of t(4;14) and the gene expression-based proliferation index as covariables, no association with survival could be found (on TG and VG only resulting from lack of t(4;14) data for the Arkansas group; supplemental Figure 5).
Discussion
Current hypotheses about induction of angiogenesis in multiple myeloma
Several hypotheses have been formulated to explain the induction of angiogenesis in MM:
Angiogenesis in MM is the result of the tumor burden and mediated by proangiogenic factors appearing at the MGUS stage (expression of VEGF, bFGF, and their receptors at a similar level in MGUS, smoldering [SMM], and active MM).41 However, bFGF is neither expressed by BMPCs nor a larger proportion of MMCs (Table 1); it is, however, expressed in all mesenchymal stromal cell samples (n = 19, data not shown); thus, lack of expression cannot be attributed to a defective probe set. VEGFA, in turn, is already expressed in BMPCs.
An “angiogenic switch” takes place at the MMC stage resulting from the expression of oncogenes (c-myc, c-fos, c-jun, ets-1) coding for angiogenic factors as a consequence of immunoglobulin-translocations and genetic instability of plasma cells,42 leading to an increased bFGF expression by MMCs. Whereas almost all MMCs show chromosomal aberrations,43 c-myc, c-fos, and c-jun are already expressed at BMPC stage and do not show a significant up-regulation in MMCs (Table 1). Ets-1 is not expressed in any of the BMPC or MMC samples.
A loss of antiangiogenic activity mediated by down-regulation of antiangiogenic factors (in MMCs or indirectly the BMME) is necessary for switch MGUS to MM.41 Jakob et al44 had derived the hypothesis from the fact that the angiogenic potential of BM sera is not completely abrogated by antibodies against bFGF45 or VEGF.46 Further evidence was indicated by a reported similar expression level of bFGF and VEGF by MMCs between MGUS, SMM, active MM,41 and an in vitro inhibitory effect of MGUS samples compared with SMM or active MM.41 However, in the latter case, no comparison was made to BMPC serum, and there has not been shown an inhibitory effect compared with medium. In general, the expression level of antiangiogenic genes remains fairly constant, with a surplus of proangiogenic over antiangiogenic genes (Tables 1, 3).
A further discussion is whether the induction of angiogenesis (ie, MVD) correlates with tumor load (plasma cell infiltration [PCI] in the BM) or MMC proliferation.2,8 Vacca et al reported a correlation of MVD with the labeling index (LI) but not with the PCI.2 Rajkumar et al reported initially the same result8 ; in a larger series of patients, however, they found a correlation of MVD with PCI.47 Niemoller et al found MVD to increases with disease progression and to correlate with PCI and B2M.48 Both hypotheses sound convincing: Angiogenesis is needed for increased proliferation and infiltration, as both rely on nutrition supply. In our data, however, the PCI does not significantly correlate with the expression of any proangiogenic or antiangiogenic gene, neither does the LI (n = 67) but for BIRC5 (survivin), a gene associated with proliferation. A possible explanation is that increased LI and PCI are independent surrogates for advanced (vs MGUS or SMM) disease: PCI correlates with Salmon/Durie-stage, as does LI.49
Angiogenesis in multiple myeloma: hypothetic model
Based on the data presented in this paper, we propose a new model of angiogenesis in MM comprising 3 hypotheses (H1-H3).
H1: BMPCs induce controlled angiogenesis.
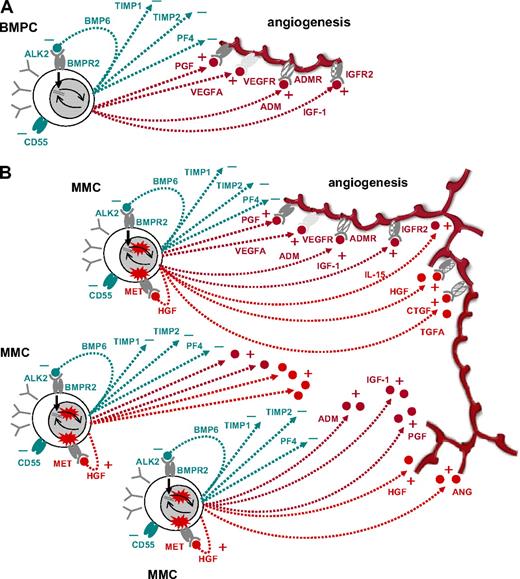
The function of BMPCs is to survive for several years and to produce huge quantities of antibodies (“antibody factories”). BMPCs therefore induce, in interaction with the BMME, a favorable microenvironment (“niche”), including blood-vessel supply, by producing and inducing the production of a slight surplus of proangiogenic over antiangiogenic factors. The concomitant expression of the latter allows (1) limiting the extent of the angiogenic stimulus to the vicinity of the BMPCs and (2) increasing angiogenesis in case of need by concomitant loosening of antiangiogenic breaks and increased production of proangiogenic factors.
Five lines of evidence support this hypothesis: (E1) BMPCs express proangiogenic factors, such as VEGFA, ADM, and IGF-1, and antiangiogenic factors, such as TIMP1, TIMP2, or BMP6 (Table 1; Figure 2). (E2) BMPCs are found in close proximity to blood vessels in the BM,50 indicating an interaction between the 2 cell types. (E3) Angiogenic factors are relevant for endothelial cell survival and maintenance of blood vessel integrity.51 (E4) The strongest evidence is given by the fact that supernatants of BMPCs induce angiogenesis in our in vitro assay compared with medium controls (Figure 4). (E5) This induction cannot be seen for supernatants of MBCs and is therefore not a general characteristic of cells (of B-cell lineage). At gene expression level, MBCs show a significantly lower expression of several proangiogenic cytokines compared with BMPCs, but likewise of antiangiogenic factors, such as TIMP1 (Table 2; Figure 2). A possible explanation for the latter is the lack of a necessity for antiangiogenic regulation if no angiogenesis induction is present (see Figure 4).
H2: An increase in the number of (BM) PCs increases angiogenesis.
Given a slight excess of production of proangiogenic over antiangiogenic factors in BMPCs (H1), it follows that an increase in plasma cell number yields an increase of the absolute surplus of proangiogenic factors produced in the BM. (Despite that the relative quantities remain the same, the absolute surplus in proangiogenic factors increases.) In this line of argumentation, it would not be necessary that accumulating MMCs show a differential expression of proangiogenic and antiangiogenic genes compared with BMPCs.
This hypothesis is supported by several lines of evidence: (E1) Comparing the percentage of plasma cells in the normal BM of approximately 0.5% with the infiltration rates seen in advanced MM of more than 50% and the increase in BM cellularity, the amount of plasma (myeloma) cells can be estimated to be a factor of at least 100 times higher compared with that in normal BM. Hence, a surplus in proangiogenic over antiangiogenic stimulation (on the basis of H1) would follow. (E2) If an increased rate of angiogenesis is the result of a slight excess of production of proangiogenic over antiangiogenic factors present already in BMPCs, all MM patients should show increased BM angiogenesis, which is the case. (E3) MMCs do not show a significantly higher median number of expressed proangiogenic (45) or antiangiogenic (31) genes, and neither a single factor nor a factor combination is aberrantly expressed in all MMCs (Table 1; see also point E1 in “H3”). (E4) The BMME mirrors presence of (malignant) plasma cells, evidenced by ND-WBM clustering together (Figure S2) and the predictability of “being” ND- versus MM-WBM with an error rate of 9% (Table S5B). Indeed, of 12 genes differentially expressed between ND- and MM-WBM, 10 are already expressed by BMPCs and 7 of these correlate positively with PCI (Table 4). (E5) There is no significant association with chromosomal aberrations detected by iFISH of a single proangiogenic or antiangiogenic factor but for the association of FGFR3 expression and t(4;14). (E6) There is no association with surrogates of tumor mass, such as B2M or International Staging System-stage, or clinical parameters. (E7) Despite a well-known increase of surrogates of MVD or BM perfusion as assessed by dce-MRI,4 no association with angiogenic gene expression could be found.
H3: Aberrantly expressed angiogenic factors by MMCs further increase BM angiogenesis in MM and might lead to different angiogenic patterns.
Evidence is given by the following observations: (E1) Despite the lack of a single aberrantly expressed factor or factor combination, 89% to 97% of MMC samples in different cohorts (TG, VG) show an aberrant expression of at least one of the angiogenic factors HGF, IL-15, ANG, APRIL, CTGF, or TGFA (Table 1). (E2) Based on expression of (anti)angiogenic genes, “being” MBC/BMPC/MMC/HMCL can be predicted fairly well (error rate: 3% TG, 3% VG) and populations separate in an unsupervised clustering (Figure 2D, supplemental Figure 1D; supplemental Table 5A) denoting a characteristic expression difference. (E3) Supernatants of MMCs and HMCLs induce higher in vitro angiogenesis compared with BMPCs (Figure 4). HMCLs here retain the proangiogenic pattern of MMCs, in analogy with HMCLs conserving signatures of BM dependence or independence.26
Based on these observations, angiogenesis seems to be a general feature of MM, not an (additional) risk factor per se for patients treated with HDT and ASCT. Another subject for further studies given these findings is whether angiogenesis may not be critical for MM pathogenesis, but just an epiphenomenon driven by the accumulation of (malignant) plasma cells and a production of proangiogenic cytokines that have a dual role as growth and survival factors for MMCs, eg, IGF-1.52 Likewise, aberrant expression of the HGF receptor MET by 70% of MMCs (Table 1; Figure 2) might allow these to make use of the HGF levels present within the BM (Table 1). This possibility would arguably explain the lack of major differences in gene expression in contrast to the striking angiogenesis induction seen in the myelomatous BM compared with normal persons. A further question is if or to what extent inflammatory cells (ie, macrophages, mast cells, lymphocytes) may also contribute substantially to angiogenesis induction in myeloma.
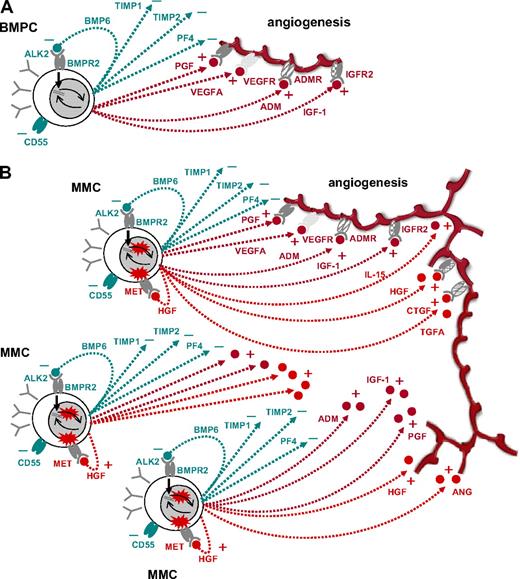
In conclusion, in contrast to MBCs, BMPCs express a surplus of proangiogenic over antiangiogenic genes transmitting to induction of in vitro angiogenesis. Thus, already an accumulation of BMPCs can induce a basal level of angiogenesis. Aberrant expression of proangiogenic genes and down-regulation of antiangiogenic genes by MMCs further increase the angiogenic stimulus already induced by BMPC genes, together explaining the presence of BM angiogenesis at various degrees in all myeloma patients (Figure 5). Chromosomal aberrations and changes in gene expression driving the evolution to MGUS and further to active MM thereby lead to the slow but progressive accumulation of plasma cells/MMCs, which “draw” their own supply with blood and nutrients by an induced and increased angiogenesis. This leads to a gradual change of the BMME (“BMME-switch,” Figure 5), providing in turn supply (nutrition, O2) and increased growth factor stimulation (proangiogenic cytokines with dual role) that help progressively overruling cell cycle breaks on the basis of altered D-type cyclin expression characteristic for MM.
Schematic representation of findings. (A) Interaction of normal BMPCs that produce proangiogenic (dark red) and antiangiogenic (dark green) factors, respectively. At a normal ratio, angiogenesis is restricted to the surrounding of BMPCs. (B) By proliferation and increasing number, aberrant as well as normal BMPC factors accumulate in the bone marrow microenvironment, inducing widespread angiogenesis.
Schematic representation of findings. (A) Interaction of normal BMPCs that produce proangiogenic (dark red) and antiangiogenic (dark green) factors, respectively. At a normal ratio, angiogenesis is restricted to the surrounding of BMPCs. (B) By proliferation and increasing number, aberrant as well as normal BMPC factors accumulate in the bone marrow microenvironment, inducing widespread angiogenesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Bart Barlogie and John D. Shaughnessy for the opportunity to validate our expression data on their cohort of patients; Stefan Delorme for participation in obtaining the dce-MRI data; Véronique Pantesco, Katrin Heimlich, and Maria Dörner for technical assistance; and Rainer Saffrich for help in microscopic imaging.
This work was supported in part by grants from the Hopp-Foundation, Germany; the University of Heidelberg, Germany; the National Center for Tumor Diseases, Heidelberg, Germany; the Tumorzentrum Heidelberg/Mannheim, Germany; the European Myeloma Stem Cell Network funded within the 6th Framework Program of the European Community; and the Ligue Nationale Contre Le Cancer (équipe labellisée), Paris, France. It is also part of a national program called Carte d'Identité des Tumeurs funded by the Ligue Nationale Contre le Cancer, France.
Authorship
Contribution: D.H. designed research, wrote the paper, and participated in the microarray experiments; J.M. participated in the analyzing of the data; T. Meissner and A.B. performed statistical analysis; A.S. performed experiments and participated in the writing of the paper; H.G. participated in the analyzing of the data and in the writing of the paper; K.M., T.R., and M.C. participated in the analyzing of the data; J.H. collected bone marrow samples as well as clinical data and contributed in performing the dce-MRI experiments and analyzing of the data; M.H., U.B., and J.-F.R. collected bone marrow samples and clinical data; J.D.V. participated in the microarray experiments; A.J. contributed in performing the iFISH experiments; and B.K. and T. Möhler participated in the analyzing of the data and in the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dirk Hose, Medizinische Klinik V, Universitätsklinikum Heidelberg, Im Neuenheimer Feld 410, 69120 Heidelberg, Germany; e-mail: dirk.hose@med.uni-heidelberg.de.