Abstract
Internal tandem duplication mutations in the Flt3 tyrosine kinase gene (ITD-Flt3) and overexpression of Survivin are frequently found in patients with acute myeloid leukemia (AML). We investigated whether Survivin mediates the enhanced survival of primary hematopoietic progenitor cells (HPCs) resulting from ITD-Flt3 signaling. Ectopic ITD-Flt3 mutants increased Survivin expression in Ba/F3 cells downstream of PI3-kinase/Akt. Treatment of ITD-Flt3+ human MV4-11 leukemia cells with the ITD-Flt3 inhibitor SU5416 reduced Survivin expression and inhibited cell proliferation. ITD-Flt3 dramatically increased the number of primary mouse marrow c-kit+, Sca-1+, LinNeg cells and colony-forming unit granulocyte-macrophages (CFU-GMs) able to proliferate in the absence of growth factors, whereas Survivin deletion significantly reduced growth factor–independent proliferation and increased apoptosis, which was further accentuated by SU5416. Ectopic ITD-Flt3 reduced differentiation of LinNeg marrow cells cultured with granulocyte-macrophage colony-stimulating factor (GM-CSF) plus stem cell factor, which was partially blocked by Survivin deletion. In addition, Survivin deletion decreased secondary colony formation induced by ITD-Flt3. Dominant-negative (dn)–Survivin delayed development of acute leukemia in mice that received a transplant of Ba/F3 cells expressing ITD-Flt3. These results suggest that Survivin regulates expansion of ITD-Flt3–transformed HPCs with self-renewal capability and development of ITD-Flt3+ acute leukemia and that antagonizing Survivin may provide therapeutic benefit for patients with acute leukemia expressing ITD-Flt3.
Introduction
The Flt3 type III tyrosine kinase receptor is expressed mainly in primitive hematopoietic cells and regulates proliferation, survival, adhesion, and migration.1-6 Internal tandem duplication (ITD) mutations of the Flt3 gene that autoactivate the Flt3 tyrosine kinase are found in approximately 25% to 30% of patients with acute myeloid leukemia (AML) and strongly associate with poor prognosis.7-10 Ectopic ITD-Flt3 expression in IL-3–dependent mouse Ba/F3 or 32D hematopoietic cells results in growth factor–independent proliferation and produces acute leukemia when transplanted in mice.11,12 Studies indicate that ITD-Flt3 transforms mouse hematopoietic cell lines via activation of Stat5, Ras-MAPK, and PI3-kinase/Akt pathways7,11,13 and blocks differentiation by suppressing C/EBPα.14 Transformation of human TF-1 cells by ITD-Flt3 is associated with growth factor–independent proliferation and suppression of SHP-1, but not SHP-2.15 Other studies report that Jak2 and Stat3 are tyrosine phosphorylated by constitutively active Flt3.16 However, the mechanism whereby ITD-Flt3 increases aberrant hematopoietic cell proliferation is not well understood. Several Flt3 kinase inhibitors have been developed and evaluated in clinical trials with varied success, achieving more than 50% blast reduction in 12.5% to 81.3% of patients, with the duration of response ranging from 2 weeks to 5 months,17-19 underscoring the need for additional therapeutic approaches.
Survivin is a member of the inhibitor-of-apoptosis protein (IAP) family and implicated in regulation of apoptosis, cell division, and cell cycle control.20-23 Survivin is barely detectable in most normal adult tissues but overexpressed in almost all cancers21 and hematopoietic malignancies.24,25 Survivin is the fourth most highly expressed transcript in cancer26 and increases resistance to apoptotic stimuli, primarily through caspase-dependent mechanisms,27,28 although it can also block apoptosis independent of caspase inhibition. Survivin expression is generally associated with poor prognosis of patients with solid tumors29 and AML.25 In several systems, antagonizing Survivin induces apoptosis.20,29 Oncogenes such as Bcr-abl30 and activated H-Ras31 that are absent in normal tissues can significantly increase Survivin transcription and expression. In AML cells, Survivin expression is regulated by hematopoietic cytokines,24 however AML cells often coexpress both cytokines and their receptors, suggesting that Survivin may also be elevated by autocrine or paracrine mechanisms.32 Survivin expression is also regulated by Flt3 ligand, stem cell factor, and thrombopoietin in normal CD34+ cells,33 suggesting the presence of a functional link between cytokine receptor expression and Survivin, however, an association between ITD-Flt3 and Survivin has not been investigated.
In this study, we evaluated whether ITD-Flt3 regulates Survivin expression and whether Survivin is involved in the aberrant proliferation of hematopoietic progenitor cells (HPCs) and development of acute leukemia induced by ITD-Flt3. Our results demonstrate that Survivin lies downstream of ITD-Flt3 signaling and that Survivin deletion diminishes ITD-Flt3–mediated aberrant proliferation of HPCs with self-renewal capability, and that Survivin knockdown delays development of acute leukemia in mice that received a transplant of Ba/F3 cells expressing ITD-Flt3.
Methods
Antibodies and cytokines
Anti-FcγIII/II, PE-conjugated anti–mouse Ly-6G (Gr-1) and Ly-6C (RB6-8C5), CD45R/B220 (RA-6B2), CD3e (145-2C11), TER119 (TER-119) and CD11b (M1/70), APC-anti–mouse c-kit (2B8), rat IgG2b, biotin-conjugated anti–Sca-1 (E13-161.7), rat IgG2a, PE-conjugated annexin-V, PE-conjugated anti–active caspase-3 antibody, and streptavidin-PE-Cy7 were obtained from BD Biosciences.Antiactin antibody (I-19) was from Santa Cruz Biotechnology.Polyclonal anti-Survivin antibody (AF886), recombinant murine IL-3 (rmIL-3) and recombinant murine stem cell factor (rmSCF) were purchased from R&D Systems (Minneapolis, MN). Recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) was purchased from BioVision (Palo Alto, CA). Granulocyte colony-stimulating factor (G-CSF; filgrastim) was from Amgen (Thousand Oaks, CA). The AG1296 and SU5416 tyrosine kinase inhibitors were from BioMol Research Laboratories (Plymouth Meeting, PA) and Calbiochem (Gibbstown, NJ), respectively. The 4-hydroxy (4OH) tamoxifen and tamoxifen were from Sigma-Aldrich
Cell culture, plasmid transfection, and retrovirus transduction
Full-length wild-type human Flt3 cDNA was cloned in a MSCV-IRES-EGFP plasmid. The MSCV-IRES-EGFP plasmids containing ITD-Flt3 mutants (N51, N73, and N78) originally cloned from AML patients by Kelly et al34 and Ba/F3 cells expressing wild-type Flt3 or ITD-Flt3 (N51, N73, and N78 without GFP) were provided by Dr D. G. Gilliland (Harvard Medical School, Cambridge, MA).5,6,34 For Flt3 retroviral transduction, 24 μg purified wild-type and ITD-Flt3 (N51) in MSCV-IRES-EGFP or empty vector was transfected into Phoenix Eco cells35,36 using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Retrovirus transduction into mouse bone marrow cells was performed as described.36 After sequential infection, cells were sorted by fluorescence-activated cell sorting (FACS) and colony formation by 50 000 GFP+ cells in semisolid agar-McCoy culture with 30% heat-inactivated fetal bovine serum (HI-FBS; Hyclone Sterile Systems, Logan, UT) scored after 14 days. In some experiments, colonies were harvested from the primary plates and replated for secondary colony-forming unit (CFU). In replicate liquid cultures, cells were stained for Sca-1, c-kit, and lineage markers at the time of plating or at the end of the liquid culture. Primary AML cells were obtained with IRB approval of Indiana University School of Medicine.
Animals
Generation of mice with the Survivin gene flanked by loxP sites was reported elsewhere.37 The mice were bred with mice expressing the tamoxifen-inducible form of Cre (Cre-ER; Tg(cre/Esr1)5Amc; Jackson Laboratory)38 and offspring crossed to establish mice homozygous for the Survivin flox allele and harboring the Cre-ER transgene (Cre-ER Survivinflox/flox). Genotyping for Cre was performed by polymerase chain reaction (PCR) using the primers 5′-GGT TTC CCG CAG AAC CTG AAG-3′ and 5′-GCT AAG TGC CTT CTC TAC ACC-3′ with 30-second denaturation at 95°C, annealing at 55°C, and extension at 72°C (30 seconds each) for 40 cycles. Genotyping for the flox allele was determined using primers as previously described.37 All mice were housed in microisolators with continuous access to rodent chow and acidified water. The Indiana University School of Medicine Institutional Animal Care and Use Committee (IACUC) approved all experimental procedures.
Statistics
Data are expressed as mean plus or minus standard error (SEM). Statistical differences between single agents and control were determined using the 2-tailed Student t test in Microsoft Excel (Microsoft, Seattle, WA).
Results
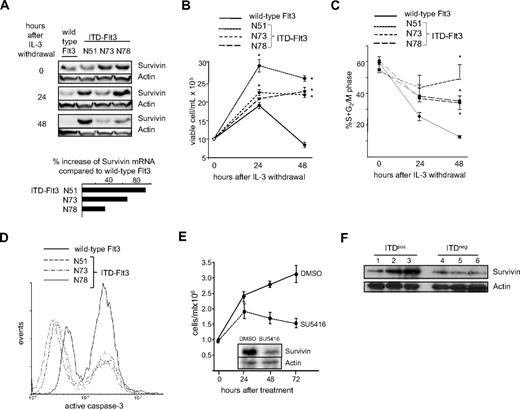
Expression of ITD-Flt3 mutations in Ba/F3 cells increases Survivin expression, enhances cell proliferation, and reduces active caspase-3
We reported that the combination of Flt3 ligand (FL), stem cell factor (SCF), and thrombopoietin (TPO) induces Survivin expression in human CD34+ cells,33 suggesting that Survivin lies downstream of Flt3 signaling. We therefore evaluated the effects of constitutive ITD-Flt3 signaling on Survivin expression. Survivin protein levels were comparable in Ba/F3 cells expressing ITD-Flt3 and wild-type Flt3 when maintained with maximal levels of IL-3, however upon IL-3 withdrawal, ITD-Flt3 prevented downmodulation of Survivin expression (Figure 1A). Survivin up-regulation by ITD-Flt3 was observed during G0/G1 phase of cell cycle, determined by dual staining for intracellular Survivin and DNA content (Table 1). Consistent with protein levels, quantitative reverse-transcription (QRT)–PCR detected higher Survivin mRNA in Ba/F3 cells transduced with ITD-Flt3 constructs, compared with cells transduced with wild-type Flt3 (Figure 1A insert). We next investigated whether an association between Survivin and ITD-Flt3 affected cell proliferation, cell cycle, and apoptosis. Ba/F3 cells expressing 3 different ITD-Flt3 mutants showed significantly enhanced IL-3–independent proliferation compared with wild-type Flt3, as previously reported (Figure 1B).34 In addition, the percentage of cells in S+G2/M phase of the cell cycle was significantly higher in ITD-Flt3–transduced cells, compared with wild-type Flt3 (Figure 1C). Analysis of active caspase-3 in Ba/F3 cells after IL-3 withdrawal identified low- and high-expressing populations, corresponding to viable cells and cells undergoing apoptosis, respectively (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Active caspase-3 expression was substantially reduced and the total proportion of cells undergoing apoptosis was lower in ITD-Flt3–expressing cells compared with cells expressing wild-type Flt3 (Figure 1D), consistent with the caspase inhibitory activity of Survivin.28
ITD-Flt3 mutations increase Survivin expression in Ba/F3 cells coincident with enhanced cell proliferation and reduction in active caspase-3. (A) Ba/F3 cells transduced with wild-type or ITD-Flt3 were cultured in RPMI-1640 with 1% HI-FBS in the absence of IL-3 for 24 and 48 hours. Survivin protein was determined by Western analysis. Representative data from 1 of 2 experiments are shown. The inset shows the percentage increase in Survivin mRNA expression in Ba/F3 cells ectopically expressing 3 different ITD-Flt3 constructs (N51, N73, and N78) compared with cells expressing wild-type Flt3 as determined by QRT-PCR. QRT-PCR was performed using Platinum SYBR Green qPCR SuperMix UDG. The primers for mouse Survivin were 5′-TGG CAG CTG TAC CTC AAG AA-3′ and 5′-AGC TGC TCA ATT GAC TGA CG-3′. The sequences for the mouse GAPDH primers were 5′-ATG GTG AAG GTC GGT GTG AAC G-3′ and 5′-GTT GTC ATG GAT GAC CTT GGC C-3′. (B) Proliferation of Ba/F3 cells expressing wild-type or ITD-Flt3 after IL-3 withdrawal. One million cells were seeded in RPMI-1640 plus 1% HI-FBS and total cell number was enumerated after 24 and 48 hours using trypan blue. Data shown are mean ± SEM for 1 of 3 experiments with identical results. *P < .05 compared with wild-type Flt3. (C) Percentage of Ba/F3 cells described in panel B in S+G2/M phase of the cell cycle. Cells were fixed in 1% paraformaldehyde and stained with 1 μg/mL propidium iodide. Cell cycle was analyzed by flow cytometry and ModFit software (Verity Software House, Topsham, ME). Data are mean ± SEM from 3 experiments. *P < .05 compared with wild-type Flt3. (D) Active caspase-3 in Ba/F3 cells expressing wild-type or ITD-Flt3 after IL-3 withdrawal for 48 hours. Cells were fixed in 1% paraformaldehyde and stained in 0.25% Triton X-100/1% BSA/PBS using PE conjugated anti–active caspase-3 antibody (BD Biosciences). Representative histogram for 1 of 2 experiments with identical results is shown. (E) Total cell proliferation and Survivin expression of ITD-Flt3+ human acute leukemia MV4-11 cells treated with the ITD-Flt3 inhibitor SU5416 for 72 hours. (F) Survivin expression in human primary ITD-Flt3+ or ITD-Flt3− AML cells. Lane 1: ITD-Flt3+ AML; normal cytogenetics. Lane 2: ITD-Flt3+ AML; normal cytogenetics. Lane 3: ITD-Flt3+ AML; normal cytogenetics. Lane 4: ITD-Flt3− AML; normal cytogenetics. Lane 5: ITD-Flt3− MLL (11q23). Lane 6: ITD-Flt3− AML; trisomy 13.
ITD-Flt3 mutations increase Survivin expression in Ba/F3 cells coincident with enhanced cell proliferation and reduction in active caspase-3. (A) Ba/F3 cells transduced with wild-type or ITD-Flt3 were cultured in RPMI-1640 with 1% HI-FBS in the absence of IL-3 for 24 and 48 hours. Survivin protein was determined by Western analysis. Representative data from 1 of 2 experiments are shown. The inset shows the percentage increase in Survivin mRNA expression in Ba/F3 cells ectopically expressing 3 different ITD-Flt3 constructs (N51, N73, and N78) compared with cells expressing wild-type Flt3 as determined by QRT-PCR. QRT-PCR was performed using Platinum SYBR Green qPCR SuperMix UDG. The primers for mouse Survivin were 5′-TGG CAG CTG TAC CTC AAG AA-3′ and 5′-AGC TGC TCA ATT GAC TGA CG-3′. The sequences for the mouse GAPDH primers were 5′-ATG GTG AAG GTC GGT GTG AAC G-3′ and 5′-GTT GTC ATG GAT GAC CTT GGC C-3′. (B) Proliferation of Ba/F3 cells expressing wild-type or ITD-Flt3 after IL-3 withdrawal. One million cells were seeded in RPMI-1640 plus 1% HI-FBS and total cell number was enumerated after 24 and 48 hours using trypan blue. Data shown are mean ± SEM for 1 of 3 experiments with identical results. *P < .05 compared with wild-type Flt3. (C) Percentage of Ba/F3 cells described in panel B in S+G2/M phase of the cell cycle. Cells were fixed in 1% paraformaldehyde and stained with 1 μg/mL propidium iodide. Cell cycle was analyzed by flow cytometry and ModFit software (Verity Software House, Topsham, ME). Data are mean ± SEM from 3 experiments. *P < .05 compared with wild-type Flt3. (D) Active caspase-3 in Ba/F3 cells expressing wild-type or ITD-Flt3 after IL-3 withdrawal for 48 hours. Cells were fixed in 1% paraformaldehyde and stained in 0.25% Triton X-100/1% BSA/PBS using PE conjugated anti–active caspase-3 antibody (BD Biosciences). Representative histogram for 1 of 2 experiments with identical results is shown. (E) Total cell proliferation and Survivin expression of ITD-Flt3+ human acute leukemia MV4-11 cells treated with the ITD-Flt3 inhibitor SU5416 for 72 hours. (F) Survivin expression in human primary ITD-Flt3+ or ITD-Flt3− AML cells. Lane 1: ITD-Flt3+ AML; normal cytogenetics. Lane 2: ITD-Flt3+ AML; normal cytogenetics. Lane 3: ITD-Flt3+ AML; normal cytogenetics. Lane 4: ITD-Flt3− AML; normal cytogenetics. Lane 5: ITD-Flt3− MLL (11q23). Lane 6: ITD-Flt3− AML; trisomy 13.
In human acute leukemia MV4-11 cells that express endogenous ITD-Flt3, treatment with SU5416, an inhibitor of ITD-Flt3 activity,39 reduced Survivin expression coincident with a reduction in total viable cells (Figure 1E). Similar results were obtained using an additional Flt3 inhibitor AG1296 (Not shown). In bone marrow samples from patients with AML, Survivin expression was generally higher in patients with ITD-Flt3+ AML, compared with samples from patients with ITD-Flt3− AML (Figure 1F).
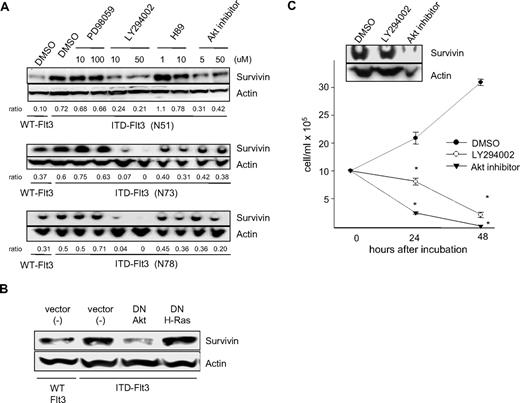
The PI3-kinase/Akt pathway is involved in ITD-Flt3–mediated up-regulation of Survivin
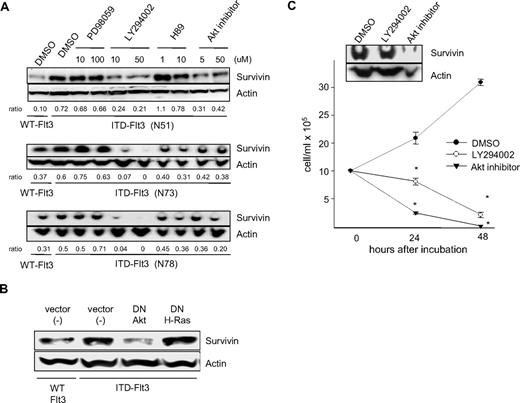
Studies suggest that ITD-Flt3 induces transformation of mouse hematopoietic cell lines via activation of Stat5, Ras-MAPK, and PI3-kinase/Akt pathways.11,13 We previously reported that Survivin expression is regulated by hematopoietic growth factors downstream of the MAPKp42/p44 and PI3-kinase pathways.36 To identify the signaling pathways involved in Survivin up-regulation by ITD-Flt3, Ba/F3 cells expressing 3 different ITD-Flt3 mutations were incubated with inhibitors for MAPKp42/p44 (PD98059), PI3-kinase (LY294002), protein kinase A (PKA; H89), and Akt (Akt inhibitor). Survivin protein expression was significantly reduced by the PI3-kinase inhibitor LY294002 and the Akt inhibitor. In contrast, the MAPK pathway inhibitor PD98059 had no effect on Survivin protein. The effect of the PKA inhibitor H89 varied among the cell lines, having no significant effect on Survivin protein in Ba/F3 cells expressing the N51 ITD mutant, but a marginal inhibitory effect in cells expressing the N73 or N78 ITD mutant (Figure 2A). To validate the specificity of Survivin inhibition by the PI3-kinase or Akt inhibitor, dominant negative (dn)–Akt and dn-H-Ras cDNAs were transfected into Ba/F3 cells expressing ITD-Flt3. Survivin protein in ITD-Flt3 Ba/F3 cells was significantly reduced by dn-Akt, but not dn-H-Ras (Figure 2B). Blocking PI3-kinase or Akt also reduced proliferation and Survivin expression in MV4-11 cells (Figure 2C). These results suggest that the PI3-kinase/Akt pathway is involved in Survivin up-regulation by ITD-Flt3 and is consistent with our previous results of hematopoietic growth factor–mediated regulation of Survivin expression through the PI3-kinase pathway.
Effects of selective signaling pathway inhibitors or dn- cDNAs on Survivin expression. (A) Ba/F3 cells expressing ITD-Flt3 (N51, N73, and N78) were incubated with 10 and 100 μM PD98059, 10 and 50 μM LY294002, 1 and 10 μM H89, 5 and 50 μM Akt inhibitor, or control DMSO for 24 hours. Ba/F3 cells expressing wild-type Flt3 were included as a control. Survivin expression was determined by Western analysis. Ratio of Survivin/actin relative to DMSO control in ITD-Flt3+ cells determined using densitometer analysis is shown beneath the blot. The selective pathway inhibitors PD-98059 (MAPKp42/44), LY-294002 (PI3-kinase), H89 (PKA), and Akt inhibitor were purchased from Wako Chemicals (Osaka, Japan). (B) dn-Akt or dn-H-Ras cDNAs were transfected into Ba/F3 cells expressing ITD-Flt3 (N51). Ba/F3 cells expressing wild-type Flt3 or ITD-Flt3 were also transfected with vector alone. dn-Akt in pcDNA3 was a kind gift from Drs R. A. Roth and H. Nakshatri (Stanford University School of Medicine, Stanford, CA, and Indiana University School of Medicine, respectively). Transfection of dn-Akt or dn-H-Ras cDNA into Ba/F3 cells expressing Flt3 were performed using a Nucleofector transfection kit (Amaxa, Gaithersburg, MD). Twenty-four hours after transfection, cells were subjected to Western analysis for Survivin. (C) MV4-11 cells were incubated with control DMSO, 50 μM LY290042, or 50 μM Akt inhibitor up to 72 hours in 10% HI-FBS plus RPMI-1640. Cell counts were enumerated by trypan blue exclusion in triplicate. Survivin expression was determined by Western analysis at 24 hours. *P < .05 compared with DMSO control. Representative data for 1 of 2 experiments are shown and are expressed as mean ± SEM.
Effects of selective signaling pathway inhibitors or dn- cDNAs on Survivin expression. (A) Ba/F3 cells expressing ITD-Flt3 (N51, N73, and N78) were incubated with 10 and 100 μM PD98059, 10 and 50 μM LY294002, 1 and 10 μM H89, 5 and 50 μM Akt inhibitor, or control DMSO for 24 hours. Ba/F3 cells expressing wild-type Flt3 were included as a control. Survivin expression was determined by Western analysis. Ratio of Survivin/actin relative to DMSO control in ITD-Flt3+ cells determined using densitometer analysis is shown beneath the blot. The selective pathway inhibitors PD-98059 (MAPKp42/44), LY-294002 (PI3-kinase), H89 (PKA), and Akt inhibitor were purchased from Wako Chemicals (Osaka, Japan). (B) dn-Akt or dn-H-Ras cDNAs were transfected into Ba/F3 cells expressing ITD-Flt3 (N51). Ba/F3 cells expressing wild-type Flt3 or ITD-Flt3 were also transfected with vector alone. dn-Akt in pcDNA3 was a kind gift from Drs R. A. Roth and H. Nakshatri (Stanford University School of Medicine, Stanford, CA, and Indiana University School of Medicine, respectively). Transfection of dn-Akt or dn-H-Ras cDNA into Ba/F3 cells expressing Flt3 were performed using a Nucleofector transfection kit (Amaxa, Gaithersburg, MD). Twenty-four hours after transfection, cells were subjected to Western analysis for Survivin. (C) MV4-11 cells were incubated with control DMSO, 50 μM LY290042, or 50 μM Akt inhibitor up to 72 hours in 10% HI-FBS plus RPMI-1640. Cell counts were enumerated by trypan blue exclusion in triplicate. Survivin expression was determined by Western analysis at 24 hours. *P < .05 compared with DMSO control. Representative data for 1 of 2 experiments are shown and are expressed as mean ± SEM.
Antagonizing Survivin reduces growth factor–independent HPC proliferation induced by ITD-Flt3
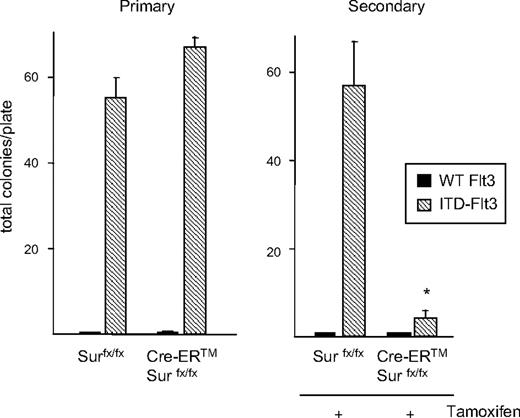
Primary HPCs require hematopoietic growth factors to proliferate, whereas ITD-Flt3–transformed hematopoietic cell lines demonstrate growth factor–independent growth.34 We evaluated whether Survivin plays a role in aberrant growth factor–independent primary HPC proliferation upon transformation by ITD-Flt3 (Figure 3). Overexpression of human wild-type Flt3 in CFU–granulocyte macrophages (GMs) from control Survivinflox/flox or Survivinflox/flox mice harboring a tamoxifen-inducible Cre recombinase (Cre-ER) did not produce growth factor–independent proliferation in vitro, whereas ectopic expression of ITD-Flt3 mutants resulted in significant growth of growth factor–independent CFU-GMs from both mice. Treatment with 4OH-tamoxifen during the culture to delete Survivin resulted in a significant reduction in growth factor–independent proliferation of CFU-GMs induced by ITD-Flt3, which was directly comparable with the degree of Survivin gene deletion observed. Tamoxifen treatment had no effect on growth factor–independent CFU-GM proliferation in littermate Survivinflox/flox ITD-Flt3 cells. These results suggest that Survivin is involved in the growth factor–independent proliferation of HPCs induced by ITD-Flt3.
Survivin mediates enhanced proliferation and growth factor–independent growth of CFU-GMs induced by ITD-Flt3. Bone marrow cells from littermate control Survivinflox/flox or Cre-ER Survivinflox/flox mice were transduced with wild-type Flt3, ITD-Flt3 (N51) in MSCV-IRES-EGFP vector, or vector alone. GFP+-transduced cells were FACS sorted and 50 000 GFP+ cells plated in 0.3% agar McCoy medium with 30% HI-FBS without growth factors in the presence or absence of 1 μM 4OH-tamoxifen. Colonies were scored on day 14. Data are expressed as mean ± SEM for 5 independent experiments. The bottom panel shows Survivin gene deletion induced by 4OH-tamoxifen in replicate liquid cultures incubated without cytokines.
Survivin mediates enhanced proliferation and growth factor–independent growth of CFU-GMs induced by ITD-Flt3. Bone marrow cells from littermate control Survivinflox/flox or Cre-ER Survivinflox/flox mice were transduced with wild-type Flt3, ITD-Flt3 (N51) in MSCV-IRES-EGFP vector, or vector alone. GFP+-transduced cells were FACS sorted and 50 000 GFP+ cells plated in 0.3% agar McCoy medium with 30% HI-FBS without growth factors in the presence or absence of 1 μM 4OH-tamoxifen. Colonies were scored on day 14. Data are expressed as mean ± SEM for 5 independent experiments. The bottom panel shows Survivin gene deletion induced by 4OH-tamoxifen in replicate liquid cultures incubated without cytokines.
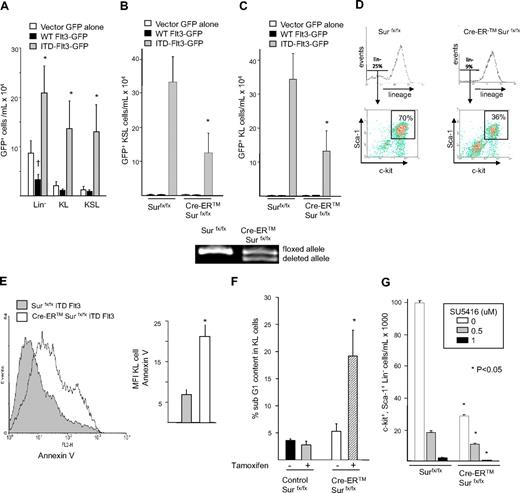
Ectopic ITD-Flt3 enhances expansion of primary KSL cells that is abrogated by Survivin deletion
ITD-Flt3 knock-in mouse models show expansion and enhanced cell cycle of c-kit+, Sca-1+, LinNeg (KSL) cells,40 the population of immunophenotypically defined cells containing primitive long-term repopulating cells. We evaluated whether retroviral transduction with ITD-Flt3 increases primary KSL cell expansion and whether this effect is mediated through Survivin. Consistent with genetic mouse models, retroviral transduction of primary mouse marrow cells with ITD-Flt3 significantly expanded the total number of KSL cells present at the end of the transduction procedure compared with transduction with wild-type Flt3 (Figure 4A). Similarly, ITD-Flt3 induced significant expansion of KL cells. Interestingly, at the end of retrovirus transduction, the majority of transduced KL cells also expressed Sca-1, further suggesting an effect on KSL cell expansion.
Expansion of primitive hematopoietic cells by ITD-Flt3 requires Survivin. (A) Two million bone marrow cells were infected with retrovirus containing wild-type (WT) human Flt3, ITD-Flt3 (N51) in MSCV-IRES-EGFP vector, or vector control for 2 days in the presence of 100 ng/mL rmSCF, rhTPO, and rhG-CSF. After infection, the medium was replaced with the same cytokine cocktail and incubated for an additional 3 days. Total cells were counted and stained to determine the proportion and the absolute number of GFP+ lineage LinNeg, c-kit+, LinNeg (KL) or c-kit+, Sca-1+, LinNeg (KSL) cells. Data are expressed as mean ± SEM from 16 independent experiments. *P < .05 compared with wild-type Flt3; †P < .05 compared with vector control. (B,C) Number of GFP+ KSL (B) and KL (C) cells transduced with vector control (EGFP+), wild-type (WT) Flt3, or ITD-Flt3 (N51) in cultures of littermate control Survivinflox/flox or Cre-ER Survivinflox/flox cells after culture for 2 weeks with 4OH-tamoxifen without hematopoietic growth factors. Data are the average of 5 experiments and expressed as mean ± SEM. *P < .05 compared with Survivinflox/flox. The bottom panel shows the gene deletion of Survivin allele by 4OH-tamoxifen. (D) Representative plots showing the proportion of KSL cells in Survivinflox/flox and Cre-ER Survivinflox/flox mouse bone marrow cells overexpressing ITD-Flt3 (N51) and cultured with 1 μM 4OH-tamoxifen for 2 weeks. The box represents c-kit+, Sca-1+ cells in the LinNeg fraction. (E) Annexin-V staining of ITD-Flt3 (N51)–transduced GFP+ KL cells, from cultures of Survivinflox/flox or Cre-ER Survivinflox/flox marrow cells after culture for 2 weeks with 4OH-tamoxifen without hematopoietic growth factors. The left panel shows representative data from 1 of 2 experiments. The mean ± SEM of annexin V fluorescence intensity (MFI) from 2 experiments is shown in the right panel. *P < .05 compared with Survivinflox/flox. (F) Sub G1 content of ITD-Flt3 (N51)–transduced KL cells from cultures of Survivinflox/flox or Cre-ER Survivinflox/flox cells after culture in the absence of growth factors with or without 4OH-tamoxifen for 14 days. Data are the mean ± SEM from 3 experiments. *P < .05 compared with Survivinflox/flox. (G) The effect of Survivin deletion and culture with increasing concentration of SU5416 on KSL cell number. Marrow cells from control Survivinflox/flox or Cre-ER Survivinflox/flox mice were retrovirally transduced with ITD-Flt3 (N51) and cultured with 1 μM 4OH-tamoxifen and escalating doses of SU5416 in the absence of hematopoietic growth factors for 14 days. Data represent 1 of the 2 experiments with similar results and are expressed as mean ± SEM. *P < .05 compared with Survivinflox/flox.
Expansion of primitive hematopoietic cells by ITD-Flt3 requires Survivin. (A) Two million bone marrow cells were infected with retrovirus containing wild-type (WT) human Flt3, ITD-Flt3 (N51) in MSCV-IRES-EGFP vector, or vector control for 2 days in the presence of 100 ng/mL rmSCF, rhTPO, and rhG-CSF. After infection, the medium was replaced with the same cytokine cocktail and incubated for an additional 3 days. Total cells were counted and stained to determine the proportion and the absolute number of GFP+ lineage LinNeg, c-kit+, LinNeg (KL) or c-kit+, Sca-1+, LinNeg (KSL) cells. Data are expressed as mean ± SEM from 16 independent experiments. *P < .05 compared with wild-type Flt3; †P < .05 compared with vector control. (B,C) Number of GFP+ KSL (B) and KL (C) cells transduced with vector control (EGFP+), wild-type (WT) Flt3, or ITD-Flt3 (N51) in cultures of littermate control Survivinflox/flox or Cre-ER Survivinflox/flox cells after culture for 2 weeks with 4OH-tamoxifen without hematopoietic growth factors. Data are the average of 5 experiments and expressed as mean ± SEM. *P < .05 compared with Survivinflox/flox. The bottom panel shows the gene deletion of Survivin allele by 4OH-tamoxifen. (D) Representative plots showing the proportion of KSL cells in Survivinflox/flox and Cre-ER Survivinflox/flox mouse bone marrow cells overexpressing ITD-Flt3 (N51) and cultured with 1 μM 4OH-tamoxifen for 2 weeks. The box represents c-kit+, Sca-1+ cells in the LinNeg fraction. (E) Annexin-V staining of ITD-Flt3 (N51)–transduced GFP+ KL cells, from cultures of Survivinflox/flox or Cre-ER Survivinflox/flox marrow cells after culture for 2 weeks with 4OH-tamoxifen without hematopoietic growth factors. The left panel shows representative data from 1 of 2 experiments. The mean ± SEM of annexin V fluorescence intensity (MFI) from 2 experiments is shown in the right panel. *P < .05 compared with Survivinflox/flox. (F) Sub G1 content of ITD-Flt3 (N51)–transduced KL cells from cultures of Survivinflox/flox or Cre-ER Survivinflox/flox cells after culture in the absence of growth factors with or without 4OH-tamoxifen for 14 days. Data are the mean ± SEM from 3 experiments. *P < .05 compared with Survivinflox/flox. (G) The effect of Survivin deletion and culture with increasing concentration of SU5416 on KSL cell number. Marrow cells from control Survivinflox/flox or Cre-ER Survivinflox/flox mice were retrovirally transduced with ITD-Flt3 (N51) and cultured with 1 μM 4OH-tamoxifen and escalating doses of SU5416 in the absence of hematopoietic growth factors for 14 days. Data represent 1 of the 2 experiments with similar results and are expressed as mean ± SEM. *P < .05 compared with Survivinflox/flox.
To evaluate the effect of Survivin deletion on ITD-Flt3–mediated KSL cell expansion, ITD-Flt3–transduced GFP+ cells from Cre-ER Survivinflox/flox and littermate control Survivinflox/flox mice were cultured ex vivo in the absence of growth factors and in the presence of 1 μM 4OH-tamoxifen. ITD-Flt3 dramatically increased total cell expansion and the proportion and absolute number of KSL (Figure 4B) and KL cells (Figure 4C) compared with either vector alone or wild-type Flt3 in control Survivinflox/flox cells. In contrast, expansion of total GFP+ ITD-Flt3–transduced cells and the absolute number of ITD-Flt3+ KL and KSL cells from Cre-ER Survivinflox/flox cells was significantly reduced upon Survivin gene deletion (Figure 4B-C). Analysis of representative KSL gates indicated that ITD-Flt3+ LinNeg cells comprised 25% of total control Survivinflox/flox marrow cells, 70% of which were double positive for Sca-1 and c-kit. In contrast, only 9% of whole ITD-Flt3+ Cre-ER Survivinflox/flox cells were LinNeg, of which only 36% were Sca-1+, c-kit+ cells after tamoxifen treatment (Figure 4D). The effect of Survivin gene deletion was also associated with increased apoptosis. After tamoxifen treatment, the mean fluorescent intensity (MFI) of annexin-V (Figure 4E) and the proportion of GFP+ Cre-ER Survivinflox/flox KL cells with hypodiploid DNA (Figure 4F) were significantly higher compared with Survivinflox/flox cells. In addition, a small but significant increase in KL cells in G0/G1 phase was observed after Survivin deletion (58% ± 1% in control versus 69% ± 2% in Survivin-deleted cells: P < .05).
Because Survivin is also regulated by other signaling pathways, in addition to ITD-Flt3, we evaluated whether the combination of Survivin reduction plus inhibition of ITD-Flt3 activity would provide an additive or greater inhibition of ITD-Flt3–mediated KSL cell expansion. ITD-Flt3–mediated KSL expansion was significantly decreased upon Survivin deletion as expected and was further enhanced by the Flt3 inhibitor SU5416 in a dose-dependent manner (Figure 4G). Conditioned medium from ITD-Flt3–expressing cells did not support proliferation of KSL or KL cells from control mice (data not shown), making it unlikely that a paracrine mechanism is responsible for the autonomous cell growth of KSL cells. Although enhanced production and survival of KSL cells expressing ITD-Flt3 were maintained for more than 5 weeks in the absence of growth factors ex vivo, ITD-Flt3–transduced cells were not immortalized and cultures were subsequently exhausted.
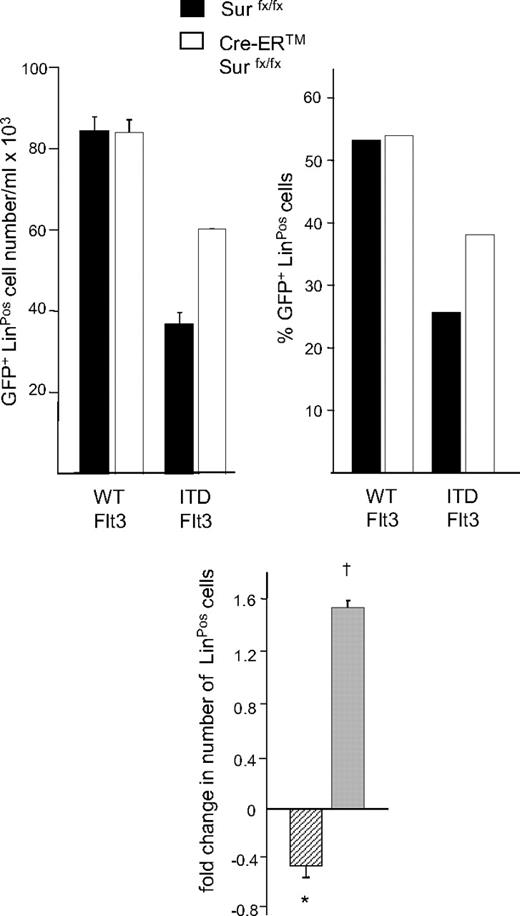
ITD-Flt3 inhibits expansion of LinPos cells
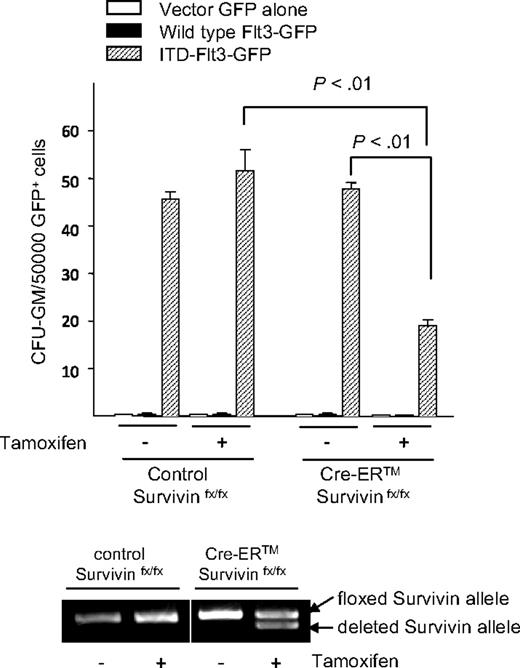
ITD-Flt3 blocks differentiation of hematopoietic cells14 and we have previously shown that Survivin plays a role in hematopoietic stem cell proliferation.41 We therefore investigated whether Survivin is involved in the inhibition of lineage commitment induced by ITD-Flt3. Primary bone marrow cells from Survivinflox/flox and Cre-ER Survivinflox/flox mice were transduced with wild-type Flt3 or ITD-Flt3 and GFP+ LinNeg cells incubated with GM-CSF and SCF. In cultures of ITD-Flt3–transduced control Survivinflox/flox cells, the absolute number and proportion of LinPos cells was significantly lower compared with wild-type Flt3 after 14 days (Figure 5). In 2 independent experiments, LinPos cells were reduced by 0.43-fold plus or minus 0.1-fold (P < .05; Figure 5 inset, hatched bar). In contrast, Survivin deletion resulted in a significant increase in the proportion and absolute number of LinPos cells in cultures of ITD-Flt3–transduced Cre-ER Survivinflox/flox cells (Figure 5). In 2 experiments, a 1.5-fold plus or minus 0.1-fold increase in total LinPos cells was observed (Figure 5 inset, gray bar; P < .05) compared with control Survivinflox/flox cells expressing ITD-Flt3. These results indicate that the inhibition of expansion or differentiation of lineage-negative cells by ITD-Flt3 is mediated through Survivin.
Effect of ITD-Flt3 and Survivin on expansion/differentiation of LinNeg cells. Absolute number and proportion of LinPos cells in cultures of LinNeg cells derived from Survivinflox/flox and Cre-ER Survivinflox/flox cells transduced with wild-type (WT) or ITD-Flt3 (N51) are shown. After retrovirus transduction, LinPos cells were depleted by magnetic-activated cell sorting (MACS) column (Miltenyi Biotec) and LinNeg cells were cultured with 50 ng/mL rmSCF and 10 ng/mL rmGM-CSF for 14 days in the presence of 1 μM 4OH-tamoxifen to induce Survivin deletion. The number and the proportion of GFP+ LinPos cells were determined by cell enumeration and flow cytometry. The inset shows fold change in LinPos cells in cultures of lineage-depleted control marrow cells expressing ITD-Flt3 versus wild-type Flt3 (▫) and fold change in LinPos cells in cultures of lineage-depleted Cre-ER Survivinflox/flox cells expressing ITD-Flt3 after Survivin deletion versus control Survivinflox/flox cells expressing ITD-Flt3 ( ). Data are mean ± SEM of 2 experiments. *P < .05 compared with wild type Flt3; †P < .05 compared with Survivinflox/flox.
). Data are mean ± SEM of 2 experiments. *P < .05 compared with wild type Flt3; †P < .05 compared with Survivinflox/flox.
Effect of ITD-Flt3 and Survivin on expansion/differentiation of LinNeg cells. Absolute number and proportion of LinPos cells in cultures of LinNeg cells derived from Survivinflox/flox and Cre-ER Survivinflox/flox cells transduced with wild-type (WT) or ITD-Flt3 (N51) are shown. After retrovirus transduction, LinPos cells were depleted by magnetic-activated cell sorting (MACS) column (Miltenyi Biotec) and LinNeg cells were cultured with 50 ng/mL rmSCF and 10 ng/mL rmGM-CSF for 14 days in the presence of 1 μM 4OH-tamoxifen to induce Survivin deletion. The number and the proportion of GFP+ LinPos cells were determined by cell enumeration and flow cytometry. The inset shows fold change in LinPos cells in cultures of lineage-depleted control marrow cells expressing ITD-Flt3 versus wild-type Flt3 (▫) and fold change in LinPos cells in cultures of lineage-depleted Cre-ER Survivinflox/flox cells expressing ITD-Flt3 after Survivin deletion versus control Survivinflox/flox cells expressing ITD-Flt3 ( ). Data are mean ± SEM of 2 experiments. *P < .05 compared with wild type Flt3; †P < .05 compared with Survivinflox/flox.
). Data are mean ± SEM of 2 experiments. *P < .05 compared with wild type Flt3; †P < .05 compared with Survivinflox/flox.
Survivin deletion abrogates ITD-Flt3–mediated secondary colony formation
Marrow HPCs from Flt3ITD/wild knock-in mice demonstrate serial replating capability in the presence of growth factors,42 which is considered a surrogate measure of self-renewal. To determine whether Survivin plays a role in HPC self-renewal induced by ITD-Flt3, we evaluated secondary colony formation of wild-type Flt3 and ITD-Flt300transduced bone marrow cells from Survivinflox/flox and Cre-ER Survivinflox/flox mice. In the absence of growth factors, no primary or secondary colonies were observed in wild-type Flt3-transduced hematopoietic stem and progenitor cells (HSPCs). As described earlier, ITD-Flt3–transduced Survivinflox/flox and Cre-ER Survivinflox/flox marrow cells showed substantial colony formation in the absence of growth factors in the primary plates (Figure 6 left panel). Replating colonies of ITD-Flt3–transduced control Survivinflox/flox HPCs produced substantial numbers of colonies in secondary plates after 14 days of incubation in the absence of any growth factors, and secondary colony formation was almost totally eliminated upon Survivin deletion (Figure 6 right panel). Replating of ITD-Flt3 secondary colonies did not produce tertiary colonies regardless of the presence or absence of Survivin (data not shown). These results suggest that ectopic ITD-Flt3 supports growth factor–independent proliferation of HPCs with self-renewal capability, which is dependent on Survivin expression.
Survivin deletion abrogates secondary colony formation induced by ITD-Flt3. Fifty thousand GFP+ bone marrow cells transduced with wild-type (WT) Flt3 or ITD-Flt3 (N51) were cultured in soft agar with 30% FBS for 2 weeks in the absence of hematopoietic growth factors. Total colonies were counted and colonies were harvested, mechanically dispersed, and plated in secondary soft agar cultures with 30% FBS but without growth factors in the presence of 1 μM 4OH-tamoxifen. Secondary colonies were scored on day 14. Data are expressed as mean ± SEM of 3 independent experiments. *P < .05 compared with Survivinflox/flox.
Survivin deletion abrogates secondary colony formation induced by ITD-Flt3. Fifty thousand GFP+ bone marrow cells transduced with wild-type (WT) Flt3 or ITD-Flt3 (N51) were cultured in soft agar with 30% FBS for 2 weeks in the absence of hematopoietic growth factors. Total colonies were counted and colonies were harvested, mechanically dispersed, and plated in secondary soft agar cultures with 30% FBS but without growth factors in the presence of 1 μM 4OH-tamoxifen. Secondary colonies were scored on day 14. Data are expressed as mean ± SEM of 3 independent experiments. *P < .05 compared with Survivinflox/flox.
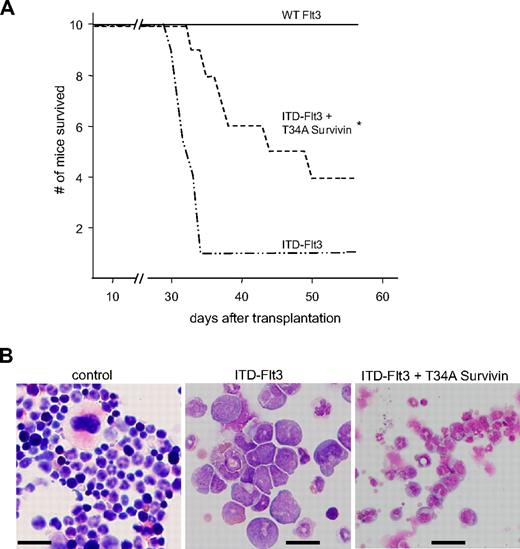
dn-Survivin delays development of acute leukemia in mice that received a transplant of Ba/F3 cells expressing ITD-Flt3
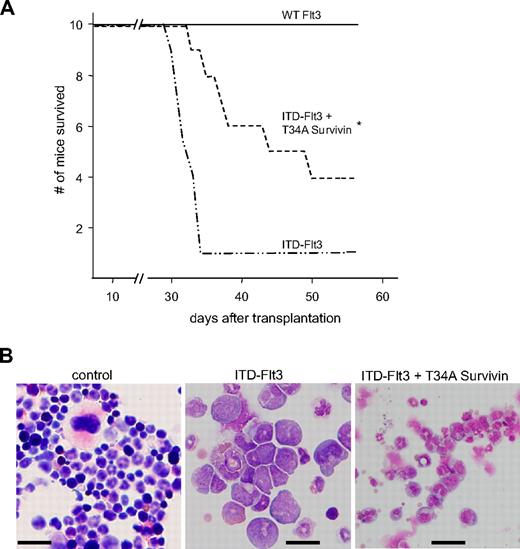
ITD-Flt3–expressing Ba/F3 cells produce acute leukemia in mice.12 Our current findings that Ba/F3 cells expressing ITD-Flt3 but not wild-type Flt3 proliferate in the absence of IL-3, and our previous findings that Survivin knockdown in primary HPCs or Ba/F3 cells by overexpression of a dn-T34A Survivin construct inhibits Survivin-mediated enhanced survival upon IL-3 withdrawal,31,35 suggest that antagonizing Survivin in ITD-Flt3–expressing Ba/F3 cells could reduce or block development of acute leukemia. Transplantation of cells overexpressing ITD-Flt3 in mice produced a lethal acute leukemia within 5 weeks as reported.12 Most of the cells observed in the recipient marrow transplanted with Ba/F3 cells expressing ITD-Flt3 were myeloid blasts, suggesting that mice developed acute myeloid leukemia (Figure 7B). However the presence of lymphoid-like blasts was also noted, consistent with detection of transplantable T- and B-lymphoid disease seen in the transgenic ITD-Flt3 mouse model.43 In contrast, significantly improved survival of mice that received a transplant of Ba/F3 cells overexpressing ITD-Flt3 plus a dn-T34A Survivin was observed (Figure 7A). In addition, in mice that received a transplant of ITD-Flt3 plus dn-T34A-Survivin–transduced Ba/F3 cells, nuclear fragmentation and maturation of bone marrow myeloid cells were significantly improved, albeit not totally normalized, as a consequence of Survivin knockdown (Figure 7B).
Effect of dn-Survivin on development of acute leukemia induced by ITD-Flt3. (A) Ba/F3 cells expressing MSCV wild-type or ITD-Flt3 were retrovirally transduced with MIEG3 vector or dn-mouse T34A Survivin in MIEG3, as described.31 Three million Ba/F3 cells expressing wild-type or ITD-Flt3 plus MIEG3 vector or dn-T34A Survivin were injected into BALB/c mice (Harlan) as described.12 Kaplan-Meier survival curve for BALB/c mice that received a transplant of 3 × 106 Ba/F3 cells expressing human wild-type (WT) Flt3, ITD-Flt3 (N73), or ITD-Flt3 plus dn-T34A mouse Survivin. *P < .05 compared with ITD-Flt3 alone. N = 10 mice/group. (B) Representative histology of bone marrow from recipients of control, ITD-Flt3 cells, or ITD-Flt3 plus dn-T34A-Survivin–transduced Ba/F3 cells. Infiltration of blasts and lack of megakaryocytes are observed in the bone marrow of recipients of transplanted ITD-Flt3–transduced Ba/F3 cells, whereas the nuclear fragmentation and cell maturation of the myeloid cells were significantly improved in the recipient mice of transplanted Ba/F3 cells overexpressing ITD-Flt3 plus dn-T34A Survivin. The image was taken using a BX60 microscope UPlanF1, DP 71 camera, and DP Controller software (Olympus, Melville, NY). Original magnification: 40 ×. Size: 100 × 100 μm2. Scale: 20 μm for all photographs.Numeric aperture 0.75. Total magnification is expressed as the objective times the eyepiece: 40 (objective)×10 (eyepiece) =400 (total). Staining: Giemsa.
Effect of dn-Survivin on development of acute leukemia induced by ITD-Flt3. (A) Ba/F3 cells expressing MSCV wild-type or ITD-Flt3 were retrovirally transduced with MIEG3 vector or dn-mouse T34A Survivin in MIEG3, as described.31 Three million Ba/F3 cells expressing wild-type or ITD-Flt3 plus MIEG3 vector or dn-T34A Survivin were injected into BALB/c mice (Harlan) as described.12 Kaplan-Meier survival curve for BALB/c mice that received a transplant of 3 × 106 Ba/F3 cells expressing human wild-type (WT) Flt3, ITD-Flt3 (N73), or ITD-Flt3 plus dn-T34A mouse Survivin. *P < .05 compared with ITD-Flt3 alone. N = 10 mice/group. (B) Representative histology of bone marrow from recipients of control, ITD-Flt3 cells, or ITD-Flt3 plus dn-T34A-Survivin–transduced Ba/F3 cells. Infiltration of blasts and lack of megakaryocytes are observed in the bone marrow of recipients of transplanted ITD-Flt3–transduced Ba/F3 cells, whereas the nuclear fragmentation and cell maturation of the myeloid cells were significantly improved in the recipient mice of transplanted Ba/F3 cells overexpressing ITD-Flt3 plus dn-T34A Survivin. The image was taken using a BX60 microscope UPlanF1, DP 71 camera, and DP Controller software (Olympus, Melville, NY). Original magnification: 40 ×. Size: 100 × 100 μm2. Scale: 20 μm for all photographs.Numeric aperture 0.75. Total magnification is expressed as the objective times the eyepiece: 40 (objective)×10 (eyepiece) =400 (total). Staining: Giemsa.
Disrupting Survivin reduces proliferation of normal HSPCs
To further evaluate the physiologic role of Survivin in proliferation of normal HSPCs, we examined growth factor–dependent in vitro proliferation of HSPCs and quantitated HSPC number in vivo using Cre-ER Survivinflox/flox mice. Culture of bone marrow cells from control Survivinflox/flox mice with 1 μM 4OH-tamoxifen had no effect on CFU-GM proliferation stimulated by SCF plus GM-CSF. However, culture of bone marrow cells from Cre-ER Survivinflox/flow mice with 4OH-tamoxifen resulted in a significant reduction in CFU-GM proliferation (supplemental Figure 2A). Ex vivo expansion of KSL cells from Survivinflox/flox mice cultured with FL, Tpo, and SCF was unaffected by culture in the presence of 1 μM 4OH-tamoxifen for 5 days, whereas culture with tamoxifen produced a dramatic reduction in the number of KSL marrow cells from Cre-ER Survivinflox/flox (supplemental Figure 2B). In addition, a significant reduction in total marrow KSL cells and common myeloid progenitor cells (CMPs) was observed in Cre-ER Survivinflox/flox mice treated with tamoxifen in vivo, with no effect observed in tamoxifen-treated Survivinflox/flox (supplemental Figure 3).
Discussion
Our studies suggest that aberrant proliferation of primary hematopoietic cells induced by ITD-Flt3 and the development of acute leukemia in mice that received a transplant of Ba/F3 cells expressing ITD-Flt3 are mediated at least in part through the inhibitor-of-apoptosis protein Survivin and this can be substantially blocked by Survivin disruption. Overexpression of wild-type Flt3 does not induce growth factor–independent proliferation of primary marrow CFU-GMs, and KL and KSL cells, however, ectopic expression of ITD-Flt3 significantly expands KSL cells and allows for growth factor–independent KSL cell and CFU-GM proliferation. Moreover, HPCs expressing ITD-Flt3 but not ectopic wild-type Flt3 form secondary colonies in the absence of growth factors, a measure of self-renewal potential. Survivin deletion significantly reduces growth factor–independent HPC proliferation as well as secondary colony formation generated by ITD-Flt3, coincident with induction of apoptosis and G0/G1 arrest. In mice, transplantation of Ba/F3 cells expressing ITD-Flt3 produced a lethal acute leukemia, within 5 weeks, however coexpression of a dominant-negative Survivin construct reduced nuclear fragmentation, improved myeloid maturation, and significantly prolonged survival.
Survivin up-regulation by ITD-Flt3 is consistent with regulation of Survivin expression by hematopoietic cytokines in normal human CD34+ cells33,36 or in AML cells.24 Survivin expression is generally associated with cell cycle progression but is regulated in all phases of cell cycle in CD34+ cells.33,36 We observed Survivin up-regulation by ITD-Flt3 even during G0/G1 phase of cell cycle, suggesting that in transduced Ba/F3 cells, like normal CD34+ cells, up-regulation of Survivin is not solely a consequence of cell cycle progression. We observed an inverse correlation between Survivin expression and active caspase-3 expression in Ba/F3 cells, which is consistent with the role of Survivin as an endogenous caspase inhibitor.27,28 Up-regulation of Survivin was associated with proliferation and cell cycle progression was associated with decreased apoptosis in Ba/F3 cells expressing ITD-Flt3 compared with wild-type Flt3, whereas down-regulation of Survivin was associated with reduced proliferation, cell cycle arrest, and apoptosis. In human MV4-11 cells that express endogenous ITD-Flt3, treatment with the ITD-Flt3 inhibitors SU5416 or AG1296 reduced cell proliferation coincident with Survivin down-regulation. These results suggest that ITD-Flt3 up-regulates Survivin expression independent of hematopoietic growth factors and its expression is associated with autonomous cell proliferation and enhanced survival.
In primary AML samples, although Survivin expression was higher in ITD-Flt3+ AML than in ITD-Flt3− samples, one ITD+ AML showed Survivin levels more comparable with ITD-Flt3− samples (Figure 1F). Similarly, Survivin protein was lower in the ITD-Flt3+ MV4-11 cell line compared with RS4;11 cells that express endogenous wild-type Flt3 (supplemental Figure 4). The fluctuation of Survivin in primary AML cells or AML cell lines is most likely due to the presence of multiple different abnormalities that can affect Survivin level. In fact, RS4;11 cells proliferate in a growth factor–independent manner,44 suggesting the presence of other oncogenic molecules that impact growth factor independence and Survivin up-regulation. It is also known that AML cells can express wild-type Flt3 and can secrete Flt3 in an autocrine fashion, which can up-regulate Survivin.32
Analysis of signaling pathways using selective pathway inhibitors or dominant-negative constructs indicated that ITD-Flt3 up-regulates Survivin via PI3-kinase/Akt, which is consistent with previous observations that ITD-Flt3 signals through this pathway7,11 and that hematopoietic cytokines up-regulate Survivin expression through PI3-kinase.36 Survivin expression downstream of ITD-Flt3 is not regulated by Ras-MAPK, whereas Survivin is regulated by hematopoietic growth factors via the MAPKp42/44 pathway in normal human CD34+ cells.36 This differential regulation may represent a potential therapeutic strategy. Lack of an inhibitory effect on ITD-Flt3–mediated up-regulation of Survivin by the MAPK inhibitor or dn-Ras cDNA may be due to low-level activation of Ras by ITD-Flt3.11
Survivin expression is regulated by several transcriptional regulators, including Stat3,45 Rb,46 p53,47 and NFkB.48 Stat3 is tyrosine phosphorylated by constitutively active Flt3 in Ba/F3 cells16 and by Flt3 ligand in primary mouse dendritic cells,49 suggesting that ITD-Flt3–mediated Survivin up-regulation by ITD-Flt3 may be mediated by Stat3. It is well established that ITD-Flt3 transmits signals through Stat5,11,13 and Patch analysis demonstrates potential Stat5a and Stat5b binding sites on both human and mouse Survivin promoters, suggesting that Stat5a or Stat5b may be involved in ITD-Flt3–mediated Survivin up-regulation. Although there are several potential PU.1 and C/EBPα binding sites in the Survivin promoter, these transcription factors are downmodulated by ITD-Flt3,14 and therefore unlikely to be involved in Survivin up-regulation. We recently found that ITD-Flt3 increases CREB and c-myc mRNA by 2-fold, and c-jun by 1.6-fold, in Ba/F3 cells compared with wild-type Flt3 (S.F. and M.A., unpublished observations, December 2008). The Survivin promoter contains potential binding sites for CREB, c-myc, and c-jun, implicating these transcription factors in Survivin up-regulation.
The finding that ITD-Flt3 KSL cells did not proliferate in the absence of growth factors for more than 5 weeks suggests that they are not fully transformed and also explains lack of tertiary colony formation despite secondary colony formation, which is in good agreement with the fact that ITD-Flt3 requires additional mutation(s) to manifest an acute leukemia in mouse models.50 However, alternatively, our retrovirus system might have failed to infect long-term repopulating stem cells in vitro. Our finding of significant expansion of ITD-Flt3–transduced KSL cells in the absence of growth factors is consistent with the expansion of KSL cells seen in the ITD-Flt3 knock-in model.40,42 Although ITD-Flt3 increases Survivin expression and induces growth factor–independent proliferation in primary mouse marrow cells, overexpression of Survivin alone failed to allow growth factor–independent proliferation, suggesting that Survivin is required but not sufficient for ITD-Flt3 signaling. Culture of ITD-Flt3–transduced lineage-depleted cells with growth factors resulted in significant reduction of LinPos cells (Figure 5), suggesting that ITD-Flt3 facilitates expansion of primitive cells or inhibits lineage differentiation. Importantly, these effects are significantly reduced when Survivin is deleted, suggesting that Survivin plays a critical role in the expansion of KSL cells and/or the block in differentiation induced by ITD-Flt3. Moreover, Survivin deletion enhanced the antiproliferative effect of the Flt3 inhibitor SU5416, suggesting that simultaneous inhibition of Survivin and ITD-Flt3 may represent an additional therapeutic strategy for hematologic malignancies harboring ITD-Flt3 mutations. Although Survivin gene deletion efficiency of KSL cells was incomplete in the tamoxifen-conditional Survivin mouse model, the extent of gene deletion and reduction of CFU and KSL cell number induced by tamoxifen were comparable (Figures 3 and 4B,C).
Retroviral transduction34 and transgenic43 and knock-in42 mouse models of ITD-Flt3 mutations produce a myeloproliferative disease (MPD). In the knock-in model, MPD was transplantable to secondary recipients using Flt3ITD/ITD bone marrow cells, indicating a cell-autonomous effect.40 In the transgenic mice model, MPD was not transplantable, however the clonal B- or T-lymphoid disease also seen in these mice could be transplanted.43 These findings suggest that primary transformed ITD-Flt3 stem cells can self-renew, which is consistent with the fact that ITD-Flt3 is found in leukemia stem cells in patients.51 In heterozygous knock-in mice, secondary and tertiary Flt3wt/ITD CFU-GMs are observed in the presence of SCF plus IL-3,42 which is a surrogate for HPC self-renewal. In contrast, HPCs from Flt3ITD/ITD knock-in mice did not show serial replating activity.40 Our data using retroviral overexpression indicate that ITD-Flt3–transduced HPCs demonstrated growth factor–independent secondary colony-forming capability, although tertiary colonies did not grow. Variability in replating phenotype is likely a consequence of gene copy with the retrovirus-mediated overexpressing system generating multiple copies of ITD-Flt3 mRNA, which enhances the effect of ITD-Flt3, in contrast to the knock-in system that has 1 or 2 copies of target gene. It has been shown that transplantation of Ba/F3 cells expressing ITD-Flt3 induces lethal acute leukemia in BALB/c mice.12 Our data clearly show that antagonizing Survivin in this model improved myeloid differentiation and prolongs survival and are in good agreement with findings that Survivin deletion significantly decreases growth factor–independent proliferation of KSL cells. These data provide a strong rationale for the potential therapeutic manipulation of Survivin for the treatment of hematopoietic malignancies expressing ITD-Flt3.
Although Survivin regulates aberrant proliferation of HPCs induced by ITD-Flt3, our data indicate that it also plays a physiologic role in regulating HSPC proliferation (supplemental Figures S2–3). Disrupting Survivin is effective in inhibiting tumor growth in vivo without overt toxicity in several preclinical models, albeit with limited systemic exposure.29 However, because Survivin is expressed and regulated in normal CD34+ cells33 and antagonizing Survivin reduces normal HSPCs both in vitro and in vivo, it is likely that Survivin deletion will affect both normal and ITD-Flt3–transformed HSPCs in patients. This indicates that identification of specific mechanisms and signaling molecules downstream of ITD-Flt3/Survivin axis in ITD-Flt3–transformed HSPCs that are distinct from normal HSPCs is crucial and likely will be more selective/specific than disrupting Survivin itself.
In summary, we show that ITD-Flt3 mutants regulate Survivin expression via the PI3-kinase/Akt pathway. When cultured in the absence of growth factors, ITD-Flt3 dramatically increases the proportion and absolute number of primary mouse marrow KSL cells, which are significantly reduced by Survivin deletion. Furthermore, Survivin deletion dramatically diminishes the secondary replating potential of ITD-Flt3–expressing cells, a measure of self-renewal. Finally, antagonizing Survivin significantly prolongs survival of mice that received a transplant of ITD-Flt3–expressing Ba/F3 cells. These results strongly suggest that antagonizing Survivin may provide therapeutic benefit in patients with acute leukemias expressing ITD-Flt3. Continued investigation of mechanisms differentially regulating Survivin expression and function in transformed and normal HSPCs will facilitate identification of crucial differences in Survivin behavior that can be used to develop innovative strategies for selectively antagonizing Survivin.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Huimin Bian and Midori Furui for technical assistance and Suzan Rice for FACS cell sorting.
This work was supported by a Biomedical Research grant from Indiana University School of Medicine; a Research Support Funds grant from Indiana University and Purdue University, Indianapolis; Research Support funds from Mochida Memorial Foundation for Medical and Pharmaceutical Research, Sankyo Biomedical Research Foundation, and Mitsubishi Pharma Research Foundation; Japan Leukemia Research Fund; an AstraZeneca Research grant; Grant-in-Aid for Scientific Research (B) (20390298) from Japan Society for the Promotion of Science (S.F.); and US Public Health Service grants HL069669 and HL079654 from the National Institutes of Health (NIH, Bethesda, MD; L.M.P.).
National Institutes of Health
Authorship
Contribution: S.F. directed the project, designed and performed experiments, analyzed the data, and wrote the paper; P.S. performed some experiments; A.M. participated in experimental design, performing experiments, and interpretation; M.A. performed part of the experiments; E.M.C. developed and provided the conditional Survivin knockout mice and participated in interpretation of data; H.S.B. obtained characterized provided primary AML samples and participated in experimental design and interpretation; S.Y. and X.-Y.F. participated in designing the experiments; and L.M.P. participated in designing the experiments and analyzing the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Seiji Fukuda, Department of Pediatrics, Shimane University School of Medicine, 89-1 Enya-Cho, Izumo, Shimane 693-8501, Japan; e-mail: sfukuda@med.shimane-u.ac.jp.







 ). Data are mean ± SEM of 2 experiments. *P < .05 compared with wild type Flt3; †P < .05 compared with Survivinflox/flox.
). Data are mean ± SEM of 2 experiments. *P < .05 compared with wild type Flt3; †P < .05 compared with Survivinflox/flox.