Abstract
Factor XIII-A (FXIII-A) is present in the cytosol of platelets, megakaryocytes, monocytes, osteoblasts, and macrophages and may be released from cells by a nonclassical pathway. We observed that plasma FXIII-A levels were unchanged in thrombocytopenic mice (Bcl-xPlt20/Plt20 and Mpl−/−), which implicates nonclassical secretion from nucleated cells as the source of plasma FXIII-A. We, therefore, examined the intracellular targeting of FXIII-A in the THP-1 (monocyte/macrophage) cell line and in human monocyte–derived macrophages. Metabolic labeling of THP-1 cells did not show release of 35S-FXIII-A either under basal conditions or when interleukin 1-β was released in response to cell stress. However, immunofluorescence of THP-1 cells and primary macrophages showed that FXIII-A associated with podosomes and other structures adjacent to the plasma membrane, which also contain trans-Golgi network protein-46 and Golgi matrix protein-130 (GM130) but not the endoplasmic reticulum luminal protein, protein disulphide isomerase. Further, FXIII-A was present in GM130-positive intracellular vesicles that could mediate its transport, and in other contexts GM130 and its binding partner GRASP have been implicated in the delivery of nonclassically secreted proteins to the plasma membrane. Hence, this mechanism may precede FXIII-A release into the extracellular matrix from macrophages and its release into plasma from the cell type of origin.
Introduction
Coagulation factor XIII-A (FXIII-A) is an 83-kDa protransglutaminase that is present in plasma as an A2B2 heterotetramer and present in the cytoplasm of platelets/megakaryocytes, monocyte/macrophages, chondrocytes, and osteoblasts1,2 as an A2 heterodimer. The best-characterized function of plasma FXIII-A is to cross-link fibrin clots after thrombin activation,1 but extracellular FXIII-A has also been implicated in bone calcification,2 angiogenesis,3 and tissue repair.4 The intracellular roles of FXIII-A remain poorly defined, as is the mechanism by which it is secreted into the plasma or extracellular matrix (ECM).
FXIII-A does not contain an identifiable endoplasmic reticulum (ER) signal sequence and is excluded from the classical ER-Golgi pathway in nucleated cells5 ; hence, FXIII-A synthesized de novo in megakaryocytes cannot be delivered to α-granules in nascent platelets. However, certain leaderless proteins, including interleukin-1β (IL-1β), IL-18, and fibroblast growth factor-2 are secreted by nonclassical mechanisms, some of which may be specific to hematopoietic cells.6-8 In some contexts (eg, cartilage deposition), FXIII-A may be nonselectively released into the ECM on cell death and lysis.9 However, it also seems probable that FXIII-A will be actively released from cells by one or more nonclassical pathways. Furthermore, the homologous protein transglutaminase-2 TG2 may be actively released under conditions of cell stress10 under the direction of an amino-terminal peptide motif.11
The outcome of bone marrow transplantation protocols in humans has implicated platelets in releasing plasma FXIII-A.12,13 Activated platelets release procoagulant microparticles containing FXIII-A,14 and the constitutive low level release of microparticles15 could mediate secretion of FXIII-A if these particles burst and release their cargo in plasma. If platelets maintain the plasma pool, then changes in plasma FXIII-A concentrations might be predicted in thrombocytopenia. Several studies have measured FXIII-A levels in human subjects with thrombocytopenia, both idiopathic16-18 and iatrogenic, secondary to chemotherapy.18,19 However, these studies have generated conflicting results and are difficult to interpret because of the differences between the patients and the treatments involved. In the present study, we used 2 distinct mouse lines that display thrombocytopenia as a result of unrelated single-gene mutations. In Bcl-xPlt20/Plt20 mice, a homozygous missense mutation in the gene encoding the antiapoptotic protein Bcl-xL decreases platelet life span to 1 day,20 whereas in Mpl−/− mice the gene for the thrombopoietin receptor is deleted, reducing the generation of megakaryocytes.21 The effects of these platelet abnormalities on plasma FXIII-A levels have not previously been studied.
Distinct pathways of nonclassical secretion operate in monocyte/macrophages and are preserved in THP-1 (monocytic leukemic) cells, which retain the facility to differentiate into an adherent macrophage-like phenotype.6 In response to cell stress, THP-1 cells encapsulate IL-1β within microvesicles that rupture once they are released into the medium.6 In addition, TG2 is translocated to podosomes in THP-1 cells, where it may mediate cell migration and where it has been reported to be exposed on the outer face of the plasma membrane.22
To determine whether FXIII-A uses pathways implicated in nonclassical secretion from nucleated cells, we conducted metabolic labeling in THP-1 cells and immunofluorescence studies in THP-1 cells and human monocyte-derived macrophages.
Our results indicate that platelets are not the major source of plasma FXIII-A and suggest that, in nucleated hematopoietic cells, FXIII-A is directed to the plasma membrane in association with Golgi vesicles. Transport in association with peripheral Golgi proteins has been linked to the nonclassical secretion of other proteins23 and may precede the release of FXIII-A into the plasma or ECM. This pathway is distinct from that which mediates IL-1β release.
Methods
Restriction endonucleases were obtained from New England Biolabs, oligonucleotides from Invitrogen, and other reagents from Sigma unless otherwise stated.
Assays of platelet and plasma FXIII-A in mice
Mice carrying either the Plt20 allele of Bcl-x20 or a null allele of Mpl21 have been previously described. Before this study, both mutations had been backcrossed for 10 generations to wild-type C57BL/6 mice. Male and female thrombocytopenic animals and appropriate control strains between 3 and 6 months of age received a subcutaneous injection of heparin solution in phosphate-buffered saline (PBS; 30 IU/animal). After 1 hour animals were anesthetized with isoflurane or Penthrane, and blood was collected from the inferior vena cava into syringes containing citrate theophylline adenosine dipyridamole anticoagulant (BD Biosciences) with the use of a 23G or 25G needle. Where described, aliquots of whole blood were used for automated blood counting with the use of an Advia 2120 system. Plasma was separated by centrifugation at 3000g for 10 minutes. For platelet preparations, whole blood was centrifuged at 200g for 6 minutes. Platelet-rich plasma was removed, prostaglandin E1 (1 μg/mL) and apyrase (0.1 activity units/mL) were added, and plasma was then centrifuged at 1000g for 10 minutes to pellet platelets. Platelet pellets were washed twice and resuspended in Tyrode HEPES buffer (138mM NaCl, 3mM KCl, 1mM MgCl2, 5mM glucose, 0.5mM NaH2PO4, 12mM NaHCO3, 20mM HEPES pH 7.4). Plasma and platelets were assayed for FXIII-A, both by immunoprecipitation-immunoblotting (IP-IB) and also by enzyme assay measurements.24 Animal procedures were carried out with the approval of the United Kingdom Home Office and the University of Leeds.
Cell culture and transfection
Nonadherent THP-1 cells were grown in suspension culture in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotic/antimycotic solution. THP-1 cells were differentiated to an adherent phenotype by treatment with 500nM phorbol 12-myristate 13-acetate (PMA) for 3 hours and left to adhere to the culture flask overnight.6 To induce IL-1β synthesis, adherent cells were treated with 10 μg/mL lipopolysaccharide (LPS; from Escherichia coli 0111:B4) for 6 or 24 hours. To acutely induce IL-1β secretion, LPS-pretreated adherent cells were treated for 20 minutes with 200μM benzoylbenzoyl adenosine triphosphate (BzATP).6 To reinduce FXIII-A synthesis in adherent cells, IL-4 (R&D Systems) was applied at 20 ng/mL for 6 days. In some experiments, adherent IL-4–treated cells were treated with Brefeldin A at 10 μg/mL for 1 hour, before processing for immunofluorescence. Adherent THP-1 cells were transfected with a plasmid encoding FXIII-A–green fluorescent protein (GFP) with lipofectamine LTX reagent as described by the manufacturer (Invitrogen).
Protein IP
THP-1 cells or platelet fractions were lysed at 4°C in IP buffer (50mM Tris-HCl pH 8.0, 150mM NaCl, 1mM EDTA, 0.2% sodium dodecyl sulfate [SDS], 1% IGEPAL CA-630, 1 mg/mL bovine serum albumin, 3mM benzamidine, and 0.2% protease inhibitor cocktail). Insoluble matter was removed by centrifugation at 15 000g for 15 minutes. After preclearing for 2 hours with protein A or protein G sepharose, samples were incubated at 4°C overnight with 10 μg/mL of the appropriate antibody (rabbit polyclonal anti–FXIII-A Ab-2 [Labvision], rabbit polyclonal anti–TG2 Ab-4 [Labvision], rabbit polyclonal anti–IL-1β antibody [Santa Cruz Biotechnology Inc], goat polyclonal anti–lactate dehydrogenase [LDH] antibody [Chemicon]). Protein A or protein G sepharose was added for 3 hours to precipitate immune complexes. Precipitates were washed with IP buffer and then with 50mM Tris-HCl pH 8.0, 0.25M sucrose. Precipitated proteins were analyzed by IB or autoradiography. In preliminary experiments, it was verified that antibody concentrations exceeded those required for quantitative precipitation of proteins.
Immunoblotting
Proteins were resolved by SDS–polyacrylamide gel electrophoresis (PAGE) and blotted to a polyvinylidene diflouride membrane. Membranes were incubated with 5% (wt/vol) nonfat milk powder in 50mM Tris-HCl, pH 7.4, 150mM NaCl, and 0.05% Tween-20 at room temperature for 1 hour and probed with one of the following primary antibodies: anti–FXIII-A mAb AC1A1 ab1834 (Abcam), sheep anti–FXIII-A polyclonal SAF13A (Enzyme Research Laboratories), anti-TG2 mAb 14G2 ab21258 (Abcam), and anti–IL-1β mAb clone 8516.311 (Sigma). Subsequently, membranes were incubated with the appropriate horseradish peroxidase–conjugated secondary antibody (Dako) diluted in blocking buffer and were visualized with the use of West Pico reagents (Pierce Chemical) and a Kodak IS2000 imager. Samples were loaded at various dilutions to verify linearity of the signal with protein concentration.
Isolation and differentiation of human monocytes
Venous blood was collected from volunteers into 3.8% (wt/vol) trisodium citrate solution. Blood samples from human volunteers were taken with informed consent in accordance with the Declaration of Helsinki. Peripheral blood mononuclear cells were isolated with Ficoll-Paque density gradient centrifugation (400g for 40 minutes), and platelets were removed by discarding supernatants from Dulbecco PBS washes after centrifugations at 200g. Monocytes were selected by adherence to cell-culture flasks after a 2-hour incubation in RPMI medium at 37°C, followed by 2 further Dulbecco PBS washes. Subsequently, cells were grown on glass coverslips for 6 days in RPMI 1640 medium containing 10% fetal bovine serum and 20 ng/mL IL-4.
Secretion assays for THP-1 cells
Nonadherent and PMA-differentiated adherent cells were labeled in RPMI 1640 medium lacking methionine and cysteine with Tran35S-label (MP Biomedicals) added at 100 μCi (3.7 MBq)/mL for 12 hours and, when indicated, in the presence of IL-4 and LPS. FXIII-A, LDH, and IL-1β were measured in cells and medium by IP and SDS-PAGE/autoradiography.
Cytosol depletion
Nonadherent THP-1 cells were labeled with Tran35S-label as described in “Secretion assays for THP-1 cells” and were cytosol-depleted with the use of the bacterial toxin streptolysin O (SLO).25,26 SLO was used at the minimum concentration that permeabilized more than 95% of cells to Trypan blue after 15 minutes at 37°C. Labeled cells, growing in suspension, were washed twice in cold acetate buffer (115mM potassium acetate, 25mM HEPES pH 7.4, 2.5mM MgCl2). In some instances, cells were resuspended at 5 × 106/mL in acetate buffer containing 0.9mM CaCl2 and 1000 U/mL SLO at 4°C for 15 minutes, washed 3 times, and then resuspended in acetate buffer containing 10mM dithiothreitol at 37°C for 45 minutes (prebinding and washing protocol). In other cases, cells were directly resuspended and incubated for 30 minutes at 37°C in acetate buffer containing 1000 U/mL SLO and 10mM dithiothreitol, using SLO preactivated by prior incubation for 15 minutes at 37°C (preactivation protocol). Permeabilized cells were resuspended in 50 mL of saline solution, incubated at 4°C for 10 minutes with mixing, and pelleted by centrifugation at 1700g. FXIII-A was measured by IP followed by SDS-PAGE/autoradiography, whereas β-N-acetylglucosaminidase and LDH activity were assayed spectrophotometrically.
Detection of radiolabeled proteins
Radiolabeled immunoprecipitated proteins were resolved by SDS-PAGE. Methanol and acetic acid fixed gels were dried after treatment with Amplify fluorographic reagent (Amersham), and proteins were visualized by autoradiography with the use of Kodak BIOMAX MR film. Autoradiograms were scanned with a Kodak IS2000 imager, and intensities were estimated by densitometry. Replicate loadings at 5-fold dilutions were used to check linearity.
Immunofluorescence
Adherent cells were grown on coverslips, whereas suspension cells were transferred to slides with the use of the cytospin system (Thermo), before paraformaldehyde fixation. After Triton X-100 permeabilization, cells were blocked for 1 hour with 5% human serum in 0.2% fish skin gelatin/PBS and then incubated with primary antibodies for 90 minutes and secondary antibodies for 1 hour.27 Cells were extensively washed with fish skin gelatin/PBS after blocking and antibody incubation steps and then with PBS before mounting with Prolong antifade with DAPI (Invitrogen). Antibodies used were as follows: anti–FXIII-A antibodies AC-1A1, Ab-2, and SAF13A; anti-TG2 antibody 14G2, anti–protein disulphide isomerase antibody mAb 1D3 ab-2225 (Abcam). Antibodies against the Golgi proteins TGN46 and GM13028 were a kind gift from Dr S. Ponnambalam (University of Leeds). Secondary antibody conjugates were from Jackson ImmunoResearch and from Invitrogen. Cells were imaged with the use of the DMIRE2 (Leica Microsystems) and LSM510 (Carl Zeiss) microscopes with a 63×/1.4 NA oil-immersion objective, Immersol 518F immersion oil (Carl Zeiss), and with a pinhole setting of one AIRY unit unless otherwise stated. Fluorophores (DAPI, Fluorescein, DyLight 488, Texas Red, Alexa 546, DyLight 594) were excited sequentially, and filters were adjusted appropriately to eliminate any signal bleed-through between channels. Images were acquired with the use of proprietary Leica and Zeiss software packages. Brightness and contrast levels were adjusted in Corel Photo-Paint X4 version 14. Cells stained with secondary antibodies only were used routinely as negative controls.
LDH and β-N-acetylglucosaminidase activity assays
LDH activity was measured with the use of the Cytotox 96 assay kit (Promega). The linearity range of this assay was determined in preliminary experiments. To assay β-N-acetylglucosaminidase, cells were lysed in 1% Triton X-100 and were assayed spectrophotometrically with the substrate 4 nitrophenyl N-acetyl-β-d-glucosamine as described by the manufacturer (Sigma-Aldrich).
Results
The plasma pool of FXIII-A is not proportionally diminished in thrombocytopenic mice
In initial studies (not shown), we observed that the enrichment of FXIII-A relative to the cytosolic marker LDH in thrombin-elicited platelet microparticles was low (∼ 4.5-fold), suggesting that microparticle release might not be sufficiently selective to maintain the plasma pool of FXIII-A. To further address this issue, we examined 2 independent lines of thrombocytopenic mice.
We confirmed that platelet counts were reduced by approximately 75% in Bcl-xPlt20/Plt20 mice used here. Plasma FXIII-A enzyme activity was maintained at 72% ± 18% in Bcl-xPlt20/Plt20 mice relative to wild-type C57BL6 controls, whereas plasma FXIII-A antigen levels were 69% plus or minus 7% (Figure 1). We also examined Mpl knockout mice that have approximately 90% reductions in both platelet and megakaryocyte counts. These mice also maintain near-normal levels of plasma FXIII-A (89% ± 10% of enzyme activity; 87% ± 16% of antigen levels). These results suggest that plasma FXIII-A does not derive primarily (if at all) from circulating platelets and indeed may not derive from the megakaryocyte lineage (see “Discussion”).
Plasma levels of FXIII-A in thrombocytopenic mice. Blood plasma was obtained from either (A) Bcl-xPlt20/Plt20 or (B) Mpl−/− mice (■) or from strain-matched controls ( ) and was assayed for FXIII-A enzyme activity or for FXIII-A antigen levels (by IP with Ab-2 followed by IB with HRP-conjugated sheep anti–FXIII-A antiserum). Results show levels of FXIII-A in the thrombocytopenic mice relative to controls and are based on 6 animals in each group. Insets show immunoblots of immunoprecipitated FXIII-A from pooled plasma.
) and was assayed for FXIII-A enzyme activity or for FXIII-A antigen levels (by IP with Ab-2 followed by IB with HRP-conjugated sheep anti–FXIII-A antiserum). Results show levels of FXIII-A in the thrombocytopenic mice relative to controls and are based on 6 animals in each group. Insets show immunoblots of immunoprecipitated FXIII-A from pooled plasma.
Plasma levels of FXIII-A in thrombocytopenic mice. Blood plasma was obtained from either (A) Bcl-xPlt20/Plt20 or (B) Mpl−/− mice (■) or from strain-matched controls ( ) and was assayed for FXIII-A enzyme activity or for FXIII-A antigen levels (by IP with Ab-2 followed by IB with HRP-conjugated sheep anti–FXIII-A antiserum). Results show levels of FXIII-A in the thrombocytopenic mice relative to controls and are based on 6 animals in each group. Insets show immunoblots of immunoprecipitated FXIII-A from pooled plasma.
) and was assayed for FXIII-A enzyme activity or for FXIII-A antigen levels (by IP with Ab-2 followed by IB with HRP-conjugated sheep anti–FXIII-A antiserum). Results show levels of FXIII-A in the thrombocytopenic mice relative to controls and are based on 6 animals in each group. Insets show immunoblots of immunoprecipitated FXIII-A from pooled plasma.
Platelet FXIII-A was marginally increased in Bcl-xPlt20/Plt20 mice (130% of wild-type controls, using 2 preparations of pooled platelets, each from 6 mice). This small increase may represent a compensatory change in FXIII-A induced by thrombocytopenia, or equally it could be explained by a lower average age of the platelets and consequently by reduced turnover of cytosolic FXIII-A.
FXIII-A is not released by the same pathway as IL-1β
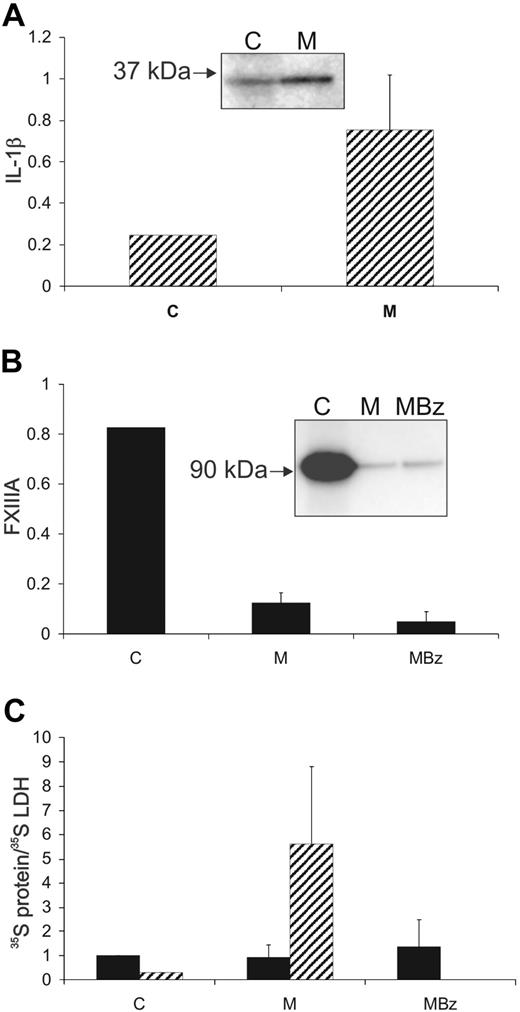
We determined whether FXIII-A could be selectively released from nucleated cells through the pathway that releases IL-1β. When nonadherent THP-1 cells were labeled with 35S–Met-plus-Cys, the release of labeled FXIII-A did not exceed that of LDH release either in the presence or absence of LPS. However, even after LPS stimulation (which induces IL-1β synthesis and secretion), we observed that nonadherent THP-1 cells released very low levels of IL-1β (< 200 pg/106 cells; supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article). In contrast, we observed that PMA-differentiated, adherent THP-1 cells efficiently released IL-1β in response to LPS (6 ng/106 cells, in agreement with values routinely obtained elsewhere; Prof Anne-Marie Surprenant, University of Manchester, oral communication, December 1, 2004). However, we also found that PMA treatment strongly down-regulated FXIII-A mRNA and protein levels. To reinduce FXIII-A expression, we treated adherent THP-1 cells with IL-4 (supplemental Figure 2). IL-4 treatment for 5 days reduced the induction of IL-1β by LPS by approximately 70%, but IL-1β and FXIII-A were both readily detectable in the cells.
When adherent THP-1 cells coexpressing FXIII-A and IL-1β were labeled, the majority of 35S–IL-1β was secreted into the medium during a 24-hour chase (75% ± 26%,). In contrast, the majority of 35S–FXIII-A (90% ± 5%) remained intracellular and was not released in excess of LDH (Figure 2). Challenge with the purinergic agonist, BzATP, did not cause significant further release of 35S–IL-1β, because most had been secreted during the chase period. However, when cells were challenged with BzATP, without a chase period, efficient release of 35S–IL-1β was confirmed (supplemental Figure 1). Because, in contrast, 35S–FXIII-A remained intracellular through the 24-hour chase period, it was possible to examine the effect of BzATP stimulation on its secretion. The small additional quantity of FXIII-A that was released on stimulation was not enriched with respect to LDH (Figure 2C). In our hands, IL-1β was secreted primarily as the 35-kDa precursor form rather than the 17-kDa processed form. It has been previously reported that THP-1 cells secrete a mixture of the 17-kDa and 35-kDa forms.6
IL-1β, but not FXIII-A, is actively secreted from adherent THP-1 cells. Adherent THP-1 cells were cultured with IL-4 for 5 days and then were metabolically labeled over a 12-hour period, with LPS present in the medium for the final 6 hours. Subsequently, cells were chased for 24 hours in complete medium and then challenged for 20 minutes with BzATP (200μM). IL-1β ( ), FXIII-A (■), and LDH were immunoprecipitated from final cell lysates (C), from the 24-hour chase medium (M) and from the medium after BzATP challenge (MBz). The distributions of radioactivity between the fractions are shown (A-B). (C) The distribution of FXIII-A and IL-1β was corrected for the distribution of immunoprecipitable 35S-LDH. Data are averaged from 4 separate experiments. Error bars indicate 1 SD. Insets show representative immunoprecipitates from a single experiment. Although the bulk of labeled IL-1β was secreted into the medium over the 24-hour chase (A), greater than 85% of labeled FXIII-A remained within the cell (B). BzATP resulted in a low level of release of FXIII-A and this was enriched less than 1.5-fold with respect to LDH.
), FXIII-A (■), and LDH were immunoprecipitated from final cell lysates (C), from the 24-hour chase medium (M) and from the medium after BzATP challenge (MBz). The distributions of radioactivity between the fractions are shown (A-B). (C) The distribution of FXIII-A and IL-1β was corrected for the distribution of immunoprecipitable 35S-LDH. Data are averaged from 4 separate experiments. Error bars indicate 1 SD. Insets show representative immunoprecipitates from a single experiment. Although the bulk of labeled IL-1β was secreted into the medium over the 24-hour chase (A), greater than 85% of labeled FXIII-A remained within the cell (B). BzATP resulted in a low level of release of FXIII-A and this was enriched less than 1.5-fold with respect to LDH.
IL-1β, but not FXIII-A, is actively secreted from adherent THP-1 cells. Adherent THP-1 cells were cultured with IL-4 for 5 days and then were metabolically labeled over a 12-hour period, with LPS present in the medium for the final 6 hours. Subsequently, cells were chased for 24 hours in complete medium and then challenged for 20 minutes with BzATP (200μM). IL-1β ( ), FXIII-A (■), and LDH were immunoprecipitated from final cell lysates (C), from the 24-hour chase medium (M) and from the medium after BzATP challenge (MBz). The distributions of radioactivity between the fractions are shown (A-B). (C) The distribution of FXIII-A and IL-1β was corrected for the distribution of immunoprecipitable 35S-LDH. Data are averaged from 4 separate experiments. Error bars indicate 1 SD. Insets show representative immunoprecipitates from a single experiment. Although the bulk of labeled IL-1β was secreted into the medium over the 24-hour chase (A), greater than 85% of labeled FXIII-A remained within the cell (B). BzATP resulted in a low level of release of FXIII-A and this was enriched less than 1.5-fold with respect to LDH.
), FXIII-A (■), and LDH were immunoprecipitated from final cell lysates (C), from the 24-hour chase medium (M) and from the medium after BzATP challenge (MBz). The distributions of radioactivity between the fractions are shown (A-B). (C) The distribution of FXIII-A and IL-1β was corrected for the distribution of immunoprecipitable 35S-LDH. Data are averaged from 4 separate experiments. Error bars indicate 1 SD. Insets show representative immunoprecipitates from a single experiment. Although the bulk of labeled IL-1β was secreted into the medium over the 24-hour chase (A), greater than 85% of labeled FXIII-A remained within the cell (B). BzATP resulted in a low level of release of FXIII-A and this was enriched less than 1.5-fold with respect to LDH.
A proportion of FXIII-A in THP-1 cells is associated with intracellular structures
To determine whether FXIII-A adopts a typical cytosolic distribution, 35S-Met-plus-Cys–labeled nonadherent THP-1 cells were treated with SLO to create pores in the plasma membrane. After cytosol depletion, cell ghosts were assayed for 35S–FXIII-A, for LDH, and for β-N-acetylglucosaminidase (to assess endomembrane integrity). When cells were permeabilized with preactivated SLO, release of FXIII-A and LDH was essentially complete, although lysosomal integrity was partially retained (Table 1). With the use of the prebinding and washing protocol to selectively permeabilize the plasma membrane, but to exclude SLO from the cytoplasm, both β-N-acetylglucosaminidase and FXIII-A were well retained, in contrast to LDH (Table 1). Hence, a significant proportion of FXIII-A is associated with SLO-sensitive intracellular structures. FXIII-A is unlikely to be present on the outer surface of the plasma membrane because SLO has equal access to this location in both protocols. Adherent THP-1 cells could not be investigated because they became very fragile after detachment, and lysosomal integrity was lost even under gentle permeabilization.
FXIII-A associates with the nucleus and with plasma membrane–associated structures in THP-1 cells
In preliminary studies, we confirmed the specificity of anti–FXIII-A antibodies. Rabbit polyclonal antibody Ab-2 and sheep polyclonal antibody SAF13A both detected a single approximately 83-kDa band by direct IB of lysates of IL-4–induced adherent THP-1 cells and specifically immunoprecipitated an approximately 83-kDa protein from metabolically labeled adherent and nonadherent THP-1 cells (not shown).
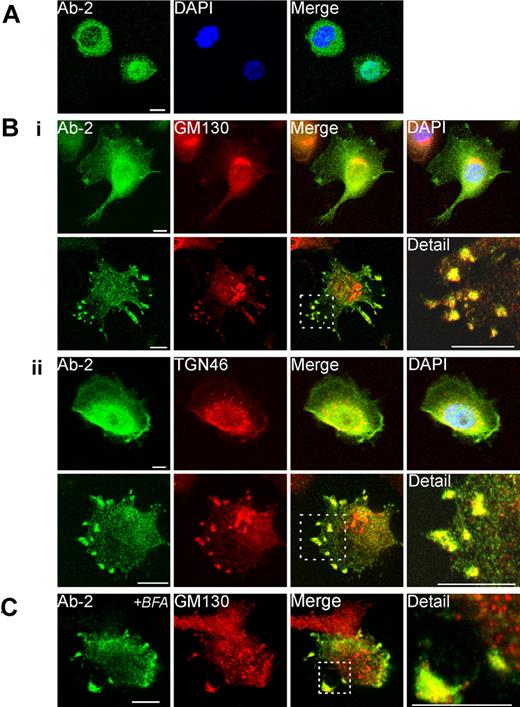
When nonadherent THP-1 cells were labeled with Ab-2, both nuclear and cytosolic immunofluorescence was apparent, with some cells showing an even distribution but with others showing a distribution biased to one or other of these compartments (Figure 3A).
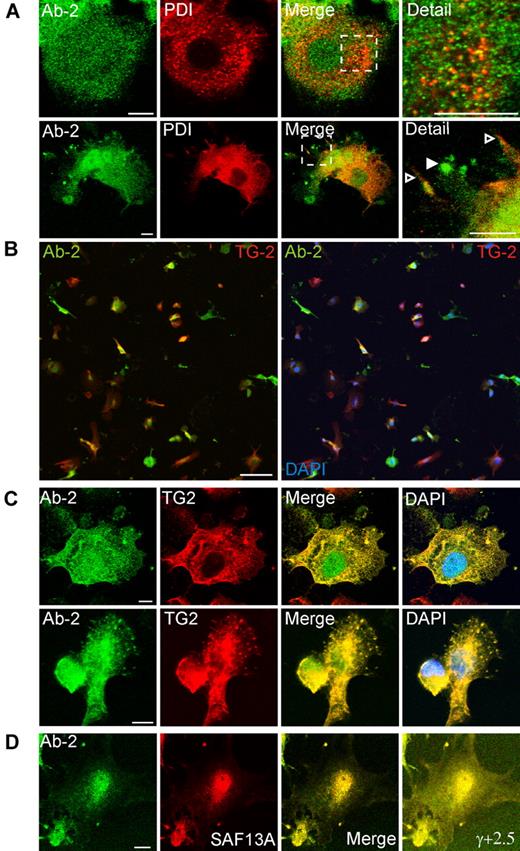
Immunofluorescent detection of FXIII-A in THP-1 cells. (A) The distribution of fluorescence for Ab-2 in fixed permeabilized nonadherent THP-1 cells was predominantly cytoplasmic in certain cells (top left) but predominantly nuclear in other cells (bottom right). (B) Adherent PMA-differentiated THP-1 cells were treated for 6 days with IL-4 (20 ng/mL). After fixation and permeabilization cells were probed with anti–FXIII-A antibody Ab-2 and with antibodies to Golgi markers GM130 (Bi) and TGN46 (Bii). In the majority of cells (shown in top rows in Bi-ii), Ab-2 fluorescence was distributed throughout the cytosol and nucleus. Ab-2 fluorescence was concentrated in the perinuclear area where it overlaid Golgi cisternae, but the regions of greatest intensity of Golgi and Ab-2 fluorescence did not correspond. In occasional cells (shown in bottom rows in Bi-ii), Ab-2 and Golgi marker fluorescence colocalized in podosome-like membrane extrusions (yellow pixels). In a high proportion of cells in which Ab-2 fluorescence was apparent in the periphery, perinuclear FXIII-A fluorescence was notably reduced. (C) THP-1 cells, grown as in panel B, were treated for 1 hour with Brefeldin A (BFA; 10 μg/mL) before fixation and permeabilization. Immunofluorescence for FXIII-A and GM130 remained within peripheral plasma membrane–associated structures despite dispersion of perinuclear Golgi elements to vesicles.
Immunofluorescent detection of FXIII-A in THP-1 cells. (A) The distribution of fluorescence for Ab-2 in fixed permeabilized nonadherent THP-1 cells was predominantly cytoplasmic in certain cells (top left) but predominantly nuclear in other cells (bottom right). (B) Adherent PMA-differentiated THP-1 cells were treated for 6 days with IL-4 (20 ng/mL). After fixation and permeabilization cells were probed with anti–FXIII-A antibody Ab-2 and with antibodies to Golgi markers GM130 (Bi) and TGN46 (Bii). In the majority of cells (shown in top rows in Bi-ii), Ab-2 fluorescence was distributed throughout the cytosol and nucleus. Ab-2 fluorescence was concentrated in the perinuclear area where it overlaid Golgi cisternae, but the regions of greatest intensity of Golgi and Ab-2 fluorescence did not correspond. In occasional cells (shown in bottom rows in Bi-ii), Ab-2 and Golgi marker fluorescence colocalized in podosome-like membrane extrusions (yellow pixels). In a high proportion of cells in which Ab-2 fluorescence was apparent in the periphery, perinuclear FXIII-A fluorescence was notably reduced. (C) THP-1 cells, grown as in panel B, were treated for 1 hour with Brefeldin A (BFA; 10 μg/mL) before fixation and permeabilization. Immunofluorescence for FXIII-A and GM130 remained within peripheral plasma membrane–associated structures despite dispersion of perinuclear Golgi elements to vesicles.
In most adherent THP-1 cells, Ab-2 fluorescence was distributed throughout both the cytosol and the nucleus. In addition, in occasional fields, groups of adherent cells extruded podosome-like structures which showed strong fluorescence both for Ab-2 and for Golgi markers (Figure 3B). In many adherent cells, cytosolic Ab-2 fluorescence was present in a diffuse perinuclear halo, although it did not appear that this fluorescence significantly colocalized with perinuclear Golgi elements. In contrast, in occasional adherent cells, tight colocalization of Ab-2 fluorescence with elements of the Golgi cisternae was apparent (supplemental Figure 3A).
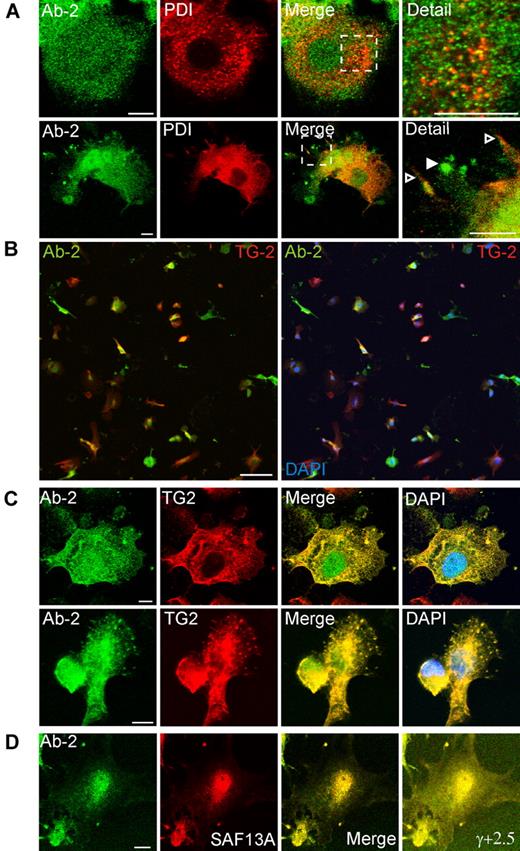
As expected, Brefeldin A treatment for 1 hour caused extensive vesiculation of perinuclear Golgi elements. However, plasma membrane–associated structures containing FXIII-A and Golgi markers were not appreciably disrupted (Figure 3C). Immunofluorescence for the ER marker, protein disulphide isomerase, did not correspond with Ab-2 fluorescence in any fields examined. Further, although protein disulphide isomerase was also present in certain plasma membrane processes, it was excluded from those structures that are most enriched in FXIII-A (Figure 4A). Immunofluorescence with an antibody to TG2 showed that many cells preferentially expressed either TG2 or FXIII-A (Figure 4B). In cells expressing both enzymes, FXIII-A was translocated to the nucleus under conditions whereby TG2 was excluded, but immunofluorescence showed that the 2 enzymes colocalized within podosome-like structures (Figure 4C). Immunofluorescence for Ab-2 was substantially lower in adherent cells that had not been treated with IL-4 to induce FXIII-A expression (not shown). Further, immunofluorescence with an affinity-purified sheep polyclonal anti–FXIII-A antibody SAF13A was shown to colocalize with that deriving from Ab-2 (Figure 4D), supporting the specificity of both antibodies.
Association of FXIII-A with TG2 but not protein disulphide isomerase in podosomes. Adherent PMA-differentiated THP-1 cells were treated for 6 days with IL-4 (20 ng/mL) and, after fixation and permeabilization, were probed with antibodies to FXIII-A, to protein disulphide isomerase (PDI), and to TG2. (A) Cytoplasmic full-length FXIII-A does not correspond in distribution to PDI (top row). PDI is excluded from podosomes that show a strong signal for Ab-2 (bottom row, filled arrowhead) but is present on other membrane processes (open arrowhead). (B) Cells were imaged with the use of a low-power objective to detect FXIII-A (green) and TG2 (red) fluorescence over the full depth of the cell. Left panel shows an overlay of red and green fluorescence, whereas right panel shows a triple overlay with DAPI fluorescence. A high proportion of cells preferentially expressed one or other transglutaminase, but some cells coexpressed FXIII-A and TG2. Both enzymes were present in the cytoplasm, whereas FXIII-A, but not TG2, was also present in the nucleus. Scale bars represent 100 μm. (C) In cells that coexpressed FXIII-A and TG2, both antigens colocalized in podosomes. (D) Immunofluorescence for Ab-2 (green) corresponded with immunofluorescence detected with a sheep polyclonal anti–FXIII-A antibody. Cell margins are apparent in an image with enhanced γ correction (γ, +2.5).
Association of FXIII-A with TG2 but not protein disulphide isomerase in podosomes. Adherent PMA-differentiated THP-1 cells were treated for 6 days with IL-4 (20 ng/mL) and, after fixation and permeabilization, were probed with antibodies to FXIII-A, to protein disulphide isomerase (PDI), and to TG2. (A) Cytoplasmic full-length FXIII-A does not correspond in distribution to PDI (top row). PDI is excluded from podosomes that show a strong signal for Ab-2 (bottom row, filled arrowhead) but is present on other membrane processes (open arrowhead). (B) Cells were imaged with the use of a low-power objective to detect FXIII-A (green) and TG2 (red) fluorescence over the full depth of the cell. Left panel shows an overlay of red and green fluorescence, whereas right panel shows a triple overlay with DAPI fluorescence. A high proportion of cells preferentially expressed one or other transglutaminase, but some cells coexpressed FXIII-A and TG2. Both enzymes were present in the cytoplasm, whereas FXIII-A, but not TG2, was also present in the nucleus. Scale bars represent 100 μm. (C) In cells that coexpressed FXIII-A and TG2, both antigens colocalized in podosomes. (D) Immunofluorescence for Ab-2 (green) corresponded with immunofluorescence detected with a sheep polyclonal anti–FXIII-A antibody. Cell margins are apparent in an image with enhanced γ correction (γ, +2.5).
In addition, we performed immunofluorescence with a widely used monoclonal antibody, clone AC-1A1 (not shown), but, as addressed in “Discussion,” this antibody yielded somewhat different results and its specificity remains to be established.
To independently confirm the subcellular distribution of FXIII-A, a FXIII-A–GFP fusion protein was expressed in adherent THP-1 cells by transfection. Although the efficiency of transfection was very low, occasional cells were observed which expressed FXIII-A–GFP, and, among these, some concentrated this protein in the tips of membrane processes (supplemental Figure 4A).
Distribution of FXIII-A in primary human monocyte–derived macrophages
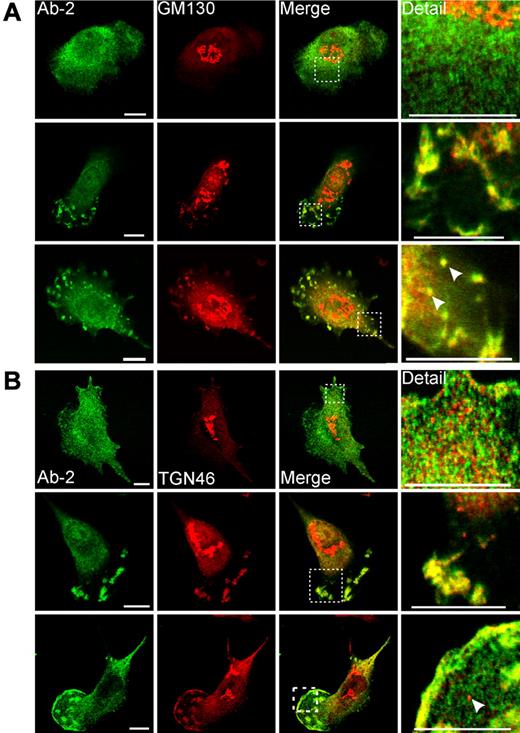
A significant proportion of human primary macrophages cultured in the presence of IL-4 also showed colocalization of FXIII-A and Golgi proteins in plasma membrane–associated structures (Figure 5). The appearance of these structures was more variable in primary cells than in THP-1 cells. In some instances, cells showed a morphology that is characteristic of migrating macrophages, having fan-like structure containing numerous podosome-like projections.29 In other cells, FXIII-A and Golgi proteins were present in apical membrane blebs or were concentrated in close apposition to the plasma membrane. Of particular interest in some cells FXIII-A and GM130 colocalized in small round structures, which were confirmed to be intracellular vesicles by imaging optical sections at serial z-planes (supplemental Figure 3B). Again, in agreement with observations in THP-1 cells, in rare cases in monocyte-derived macrophages, FXIII-A was associated with structures clearly continuous with Golgi cisternae (supplemental Figure 3A).
Immunofluorescent detection of FXIII-A in primary monocyte-derived macrophages. Macrophages were treated for 6 days with IL-4 (20 ng/mL). After fixation and permeabilization, cells were probed with anti–FXIII-A antibody Ab-2 and with antibodies to Golgi markers GM130 (A) and TGN46 (B). (A-B top rows) The morphology represents the majority of cells present, and in these cells Ab-2 fluorescence was distributed throughout the cytosol. (A-B middle rows) Macrophages are shown that exhibited a morphology characteristic of migrating cells. In these cells, Ab-2 and Golgi marker fluorescence were colocalized in plasma membrane–associated ruffles and podosome-like structures. (A bottom row) A cell shows podosomes and membrane blebs containing both FXIII-A and GM130. In addition, candidate transport vesicles are present (arrowheads). The intracellular topology of these vesicles is established in supplemental Figure 3B. (B bottom row) A cell shows extensive colocalization of FXIIIA and TGN46 to the plasma membrane in addition to their presence in podosome-like structures. A representative TGN46-positive but FXIII-A–negative vesicle is shown with an arrowhead. TGN46-positive vesicles were apparent in most macrophages examined but were negative for FXIII-A in all fields examined.
Immunofluorescent detection of FXIII-A in primary monocyte-derived macrophages. Macrophages were treated for 6 days with IL-4 (20 ng/mL). After fixation and permeabilization, cells were probed with anti–FXIII-A antibody Ab-2 and with antibodies to Golgi markers GM130 (A) and TGN46 (B). (A-B top rows) The morphology represents the majority of cells present, and in these cells Ab-2 fluorescence was distributed throughout the cytosol. (A-B middle rows) Macrophages are shown that exhibited a morphology characteristic of migrating cells. In these cells, Ab-2 and Golgi marker fluorescence were colocalized in plasma membrane–associated ruffles and podosome-like structures. (A bottom row) A cell shows podosomes and membrane blebs containing both FXIII-A and GM130. In addition, candidate transport vesicles are present (arrowheads). The intracellular topology of these vesicles is established in supplemental Figure 3B. (B bottom row) A cell shows extensive colocalization of FXIIIA and TGN46 to the plasma membrane in addition to their presence in podosome-like structures. A representative TGN46-positive but FXIII-A–negative vesicle is shown with an arrowhead. TGN46-positive vesicles were apparent in most macrophages examined but were negative for FXIII-A in all fields examined.
Discussion
Transglutaminases have both intracellular and extracellular functions and they lack ER-Golgi signal sequences.30 FXIII-A has been proposed to be secreted into the plasma from platelets,12,13 but our observations on thrombocytopenic mice call this into question. In Bcl-xPlt20/Plt20 mice, the number of platelets produced per day (and hence the number of platelets cleared) is essentially normal, but a marked decrease in platelet life span greatly reduces steady-state platelet counts.20 Bcl-xPlt20/Plt20 mice maintained plasma FXIII-A at near normal levels. There was no evidence that platelet FXIII-A levels had been sufficiently up-regulated to offset thrombocytopenia, and it would be surprising if this had occurred because FXIII-A+/− heterozygous mice do not up-regulate FXIII-A production from the functional allele.31 Although this excludes continuous secretion throughout the platelet lifetime as the main source of plasma FXIII-A, it does not exclude early release from nascent platelets or late release from senescent platelets. Therefore, we also examined Mpl−/− mice, in which thrombocytopenia results from severely decreased biogenesis of megakaryocytes; hence, the numbers of platelets produced and cleared per day are both reduced.21 FXIII-A plasma levels were again normal, further excluding the possibility that platelets are the main source of plasma FXIII-A. These results also suggest that megakaryocytes cannot be the source, unless there is an unexpected increase in megakaryocyte FXIII-A concentrations in Mpl−/− mice. Further studies are planned to address this issue. However, our results are consistent with observations in human subjects that thrombocytopenia is not invariably associated with decreased plasma FXIII-A levels.17,18 Although the cell type responsible for maintaining plasma FXIII-A remains to be identified, these findings implicate nucleated cells in its release. We therefore examined the intracellular targeting and possible release of FXIII-A in THP-1 (monocytic leukemic) cells.
Under certain circumstances, FXIII-A appears to show reciprocal expression with the homologous transglutaminase, TG2.22 Accordingly, we confirmed that FXIII-A is down-regulated and TG2 is induced, when THP-1 cells become adherent. IL-4 polarizes primary macrophages to the M2 phenotype (which is reparative rather than inflammatory) and causes them to up-regulate FXIII-A expression.32-34 Here, we have shown that IL-4 acts similarly in adherent THP-1 cells (supplemental Figure 2A-B), allowing us to examine the intracellular distribution and secretion of FXIII-A in adherent as well as in nonadherent cells.
Metabolic labeling of THP-1 cells did not detect constitutive secretion of 35S–FXIII-A. However, it seemed possible that FXIII-A would be secreted from these cells under conditions of cell stress when local release of FXIII-A into the ECM may be required for wound healing. We confirmed that LPS-induced stress caused the release of the inflammatory mediator IL-1β, which has been described as occurring by one or more nonclassical pathways.6,35-37 However, FXIII-A was not released under these circumstances. These results may imply that FXIII-A does not have the targeting information to enter the IL-1β pathway, but, bearing in mind the heterogeneous distribution of TG2 and FXIII-A expression between individual cells (Figure 4B), it may also transpire that FXIII-A is not expressed in those cells in which the IL-1β pathway is active. In either case, FXIII-A must be released by a separate pathway.
The partial retention of FXIII-A in cytosol-depleted THP-1 cell ghosts (Table 1) suggests that FXIII-A binds to, or is contained within, intracellular structures. Immunofluorescence with rabbit polyclonal antibody Ab-2 in THP-1 cells (independently confirmed with a sheep polyclonal anti–FXIII-A antibody) showed the presence of antigen in the cytosol and the nucleus of both adherent and nonadherent cells. Nuclear localization may account for the significant proportion of FXIII-A that remains cell associated after SLO treatment. We did not observe convincing accumulation of FXIII-A in the nuclei of adherent primary macrophages (data not shown), which is consistent with a previous report that FXIII-A localizes transiently to the nucleus of primary monocyte/macrophages during adherence and differentiation.38 Possibly, persistent nuclear localization is a particular feature of transformed or dividing cells, because a dual cytosolic and nuclear distribution of FXIII-A was also observed in transfected COS-1 cells (supplemental Figure 4B). The functional significance of FXIII-A translocation to the nucleus remains unclear. Of particular note, however, we found that in a proportion of adherent THP-1 cells FXIII-A was concentrated in structures adjacent to the plasma membrane. Some of these structures resembled podosomes, structures implicated in cell migration.39 We also found that primary macrophages concentrated FXIII-A in podosome-like structures, but, in addition, other plasma membrane–associated structures resembling blebs and ruffles contained FXIII-A in these cells.
We also examined immunofluorescence in THP-1 cells with the use of AC-1A1, a monoclonal antibody raised to recombinant FXIII-A. This immunofluorescence also colocalized with Golgi proteins in podosome-like structures and was present in the cytosol (not shown). Zaba et al40 observed correspondence between dermal cells that were stained with AC-1A1 and those stained with a polyclonal anti–FXIII-A antiserum, apparently confirming the specificity of AC-1A1. However, Nakano et al41 and Al Jallad et al42 have shown that AC-1A1 preferentially detects a 37-kDa protein in mouse osteoblasts, macrophages and in bone protein extracts. This group has assigned this protein as a catalytically active proteolytic fragment of FXIII-A. We similarly observed that AC-1A1 detects a 37-kDa protein in THP-1 cells but also that it detected a 37-kDa protein in bone protein extracts from FXIII-A−/− mice (data not shown). Because in FXIII-A−/− mice the exon encoding the catalytic cysteine residue is deleted, the 37-kDa protein in bone and THP-1 cells cannot be unambiguously assigned and neither can we deduce that AC-1A1 immunofluorescence is wholly due to FXIII-A–derived antigens in THP-1 cells. Further, there are differences in the distribution of AC-1A1, and immunofluorescence shows some differences in distribution to Ab-2 fluorescence, particularly in the detection of perinuclear Golgi elements but also of nuclei (not shown).
Recent interest has focused on the involvement of certain Golgi proteins in nonclassical secretion.43 For example, a member of the GRASP (Golgi reorganization and stacking protein) family mediates the secretion of acyl CoA binding protein in Dictyostelium (and possibly the equivalent protein in mammalian neurones) by delivering acyl CoA binding protein to the plasma membrane to facilitate its extrusion through membrane pores.44 Similarly, in Drosophila (d), dGRASP and its binding partner dGM (Golgi Matrix)130 (which are both conventionally considered cis-Golgi markers)45 redistribute to open zones of contact which are established between epithelial cells at a certain stage of development.46 At these sites, they mediate the Golgi-cisternae independent secretion of integrin α-subunit. In macrophages, we observed that GM130 colocalized with FXIII-A, both in plasma membrane–associated structures and also in candidate FXIII-A transport vesicles (Figure 5A, supplemental Figure 3B). In contrast, FXIII-A was not detected in classical secretory vesicles marked by TGN46 (Figure 5B), an integral membrane protein of the trans-Golgi network.47 Presumably, TGN46 is delivered to plasma membrane–associated structures separately from GM130, and it is notable that certain classically secreted proteins such as matrix metalloproteinases48 (whose presence would be expected within TGN46-positive vesicles) are also preferentially released from sites of podosome formation.
It has been shown that dGM130 and dGRASP remain associated with Drosophila epithelial open zones of contact after treatment with Brefeldin A,46 an antibiotic that inhibits ER to the Golgi, but not retrograde, transport.49 Similarly, we showed that FXIII-A and Golgi markers in plasma membrane–associated structures were not dispersed by Brefeldin A treatment under conditions in which Golgi cisternae become vesiculated, implying similarities in membrane trafficking in these 2 contexts.
Delivery of FXIII-A on the outer face of vesicles (where GM130 is exposed) would result in its accumulation on the inner face of the plasma membrane. However, in the presence of plasma membrane transporter, a proportion of FXIII-A could be either externalized or secreted. Similarly, if a transporter is present in the membrane of GM130-positive vesicles, this could mediate FXIII-A secretion if these vesicles fuse with the plasma membrane. The expression of a membrane transporter may be specific for cell type and developmental stage.
Akimov and Belkin22 previously reported the presence of TG2 in podosomes of THP-1 cells and, further, that TG2 was present on the outer surface of these structures. However, using the same methods, they deduced that a considerable proportion of total FXIII-A in THP-1 cells was surface exposed, whereas our immunofluorescence data in both nonadherent and adherent THP-1 cells suggest that FXIII-A is overwhelmingly intracellular in the majority of cells. We are currently attempting to establish methods that will unambiguously identify and quantify any extracellular pool of transglutaminases that may be present.
In summary, FXIII-A is distributed between the cytosol and the nucleus but is also delivered to plasma membrane–associated structures that appear to include podosomes. Golgi proteins GM130 and TGN46 are also delivered to these structures, but apparently in distinct vesicles, with FXIII-A present in GM130-positive vesicles. To our knowledge, accumulation of these Golgi proteins at the surface of the cell has not been previously reported, and its significance merits further studies. This Golgi-mediated delivery mechanism may only apply to nucleated cells, because morphologically identifiable Golgi elements have been shown to be excluded from proplatelets during thrombopoiesis50 ; hence, it may not be possible to generate the requisite structures within platelets. Further, we have observed considerably reduced immunofluorescence staining for GM130 and TGN46 in FXIII-A–positive platelets relative to macrophages (not shown). Golgi-mediated concentration of FXIII-A at the plasma membrane could precede its nonclassical secretion from nucleated cells, and this seems more credible as a source of plasma FXIII-A than its release from platelets on the basis of our observations of thrombocytopenic mice.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof Anne-Marie Surprenant (The University of Manchester) for useful discussions about IL-1β secretion, Drs Sreenivasan Ponnambalam and Gareth Howell (University of Leeds) for advice and assistance with microscopy, Drs Nadira Yuldasheva and Stephen Wheatcroft (University of Leeds) for assistance with mouse blood sampling, and Mr Philip Day (Leeds Teaching Hospitals Trust) for assistance with automated blood counting.
This work was supported by the British Heart Foundation (grant (PG/03/080/15 731), by a Leeds Multidisciplinary Cardiovascular Research Center Career Development Grant (P.A.C.), and by a fellowship from the Leukemia & Lymphoma Society (E.C.J.).
Authorship
Contribution: P.A.C. performed experimental work and contributed to experimental design/interpretation and to writing the manuscript; R.J.P. formulated the original study design and funding application, and contributed to ongoing data analysis and to writing the manuscript; B.T.K., K.F.S., and E.C.J. contributed to experimental design; and P.J.G. contributed to experimental design and writing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Richard J. Pease, Division of Cardiovascular and Diabetes Research, The LIGHT Laboratories, Clarendon Way, University of Leeds, Leeds LS2 9JT, United Kingdom; e-mail: r.j.pease@leeds.ac.uk.





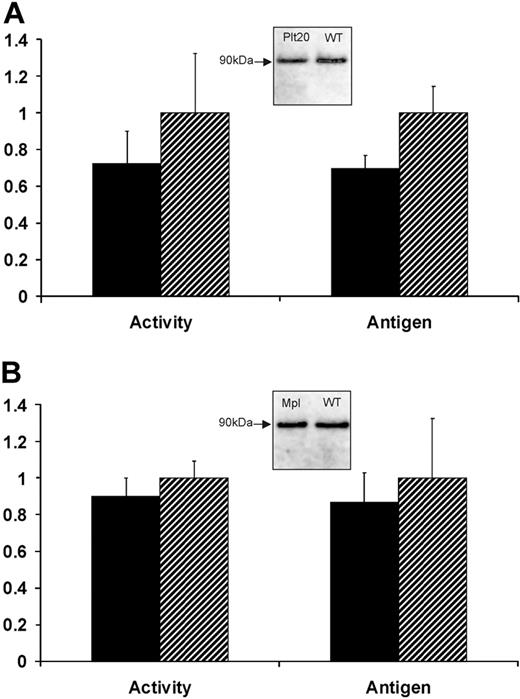
 ) and was assayed for FXIII-A enzyme activity or for FXIII-A antigen levels (by IP with Ab-2 followed by IB with HRP-conjugated sheep anti–FXIII-A antiserum). Results show levels of FXIII-A in the thrombocytopenic mice relative to controls and are based on 6 animals in each group. Insets show immunoblots of immunoprecipitated FXIII-A from pooled plasma.
) and was assayed for FXIII-A enzyme activity or for FXIII-A antigen levels (by IP with Ab-2 followed by IB with HRP-conjugated sheep anti–FXIII-A antiserum). Results show levels of FXIII-A in the thrombocytopenic mice relative to controls and are based on 6 animals in each group. Insets show immunoblots of immunoprecipitated FXIII-A from pooled plasma.