Abstract
The function of von Willebrand factor (VWF) is regulated by proteolysis, which limits its multimeric size and ability to tether platelets. The importance of ADAMTS13 metalloprotease in VWF regulation is demonstrated by the association between severe deficiency of ADAMTS13 and thrombotic thrombocytopenic purpura (TTP). However, ADAMTS13 activity levels do not always correlate with the clinical course of TTP, suggesting that other proteases could be important in regulating VWF. We identified 4 leukocyte proteases that cleave the synthetic VWF substrate FRETS-VWF73 and multimeric VWF. Elastase and proteinase 3 (PR3) cleave multimeric VWF and FRETS-VWF73 at the V1607-T1608 peptide bond; cathepsin G and matrix metalloprotease 9 cleave VWF substrates at the Y1605-M1606 and M1606-V1607 bonds, respectively. Isolated intact human neutrophils cleave FRETS-VWF73 at the V1607-T1608 peptide bond, suggesting that elastase or PR3 expressed on leukocyte surfaces might cleave VWF. In the presence of normal or ADAMTS13-deficient plasma, cleavage of FRETS-VWF73 by resting neutrophils is abolished. However, activated neutrophils retain proteolytic activity toward FRETS-VWF73 in the presence of plasma. Although the in vivo relevance remains to be established, these studies suggest the existence of a “hot spot” of VWF proteolysis in the VWF A2 domain, and support the possibility that activated leukocytes may participate in the proteolytic regulation of VWF.
Introduction
The hemostatic activity of von Willebrand factor (VWF) is regulated in blood by the metalloprotease ADAMTS13, which cleaves VWF in the A2 domain.1 The importance of VWF regulation by ADAMTS13 is demonstrated by the close association between severe deficiency of ADAMTS13 activity and thrombotic thrombocytopenic purpura (TTP).2,3 However, the association between ADAMTS13 activity and TTP is imperfect. Patients with inhibitor-mediated ADAMTS13 deficiency can achieve clinical remission despite persistent severe deficiency of ADAMTS13 activity,2,4 and not all patients with congenital deficiency of ADAMTS13 develop TTP.5 Moreover, VWF proteolysis can be paradoxically increased in TTP patients during acute episodes.6 These observations suggest the existence of other important disease-modifying factors in TTP, in addition to ADAMTS13.
Disease-modifying factors in TTP may include other VWF-cleaving proteases. Before the discovery of ADAMTS13, the proteases calpain, neutrophil elastase, and cathepsin G were known to cleave VWF.7-12 However, the exact cleavage sites were not determined, and the physiologic relevance of these proteases was unknown. It was known that normal plasma contained proteolytic fragments of VWF having masses of approximately 176 kDa and 140 kDa. The primary cleavage site that gave rise to these fragments was identified as the Y1605-M1606 peptide bond.13 In studies seeking to identify the responsible protease, Tsai et al8,9,14 observed that neutrophil proteases cleaved high-molecular-weight VWF into a series of multimers indistinguishable from those found in normal plasma. By contrast, Berkowitz et al10 concluded that VWF cleavage fragments generated by neutrophil elastase were different from the 176-kDa and 140-kDa fragments found in plasma. The role of leukocyte proteases in VWF regulation remained unresolved, and was later overshadowed by the discovery of ADAMTS13.
In this study, we demonstrate that under denaturing and fluid shear stress conditions multiple leukocyte proteases cleave VWF predominantly in the central A2 domain. We also show that activated neutrophils, but not normal neutrophils, retain VWF cleaving activity in the presence of plasma inhibitors, suggesting that leukocyte proteases may regulate VWF function under physiologic conditions.
Methods
Patients and plasma
Blood samples were obtained from volunteer subjects after informed consent, in accordance with the Declaration of Helsinki, sanctioned by the institutional human research subject committees of the BloodCenter of Wisconsin and the University of Iowa. Plasma samples were anticoagulated with sodium citrate and were stored at −80°C until use. Plasma samples included one patient who developed TTP in adulthood and suffered numerous relapses over a period of years. Plasma samples obtained from this patient at the time of a relapse were severely deficient in ADAMTS13 activity, and exhibited inhibitory activity in mixing studies.
Mouse plasma
Plasma samples were obtained by cardiac puncture in 10% acid citrate dextrose from wild-type C57BL/6 mice and mice with a targeted deletion of the Adamts13 gene as a kind gift of Dr David Motto at Carver College of Medicine, University of Iowa.15
Isolation of human neutrophils
Neutrophils were prepared from heparin anticoagulated blood samples obtained from healthy volunteers. Samples were subjected to sedimentation in 3% dextran followed by Ficoll Hypaque gradient centrifugation (40 minutes at room temperature at 410g). Remaining red blood cells were lysed by addition of sterile water, and the neutrophil cell pellet was washed and resuspended in Hanks balanced salt solution (HBSS). Neutrophil concentrations were determined by hemocytometer counting. Cells were stained with Wright stain and a differential count was performed. Cell preparations were more than 95% neutrophils. Cell preparations were kept on ice until use. To stimulate neutrophils, 100 mg/mL phorbol 12 myristate 13-acetate (PMA; Sigma) was incubated with cells for 30 minutes at 37°C before mixing with plasma samples or FRETS-VWF73 reagents.
Leukocyte proteases and ADAMTS13
Human neutrophil elastase, proteinase 3 (PR3), cathepsin G, and matrix metalloproteinase 9 (MMP9) were purchased from Sigma-Aldrich. MMP9 was activated before use by incubation for 4 hours at 37°C in 50 mM Tris buffer, pH 7.4, containing 1 mM 4 aminophenyl mercuric acetate (APMA) according to the manufacturer's recommendations. Recombinant human ADAMTS13 was expressed in stably transfected HEK293 cells and purified by Q-fast ion exchange chromatography, Ni-NTA affinity chromatography, and gel filtration according to published methods.16,17
Preparation of recombinant FRETS-VWF73
Escherichia coli expressing a VWF73 peptide (D1596-R1668) tagged with 6xHis was a kind gift from Dr John Owen at Department of Medicine, Wake Forest University (Winston-Salem, NC). The amino acid residues Q1599 and N1610 of the peptide were replaced by a cysteine residue, which was then labeled with 5′-fluorescein-maleimide. Because of the close proximity, the 2 fluorescent dyes are quenched.18 When any peptidyl bond between the 2 fluorescent dyes is cleaved, fluorescence is generated. The E coli expressing the VWF73 peptide were expanded at 37°C with LB broth and induced with IPTG at 30°C for 4 hours. Cell lysates were prepared and recombinant VWF73 was purified to homogeneity (8 kDa) with Ni-NTA affinity chromatography after being labeled with fluorescein-5-maleimide according to previously described methods.19 This recombinant fluorescein-labeled VWF73 was designated as rFRETS-VWF73.
Commercial FRETS-VWF73 (Peptide International) was also used in some experiments. Commercial FRETS-VWF73 is a chemically synthesized peptide,20 containing amino acid residues D1595-R1668, in which residues Q1599 and N1610 are replaced by A2pr (Nma) and A2pr (Dnp), respectively. Similar to rFRETS-VWF73, when any peptidyl bond between the fluorochrome and the quencher is cleaved, fluorescence is generated.
Cleavage of FRETS-VWF73 substrates by leukocyte proteases
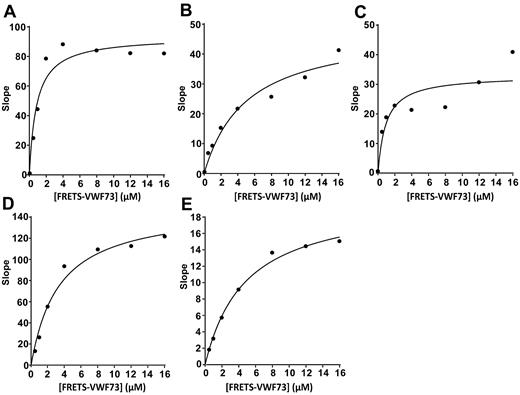
The proteolytic activity of leukocyte proteases was determined using rFRETS-VWF73 and commercial FRETS-VWF73. The rFRETS-VWF73 was used for kinetic assays because of its greater solubility at high concentrations. For kinetic determinations, leukocyte elastase (10 nM), cathepsin G (15 nM), MMP9 (10 nM), PR3 (10 nM), and rADAMTS13 (10 nM) were incubated with increasing concentrations (0-16 μM) of rFRETS-VWF73 in 5 mM Bis-Tris, pH 6.0, 25 mM CaCl2, 0.005% Tween-20. The total volume for each assay was 200 μL. The assay was performed in duplicate and repeated at least twice. Proteolysis of rFRETS-VWF73 was monitored every 5 minutes for 60 minutes to obtain the initial rate of fluorescent generation (Vmax) at the excitation 485 nm and the emission 530 nm on a Vector 3 fluorescent microtiter reader (Perkin Elmer). The values of slope were plotted against the substrate concentrations [S] and fitted into a Michaelis-Menten equation to determine the catalytic constant (kcat) and Michaelis constant (km) using a SigmaPlot software (Systat Software).
The proteolytic activity of unstimulated or stimulated neutrophils was determined using commercial FRETS-VWF73. In these experiments, cells at a final concentration of 105 cells/mL in HBSS or 5 mM Bis-Tris, 25 mM CaCl2, Tween-20 were added to the FRETS-VWF73 peptide (final concentration, 2 μM) in a total volume of 200 μL with 5 mM Bis-Tris buffer, 25 mM CaCl2, 0.0005% Tween-20, pH 6.0. Cleavage of FRETS-VWF73 was monitored every 5 minutes for 60 minutes using a SpectraMAx M5 fluorescent plate reader (Molecular Devices) with the excitation at 340 nm and the emission at 450 nm. The relative activity determined from the initial rate of fluorescent generation is reported. No effect of the buffer type was observed on cleavage of commercial FRETS-VWF73 by the neutrophils or leukocyte proteases.
Matrix assisted laser desorption ionization–time of flight mass spectrometry
The cleavage products of commercial FRETS-VWF73 were analyzed by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (Bruker BiFlex3). Samples were desalted using a ZipTip C18 column (Millipore) before analysis. The masses of the major FRETS-VWF73 peaks generated by each enzyme source were compared with calculated masses of carboxyl-terminal fragments of FRETS-VWF73 to identify matches between the observed peak size and the corresponding fragment size.
Cleavage of multimeric VWF under denaturing conditions
The cleavage of multimeric VWF by leukocyte proteases was analyzed in a modified version of the assay described by Furlan et al21 and Raife et al.22 Purified VWF (Humate P; ZLB Behring GmbH) at a final concentration of 3 U/mL was incubated with enzymes for various times at 37°C in 5 mM Tris buffer, pH 8.0, with or without 1.5 mM urea. Final enzyme concentrations in the reaction were as follows: elastase 138 nM, PR3 172 nM, and cathepsin G 8.6 nM. VWF and proteolytic products were reduced by heating with SDS/mercaptoethanol, electrophoresed into 5% polyacrylamide gel, and stained with Coomassie blue.
To identify proteolytic fragments as VWF, bands were excised from gels, treated with trypsin, and analyzed by mass spectrometry. Amino terminal protein sequencing was performed on excised cleavage fragments of VWF of approximately 170 to 180 kDa after electrophoresis in 5% acrylamide gel, transfer to a PVDF membrane, and staining with Coomassie blue. Sequencing was performed by the Protein Facility at Iowa State University using a Perkin Elmer 494 sequencer after the method of Edman.
Cleavage of multimeric VWF under fluid shear stress
Purified plasma VWF (37.5 μg/mL) was mixed with ADAMTS13 (as a control) or leukocyte proteases at concentrations ranging from 0 nM to 250 nM in 50 mM HEPES buffer containing 1 mg/mL bovine serum albumin (BSA), 150 mM NaCl, and 5 mM CaCl2 in a 0.2 mL thin-walled polymerase chain reaction tube with dome caps (Fisher Scientific). The reaction mixture (total volume 20 μL) was incubated at 25°C for 15 minutes and then subjected to vortexing at a rotational rate of 2500 rpm for 5 minutes using a minivortexer (Fisher Scientific).17 The reaction was quenched by addition of 1× SDS–polyacrylamide gel sample buffer (Bio-Rad) without β-mercaptoethanol and heated at 100°C for 5 minutes. The protein was fractionated on 5% SDS–polyacrylamide gel at 120 V for 150 minutes and transferred onto nitrocellulose membrane (0.4 μm pore size) with 25 mM Tris-HCl, pH 8.3, 192 mM glycine, 10% (vol/vol) methanol at 110 mA for 60 minutes. VWF and its cleavage products on the membrane were detected by incubation with rabbit anti-VWF IgG (Dako) diluted with TBS containing 1% casein (1:5000), followed by incubation with infrared fluorescent labeled anti–rabbit IgG (1:10 000; Li-COR Biosciences). The fluorescent signal was determined by an Odyssey imaging system and converted into gray image as previously described.23
Results
Leukocyte proteases cleaved FRETS-VWF73
To determine whether leukocyte proteases cleave VWF near the ADAMTS13 cleavage site, the major neutrophil proteases neutrophil elastase, PR3, cathepsin G, and MMP9 were analyzed for their ability to cleave 2 types of FRETS-VWF73 substrates. We showed that all 4 enzymes cleaved FRETS-VWF73 with cleaving efficiencies (ie, kcat/km) similar to or greater than ADAMTS13 (Figure 1 and Table 1). MMP9 cleaved rFRETS-VWF73 with a relative cleaving efficiency of 0.42, similar to ADAMTS13 (0.76; Table 1). Elastase, cathepsin G, and PR3 cleaved rFRETS-VWF73 with cleaving efficiencies of 1.7, 6.6, and 4.7, respectively (Table 1). These results suggest that the leukocyte proteases cleaved VWF within the amino acid region (APNLVYMVTGN) between Q1599 and P1611 in the VWF-A2 domain.
Kinetic cleavage of rFRETS-VWF73 by leukocyte proteases. Leukocyte elastase, 10 nM (A), cathepsin G, 15 nM (B), MMP9, 10 nM (C), PR3, 10 nM (D), and recombinant ADAMTS13, 10 nM (E) were incubated with increasing concentrations (0-16 μM) of rFRETS-VWF73 peptide in 5 mM Bis-Tris, pH 6.0, 25 mM CaCl2, 0.005% Tween-20. The proteolytic cleavage was monitored by the initial rate of fluorescent generation (vmax) at the excitation 485 nm and the emission 530 nm on a Vector 3 fluorescent microtiter reader. The data (slope vs substrate concentrations) were fitted into a Michaelis-Menten equation to determine the catalytic constant (kcat) and Michaelis constant (km) using SigmaPlot software. The curves represent mean values of 2 independent experiments at the same concentration of for each enzyme. Slope refers to the change in fluorescence units per second.
Kinetic cleavage of rFRETS-VWF73 by leukocyte proteases. Leukocyte elastase, 10 nM (A), cathepsin G, 15 nM (B), MMP9, 10 nM (C), PR3, 10 nM (D), and recombinant ADAMTS13, 10 nM (E) were incubated with increasing concentrations (0-16 μM) of rFRETS-VWF73 peptide in 5 mM Bis-Tris, pH 6.0, 25 mM CaCl2, 0.005% Tween-20. The proteolytic cleavage was monitored by the initial rate of fluorescent generation (vmax) at the excitation 485 nm and the emission 530 nm on a Vector 3 fluorescent microtiter reader. The data (slope vs substrate concentrations) were fitted into a Michaelis-Menten equation to determine the catalytic constant (kcat) and Michaelis constant (km) using SigmaPlot software. The curves represent mean values of 2 independent experiments at the same concentration of for each enzyme. Slope refers to the change in fluorescence units per second.
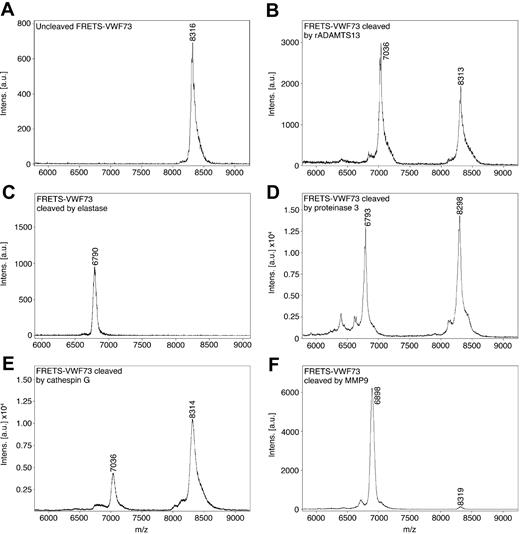
To identify the exact peptide bonds in the VWF-A2 domain cleaved by leukocyte proteases, samples of commercial FRETS-VWF73 cleaved by the leukocyte proteases were analyzed by mass spectrometry. The molecular mass of the major cleavage fragment generated by each enzyme was matched to the corresponding calculated mass of a carboxyl-terminal fragment of FRETS-VWF73. Uncleaved FRETS-VWF73 had a molecular mass of 8316 Da (margin of error = 0.2%; Figure 2A). Cleavage of FRETS-VWF73 with recombinant ADAMTS13 yielded a major fragment with a mass of 7036 Da, consistent with a carboxyl terminal fragment cleaved at the bond corresponding to Y1605-M1606 (Figure 2B). Cleavage of FRETS-VWF73 with elastase yielded a major cleavage fragment with a mass of 6790 Da, consistent with cleavage at the V1607-T1608 peptide bond (Figure 2C). PR3 cleavage of FRETS-VWF73 yielded a major fragment with a mass of 6793 Da (Figure 2D), also consistent with cleavage at the V1607-T1608 peptide bond. Cathepsin G generated a major fragment of 7036 Da, consistent with cleavage at the same Y1605-M1606 peptide bond cleaved by ADAMTS13 (Figure 2E). MMP9 yielded a major fragment having a mass of 6898 Da, consistent with cleavage at the M1606-V1607 peptide bond (Figure 2F).
Mass spectrometry analysis of FRETS-VWF73 cleaved by leukocyte proteases. Commercial FRETS-VWF73 peptide was incubated without (A), and with recombinant ADAMTS13 (rADAMTS13; B), neutrophil elastase (C), PR3 (D), cathepsin G (E), and MMP9 (F) at 30°C for 60 minutes. The digested materials were analyzed by MALDI-TOF mass spectrometry. The masses of the major peaks were compared with the calculated molecular weights of carboxyl terminus fragments of FRETS-VWF73. The mass of the major peak arising from rADAMTS13 cleavage and cathepsin G cleavage corresponds to cleavage at the M1605-Y1606 peptide bond. The major peaks resulting from neutrophil elastase cleavage and PR3 cleavage correspond to cleavage at the V1607-T1608 peptide bond. The major peak resulting from cleavage by MMP9 corresponds with cleavage at M1606-V1607. The mass spectrometry margin of error is 0.2%.
Mass spectrometry analysis of FRETS-VWF73 cleaved by leukocyte proteases. Commercial FRETS-VWF73 peptide was incubated without (A), and with recombinant ADAMTS13 (rADAMTS13; B), neutrophil elastase (C), PR3 (D), cathepsin G (E), and MMP9 (F) at 30°C for 60 minutes. The digested materials were analyzed by MALDI-TOF mass spectrometry. The masses of the major peaks were compared with the calculated molecular weights of carboxyl terminus fragments of FRETS-VWF73. The mass of the major peak arising from rADAMTS13 cleavage and cathepsin G cleavage corresponds to cleavage at the M1605-Y1606 peptide bond. The major peaks resulting from neutrophil elastase cleavage and PR3 cleavage correspond to cleavage at the V1607-T1608 peptide bond. The major peak resulting from cleavage by MMP9 corresponds with cleavage at M1606-V1607. The mass spectrometry margin of error is 0.2%.
Leukocyte proteases cleaved multimeric VWF under denaturing conditions
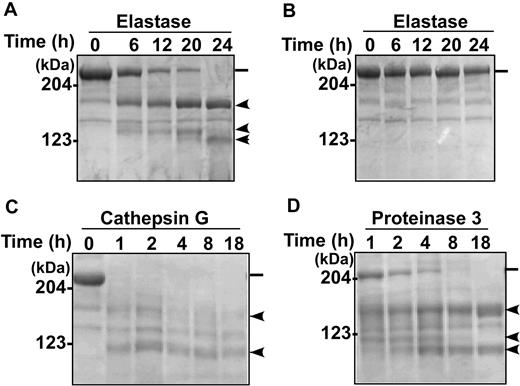
Proteolytic cleavage of multimeric VWF by ADAMTS13 requires denaturation of the substrate by chemical denaturants such as urea or guanidine HCl21 or high shear stress.24 To determine whether leukocyte proteases cleaved multimeric VWF, plasma-derived VWF was subjected to denaturization with 1.5 M urea and then incubated with proteases for various times. The cleavage products were analyzed by SDS–polyacrylamide gel electrophoresis with Coomassie blue staining. The results showed a time-dependent degradation of the denatured VWF substrate, generating a major cleavage fragment of approximately 175 kDa molecular mass and other smaller bands (Figure 3). Proteolysis by elastase (Figure 3A), cathepsin G (Figure 3C), and PR3 (Figure 3D) was clearly detectable. Proteolysis of nondenatured VWF by elastase was negligible (Figure 3B). Cleavage of multimeric VWF by MMP9 was not detected in the presence of various concentrations of urea, guanidine HCl, or sodium dodecyl sulfate (not shown), suggesting that this enzyme is inactivated by chemical denaturants.
Cleavage of denatured VWF substrate by leukocyte proteases. Purified VWF (Humate P) was incubated with elastase (138 nM; A-B), cathepsin G (8.6 nM; C), and PR3 (172 nM; D) for the times indicated in each panel at 37°C in 0.005 M Tris buffer, pH 8.0, with (A,C-D) or without (B) 1.5 M urea. Proteolytic cleavage products were analyzed by 5% SDS–polyacrylamide gel electrophoresis under denaturing and reducing conditions, and stained with Coomassie blue. The positions of molecular mass standards are marked. ━ indicates the intact VWF polypeptide (250 kDa), whereas the  indicates the cleavage products of various sizes under denaturing and reducing conditions.
indicates the cleavage products of various sizes under denaturing and reducing conditions.
Cleavage of denatured VWF substrate by leukocyte proteases. Purified VWF (Humate P) was incubated with elastase (138 nM; A-B), cathepsin G (8.6 nM; C), and PR3 (172 nM; D) for the times indicated in each panel at 37°C in 0.005 M Tris buffer, pH 8.0, with (A,C-D) or without (B) 1.5 M urea. Proteolytic cleavage products were analyzed by 5% SDS–polyacrylamide gel electrophoresis under denaturing and reducing conditions, and stained with Coomassie blue. The positions of molecular mass standards are marked. ━ indicates the intact VWF polypeptide (250 kDa), whereas the  indicates the cleavage products of various sizes under denaturing and reducing conditions.
indicates the cleavage products of various sizes under denaturing and reducing conditions.
Leukocyte proteases cleaved multimeric VWF under fluid shear stress
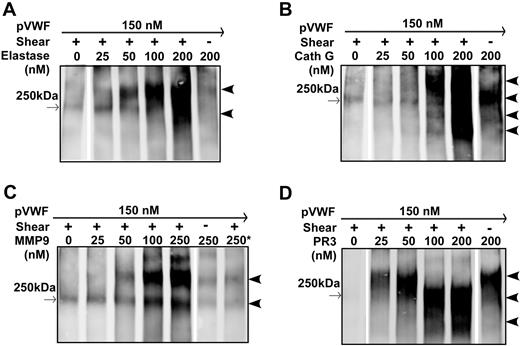
To determine whether leukocyte proteases cleave multimeric VWF under more physiologically relevant conditions, multimeric VWF was subjected to cleavage by leukocyte proteases in the presence of a buffer with physiologic pH (7.5) and salt concentration (150 mM NaCl), using vortexing-induced fluid shear stress. These conditions were previously found to be sufficient for cleavage of multimeric VWF by ADAMTS13 in the presence of factor VIII.23 Within 5 minutes of incubation under constant vortexing at 2500 rpm, elastase, cathepsin G, MMP9, and PR3 all cleaved plasma-derived VWF in a concentration-dependent manner (Figure 4). Similar to the results observed under chemical denaturing conditions, under shear stress denaturing but nonreducing conditions, the major cleavage products from elastase, cathepsin G, MMP9, and PR3 were approximately 350 kDa (dimers of two 175-kDa C-terminal fragments; Figure 4). There were also other bands smaller than 250 kDa at high concentrations of elastase (Figure 4A), cathepsin G (Figure 4B), and PR3 (Figure 4C), suggesting the existence of other cleavage sites in full-length VWF for elastase, cathepsin G, and PR3. In the absence of fluid shear stress or chemical denaturants, after 30 minutes of incubation in 50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mg/mL BSA cathepsin G (Figure 4B) and PR3 (Figure 4D cleaved multimeric VWF to generate fragments similar to that seen under fluid shear stress. No cleavage was observed when native VWF was incubated with activated MMP9 under the same conditions (Figure 4C). These data suggest that cathepsin G and PR3 may attack other surface-exposed peptidyl bonds on globular VWF, whereas MMP-9 cleaves the specific Met-Val bond at the central A2 domain of VWF.
Cleavage of multimeric VWF by leukocyte proteases under fluid shear stress. Purified plasma VWF (pVWF; 37.5 μg/mL) was incubated for 15 minutes and subjected to no vortexing (shear −) or constant vortexing at 2500 rpm for 5 minutes (shear +) in the presence of various concentrations of elastase (A), cathepsin G (B), MMP9 (C), or PR3 (D) as indicated in each panel. The reaction was performed in a polymerase chain reaction tube with a total volume of 20 μL in buffer containing 50 mM HEPES, pH 7.5, 150 mM NaCl, 5 mM CaCl2, and 1 mg/mL BSA. The proteolytic cleavage products were determined by 5% SDS–polyacrylamide gel under denaturing and nonreducing conditions. Western blotting was performed with anti-VWF IgG and infrared fluorescent dye labeled anti–rabbit IgG as described in “Cleavage of multimeric VWF under fluid shear stress.” → (left borders) indicate positions of a 250-kDa molecular weight marker.  (right borders) indicates positions of cleavage products. The asterisk in panel C denotes 20 mM EDTA added to the reaction mixture.
(right borders) indicates positions of cleavage products. The asterisk in panel C denotes 20 mM EDTA added to the reaction mixture.
Cleavage of multimeric VWF by leukocyte proteases under fluid shear stress. Purified plasma VWF (pVWF; 37.5 μg/mL) was incubated for 15 minutes and subjected to no vortexing (shear −) or constant vortexing at 2500 rpm for 5 minutes (shear +) in the presence of various concentrations of elastase (A), cathepsin G (B), MMP9 (C), or PR3 (D) as indicated in each panel. The reaction was performed in a polymerase chain reaction tube with a total volume of 20 μL in buffer containing 50 mM HEPES, pH 7.5, 150 mM NaCl, 5 mM CaCl2, and 1 mg/mL BSA. The proteolytic cleavage products were determined by 5% SDS–polyacrylamide gel under denaturing and nonreducing conditions. Western blotting was performed with anti-VWF IgG and infrared fluorescent dye labeled anti–rabbit IgG as described in “Cleavage of multimeric VWF under fluid shear stress.” → (left borders) indicate positions of a 250-kDa molecular weight marker.  (right borders) indicates positions of cleavage products. The asterisk in panel C denotes 20 mM EDTA added to the reaction mixture.
(right borders) indicates positions of cleavage products. The asterisk in panel C denotes 20 mM EDTA added to the reaction mixture.
Major cleavage sites of leukocyte proteases were at or near the ADAMTS13 cleavage site
The major 175-kDa electrophoretic bands observed under reducing conditions resulted from proteolytic cleavage of multimeric VWF by elastase, cathepsin G, and PR3 were excised from gels and analyzed by Edman amino terminal sequencing. The bands generated by elastase and PR3 both revealed the amino acid sequence TGNPASDE, confirming the V1607-T1608 peptide bond as the elastase and PR3 cleavage site in the A2 domain (Table 2). Cathepsin G cleavage yielded a band having the N-terminus sequence MVTGNPAS, confirming cleavage at Y1605-M1606 peptide bond (Table 2). Because of a loss of activity of MMP9 under chemical denaturing conditions and a limited cleavage product generated (within 5 minutes) under vortexing induced shear stress, the MMP9 cleavage site in multimeric VWF was not determined by the Edman amino terminal sequencing method.
Membrane-bound leukocyte proteases cleaved FRETS-VWF73
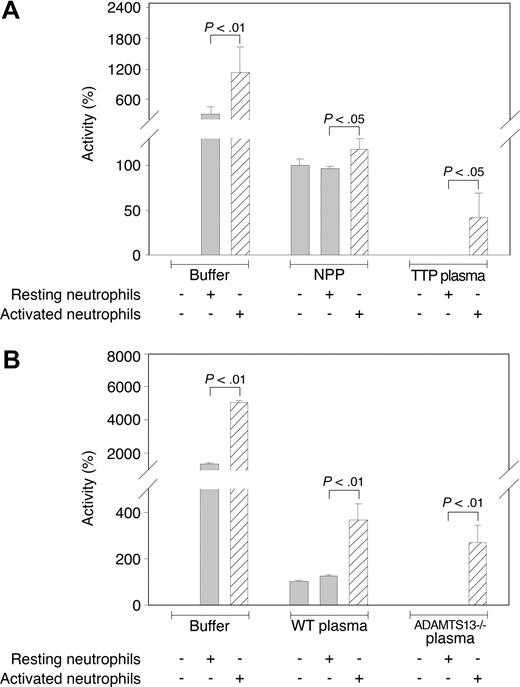
The observation that leukocyte proteases cleaved denatured VWF and VWF under fluid shear stress near the ADAMTS13 cleavage site suggested the possibility that intact leukocytes could cleave VWF near the ADAMTS13-scissile bond. We therefore analyzed the cleavage of commercial FRETS-VWF73 by isolated neutrophils in buffer alone and in the presence of human or mouse plasma. Isolated resting neutrophils from volunteer subjects readily cleaved FRETS-VWF73 (Figure 5), whereas the supernatants had negligible proteolytic activity (not shown). At a final concentration of 105/mL, resting neutrophils cleaved FRETS-VWF73 at a rate approximately 4-fold greater than the standard concentration of normal pooled plasma (2.5% by volume; Figure 5A). However, when resting neutrophils were combined with normal pooled plasma, FRETS-VWF73 cleavage was reduced to the level of the normal pooled plasma. But when resting neutrophils were combined with ADAMTS13-deficient plasma from TTP patients, no FRETS-VWF73–cleaving activity was observed (Figure 5), suggesting that TTP plasma inhibits leukocyte protease activity independent of ADAMTS13.
Cleavage of FRETS-VWF73 by membrane-bound proteases on neutrophils. (A) The (commercial) FRETS-VWF-73–cleaving activity of 2.5% normal pooled plasma (NPP) or 2.5% ADAMTS13-deficient TTP plasma in the presence of resting or PMA-activated neutrophils (105 cells/mL). Resting or activated cells were resuspended in 2.5% plasma mixed in reaction buffer. Activities are represented relative to 2.5% NPP alone, which is designated as 100% activity. (B) The cleaving activity of 2.5% wild-type (WT) or 2.5% ADAMTS13-deficient (ADAMTS13−/−) mouse plasma in the presence of resting or PMA-activated neutrophils (105 cells/mL). Activities are represented relative to WT mouse plasma, which is designated as 100% activity. Results shown in panels A and B are mean values (± SD) from 2 combined representative experiments (N = 4). ▨ represents activated neutrophils. The P values were determined by Student t tests.
Cleavage of FRETS-VWF73 by membrane-bound proteases on neutrophils. (A) The (commercial) FRETS-VWF-73–cleaving activity of 2.5% normal pooled plasma (NPP) or 2.5% ADAMTS13-deficient TTP plasma in the presence of resting or PMA-activated neutrophils (105 cells/mL). Resting or activated cells were resuspended in 2.5% plasma mixed in reaction buffer. Activities are represented relative to 2.5% NPP alone, which is designated as 100% activity. (B) The cleaving activity of 2.5% wild-type (WT) or 2.5% ADAMTS13-deficient (ADAMTS13−/−) mouse plasma in the presence of resting or PMA-activated neutrophils (105 cells/mL). Activities are represented relative to WT mouse plasma, which is designated as 100% activity. Results shown in panels A and B are mean values (± SD) from 2 combined representative experiments (N = 4). ▨ represents activated neutrophils. The P values were determined by Student t tests.
Compared with resting neutrophils, PMA-activated neutrophils exhibited a markedly increased ability to cleave FRETS-VWF73 (Figure 5A). In addition, in contrast to resting neutrophils, PMA-activated neutrophils combined with normal pooled plasma or with ADAMTS13-deficient TTP plasma exhibited substantial FRETS-VWF73–cleaving activity (Figure 5A). Similar results were obtained when neutrophils were combined with wild-type or ADAMTS13-deficient mouse plasmas. When resting neutrophils and mouse plasma samples were combined, the FRETS-VWF-73–cleaving activities were the same as the activities of the plasma samples alone (Figure 5B). However, the combination of activated neutrophils and wild-type or ADAMTS13-deficient mouse plasma exhibited markedly more FRETS-VWF-73–cleaving activity than the plasmas (Figure 5B). These results suggest that proteolytic cleavage of VWF by activated leukocytes may occur in the presence of plasma protease inhibitors.
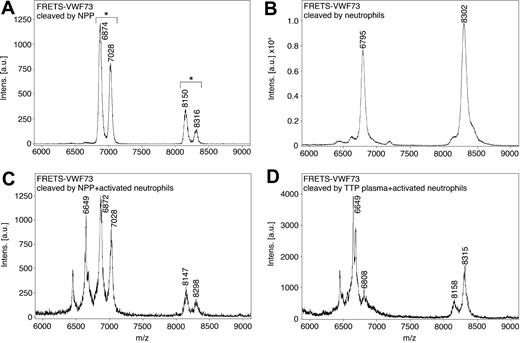
To identify the VWF cleavage sites resulting from activated neutrophils, fragments of commercial FRETS-VWF73 cleaved by activated neutrophils in the presence of plasmas were analyzed by mass spectrometry. When FRETS-VWF73 was incubated with normal pooled plasma containing ADAMTS13, a major peak consistent with cleavage at the peptide bond corresponding to Y1605-M1606 was observed (Figure 6A). In addition, peaks were observed approximately 157 Da smaller than the peaks associated with the uncleaved substrate FRETS-VWF73 and the major ADAMTS13 cleavage fragment. These “doublet” peaks are consistent with removal of the carboxyl-terminal arginine (157 Da) from the FRETS-VWF73 substrate, possibly by a plasma carboxypeptidase, such as carboxypeptidase B.25 All other plasma-containing samples analyzed by mass spectrometry exhibited these doublet peaks. Attempts to inhibit carboxypeptidase activity interfered with activated neutrophil proteolytic activity (not shown).
Mass spectrometry analysis of cleavage site on FRETS-VWF73 by membrane-bound proteases on neutrophils. A commercial FRETS-VWF73 peptide cleaved by (A) normal pooled plasma (NPP), (B) PMA-activated neutrophils in assay buffer, (C) activated neutrophils combined with NPP, (D) or activated neutrophils combined with ADAMTS13-deficient TTP plasma were analyzed by MALDI-TOF mass spectrometry. The doublet peaks (shown by asterisks above brackets) observed when NPP or TTP plasmas were present differed by 157 Da, and are consistent with the removal of a carboxyl terminal arginine of the FRETS-VWF73 substrate by plasma carboxypeptidase B. NPP alone generated a doublet peak consistent with cleavage at the Y1605-M1606 peptide bond (A). Activated neutrophils combined with NPP or TTP plasma generated a major peak of 6649 Da, consistent with cleavage at the V1607-T1608 bond and the removal of the carboxyl terminal arginine (C-D).
Mass spectrometry analysis of cleavage site on FRETS-VWF73 by membrane-bound proteases on neutrophils. A commercial FRETS-VWF73 peptide cleaved by (A) normal pooled plasma (NPP), (B) PMA-activated neutrophils in assay buffer, (C) activated neutrophils combined with NPP, (D) or activated neutrophils combined with ADAMTS13-deficient TTP plasma were analyzed by MALDI-TOF mass spectrometry. The doublet peaks (shown by asterisks above brackets) observed when NPP or TTP plasmas were present differed by 157 Da, and are consistent with the removal of a carboxyl terminal arginine of the FRETS-VWF73 substrate by plasma carboxypeptidase B. NPP alone generated a doublet peak consistent with cleavage at the Y1605-M1606 peptide bond (A). Activated neutrophils combined with NPP or TTP plasma generated a major peak of 6649 Da, consistent with cleavage at the V1607-T1608 bond and the removal of the carboxyl terminal arginine (C-D).
Activated neutrophils in buffer cleaved FRETS-VWF73, yielding a single major peak corresponding to cleavage of the V1607-T1608 peptide bond (Figure 6B). This result is consistent with cleavage by either neutrophil elastase or proteinase 3. The combination of activated neutrophils and normal pooled plasma yielded the same doublet peaks observed with cleavage of FRETS-VWF73 by normal pooled plasma, as well as an additional major peak of 6649 Da, consistent with cleavage at the V1607-T1608 peptide bond (and removal of the carboxyl-terminal arginine; Figure 6C). The combination of activated neutrophils and ADAMTS13-deficient TTP plasma also yielded a major peak of 6649 Da, consistent with cleavage at the V1607-T1608 peptide bond.
Discussion
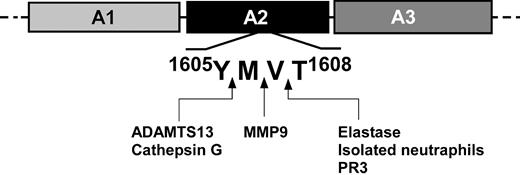
In this study, we observed cleavage of VWF by 4 neutrophil proteases at 3 peptide bonds at or adjacent to the ADAMTS13 cleavage site in the VWF A2 domain. Cleavage was observed under chemical denaturing conditions and in the presence of fluid shear stress. These 3 protease-sensitive peptide bonds include the Y1605-M1606 bond cleaved by cathepsin G, the V1607-T1608 cleaved by both neutrophil elastase and PR3, and the M1606-V1607 bond in FRETS-VWF73 cleaved by MMP9. In addition, we observed that activated neutrophils were able to cleave FRETS-VWF73 at the bond corresponding to V1607-T1608 in the presence of normal or ADAMTS13-deficient plasma. These observations suggest that activated leukocytes could contribute to the proteolytic regulation of VWF, and that this cluster of adjacent peptide bonds in the VWF A2 domain may comprise a protease-sensitive regulatory hot spot in VWF (Figure 7).
Potential cleavage sites in the VWF-A2 domain of leukocyte proteases and ADAMTS13. A schematic diagram of the VWF A domains is shown with the peptide bond cleavage sites in the A2 domain shown by arrows for neutrophil elastase, PR3, cathepsin G, ADAMTS13, MMP9, and neutrophils. The cleavage sites were determined by mass spectrometry analysis of the carboxyl terminal fragments of cleaved FRETS-VWF73, and confirmed by amino terminal sequencing of approximately 175 kDa electrophoretic bands of cleaved multimeric VWF under reducing conditions for neutrophil elastase, PR3, and cathepsin G.
Potential cleavage sites in the VWF-A2 domain of leukocyte proteases and ADAMTS13. A schematic diagram of the VWF A domains is shown with the peptide bond cleavage sites in the A2 domain shown by arrows for neutrophil elastase, PR3, cathepsin G, ADAMTS13, MMP9, and neutrophils. The cleavage sites were determined by mass spectrometry analysis of the carboxyl terminal fragments of cleaved FRETS-VWF73, and confirmed by amino terminal sequencing of approximately 175 kDa electrophoretic bands of cleaved multimeric VWF under reducing conditions for neutrophil elastase, PR3, and cathepsin G.
Several investigators have previously described that VWF could be cleaved by leukocyte proteases. Berkowitz et al used epitope-specific antibodies to identify cleavage fragments of VWF resulting from elastase proteolysis.10 It was concluded that the fragments were dissimilar to those occurring in plasma. In contrast to Berkowitz et al, Tsai et al digested VWF with neutrophil lysates and with cathepsin G and concluded that the resulting fragments analyzed by gel electrophoresis were similar to those observed in plasma.8,9 Neither investigator identified the protease cleavage sites in VWF, and the discrepancy between these investigators' conclusions remained unresolved. In the current study, we have confirmed that multiple leukocyte proteases cleave both chemically denatured VWF and VWF under fluid shear stress generating major electrophoretic fragments of approximately 175 kDa under reducing conditions (Figure 3), and approximately 350 kDa (dimer of 175 kDa) under nonreducing conditions (Figure 4), consistent with cleavage in the VWF A2 domain.
It has been suggested that neutrophil proteases are unlikely to be responsible for VWF proteolysis because protease inhibitors in normal plasma suppress their activities.26 This interpretation warrants reconsideration. A body of literature dating to the early 1980s establishes that proteases secreted by activated neutrophils remain bound to the cell surface and remain catalytically active in the presence of physiologic inhibitors. Elastase, cathepsin G, PR3, MMP9, and urokinase plasminogen activator are all expressed on activated neutrophil surfaces and have been shown to remain functional in the presence of physiologic inhibitors such as α1 proteinase inhibitor, tissue inhibitor of matrix metalloproteinase 1, tissue inhibitor of matrix metalloproteinase 2, plasminogen activator inhibitor 1, and plasminogen activator inhibitor 2.27-34 Therefore, according to current models, activated neutrophils in close contact with substrates are partially protected from plasma inhibitors and can proteolyze substrates in the presence of plasma protease inhibitors.30 Consistent with this observation, in the presence of normal or ADAMTS13-deficient plasma, we observed that resting neutrophils did not exhibit FRETS-VWF73–cleaving activity, but activated neutrophils exhibited substantial proteolytic activity. The peptide bond in FRETS-VWF73 cleaved by activated neutrophils was V1607-T1608, consistent with the FRETS-VWF73–cleaving activity of neutrophil elastase. These results are consistent with other investigators' observations that proteases expressed by activated neutrophils, especially elastase, may be protected from inhibition by plasma leukocyte protease inhibitors, and may retain the ability to cleave substrates such as VWF under physiologically relevant conditions. From these results we propose that, when in close proximity to a VWF-platelet thrombus, enzymes expressed on activated leukocyte surfaces may cleave unfolded VWF and down-regulate thrombosis. Further studies are required to test this hypothesis in vivo.
Because of the presence of protease inhibitors, it seems unlikely that leukocyte proteases in normal plasma have significant physiologic enzymatic activity. However, it is possible that these proteases could affect assays designed to specifically detect ADAMTS13 activity. This might occur when blood samples are collected or stored in a manner that promotes significant leukocyte activation or decay, and subsequent release of enzymes. Indeed, serum elastase concentrations are nearly 7-fold higher than citrate- or EDTA-anticoagulated plasma elastase levels.35
Clinical observations also support a possible role of leukocytes in down-regulating VWF. For example, high neutrophil counts have been associated with VWF proteolysis in acute promyelocytic leukemia.36 In illnesses associated with inflammation, VWF multimers were observed to be decreased and subunit fragments were increased.37,38 VWF degradation was also observed to be enhanced in direct proportion to the leukocyte count in myeloproliferative disorders.39,40 In a study of plasma VWF multimer patterns in patients with high or low neutrophil counts, 7 of 10 patients who exhibited decreased VWF multimer sizes had very high neutrophil counts, suggesting that high neutrophil counts were associated with increased VWF proteolysis.41
Disseminated intravascular coagulation is associated with severe secondary deficiency of ADAMTS13 activity and might be expected to be accompanied by unusually large VWF multimers.42 However, no significant correlation between ADAMTS13 deficiency and the presence of unusually large VWF multimers has been observed.42 To the contrary, some reports have described increased fragmentation of VWF in disseminated intravascular coagulation,43 and the VWF proteolytic fragments appear to be the same as those generated by digestion with neutrophil lysates.7
The importance of ADAMTS13 deficiency in the pathogenesis of TTP is well established. However, ADAMTS13 deficiency alone is not sufficient to cause TTP. Severe deficiency has been observed in individuals who have recovered from or never had TTP, and targeted deletion of ADAMTS13 in mice does not result in a spontaneous thrombotic phenotype.5,44 In some patients with TTP, there is enhanced or alternative cleavage of VWF.6,45 Other important disease modifiers must therefore be involved in TTP pathogenesis. Leukocyte protease cleavage of VWF may be a modifying process in TTP. We hypothesize that in individuals who have severely deficient ADAMTS13 activity without manifestations of TTP, the VWF proteolytic activity of activated leukocytes may be sufficient to prevent platelet thrombosis. Conversely, in patients with active TTP, there may be deficiencies in both leukocyte protease-mediated and ADAMTS13-mediated proteolysis of VWF.
Type 2A von Willebrand disease (VWD) is most commonly caused by mutations that enhance the susceptibility of VWF to cleavage in the A2 domain. Several VWF mutations associated with type 2A VWD have been identified near the Y1605-T1608 proteolytic hot spot.46 Although many of these mutations probably enhance ADAMTS13 cleavage of VWF, not all of them do.47 Surprisingly, some appear to reduce cleavage, including the D1614G mutation, which reduces ADAMTS13-mediated cleavage approximately 8-fold.47 Although this observation appears paradoxical, it is possible that some VWD type 2A mutations cause increased VWF proteolysis by enhancing the susceptibility to cleavage by leukocyte proteases rather than by ADAMTS13.
Other types of leukocytes besides neutrophils have the potential to regulate VWF. Monocytes express proteases similar to neutrophils, including elastase, cathepsin G, and, probably, PR3.30 Like neutrophils, activated monocytes express serine proteases on their cell membranes. Eosinophils express elastase and MMPs within granules.30 Basophils and mast cells express 3% to 20% of the amount of elastase expressed by neutrophils. It is therefore possible that several types of leukocytes could regulate VWF.
The observation that multimeric VWF requires denaturation or fluid shear stress for optimal proteolysis by leukocyte proteases supports the current model of VWF proteolytic regulation in which VWF engaged in binding platelets or tethering platelets to endothelial cells is subjected to tensile forces that change its tertiary structure and expose protease docking sites.1 Our observation that VWF proteolysis can be mediated by multiple enzymes acting within a 4–amino acid microdomain suggests that this region may be a hot spot for VWF proteolytic regulation (Figure 7). The potential importance of this A2 domain hot spot is supported by the observation that the Swiss-Prot/TrEMBL database48 includes more than 100 species having VWF homologues containing the identical sequence of 4 amino acids (VYMV) spanning the hot spot. This high degree of sequence conservation suggests that selective pressure may have favored preservation of this domain structure, perhaps because of its importance in VWF proteolytic regulation.
In summary, our studies suggest that the proteases neutrophil elastase, cathepsin G, PR3, and MMP9 may be modifying factors in VWF-dependent hemostasis when expressed on the surface of activated leukocytes. In addition, the narrow region of VWF spanning Y1605 to T1608 may be an important hot spot of VWF proteolytic regulation. Further studies using in vivo physiologic models may determine an important role of leukocyte proteases in VWF regulation. These results suggest a possible new link between inflammation and hemostasis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr William Nauseef for providing isolated neutrophils and helpful advice. The authors also thank Dr John Owen at Wake Forest University for providing E coli–expressing rFRETS-VWF73.
This work was supported by National Heart, Lung, and Blood Institute (National Institutes of Health, Bethesda, MD) grants HL 55556 (T.J.R.), HL 44612 (R.R.M.), HL 81588 (R.R.M.), HL 33721 (R.R.M.), and HL079027 (X.L.Z.).
National Institutes of Health
Authorship
Contribution: T.J.R., S.R.L., G.F.J., and X.L.Z. designed research, analyzed and interpreted data, and wrote the paper; and W.C., B.S.A., B.B., and R.R.M. performed research, analyzed data, and interpreted data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Thomas J. Raife, Department of Pathology, University of Iowa Carver College of Medicine, C250 GH, Iowa City, IA 52240; e-mail: thomas-raife@uiowa.edu; or X. Long Zheng, Department of Pathology and Laboratory Medicine, The Children's Hospital of Philadelphia, 34th St and Civic Center Blvd, 816G ARC, Philadelphia, PA 19104; e-mail: zheng@email.chop.edu.