Abstract
Preclinical data demonstrate enhanced antitumor effect when lumiliximab, an anti-CD23 monoclonal antibody, is combined with fludarabine or rituximab. Clinical data from a phase 1 trial with lumiliximab demonstrated an acceptable toxicity profile in patients with relapsed or refractory chronic lymphocytic leukemia (CLL). We therefore pursued a phase 1/2 dose-escalation study of lumiliximab added to fludarabine, cyclophosphamide, and rituximab (FCR) in previously treated CLL patients. Thirty-one patients received either 375 mg/m2 (n = 3) or 500 mg/m2 (n = 28) of lumiliximab in combination with FCR for 6 cycles. The toxicity profile was similar to that previously reported for FCR in treatment of relapsed CLL. The overall response rate was 65%, with 52% of patients achieving a complete response (CR), which compares favorably with the CR rate previously reported for the FCR regimen alone in relapsed CLL. The estimated median progression-free survival for all responders was 28.7 months. The addition of lumiliximab to FCR therapy is feasible, achieves a high CR rate, and does not appear to enhance toxicity in previously treated patients with CLL. A randomized trial comparing lumiliximab plus FCR with FCR alone is underway to define the benefit of this combination in relapsed CLL. This trial was registered at clinicaltrials.gov as NCT00103558.
Introduction
Chronic lymphocytic leukemia (CLL) is the most common type of adult leukemia.1 CLL cells express the B-cell markers CD19, CD20, CD23, and surface Immunoglobulin (dim) [sIg (dim)] with coexpression of the T-cell marker CD5.2 Recent data indicate that select genetic features, including interphase cytogenetics, immunoglobulin gene mutational status, and ZAP-70 expression, contribute to the heterogeneity of CLL and potentially influence prognosis.3-6 Several of these prognostic features may impact treatment response and response duration.7-11 Despite the identification of these important prognostic features, treatment of CLL is initiated only at time of symptomatic disease because early treatment has not been shown to convey a survival advantage.
The initial treatment of symptomatic CLL has evolved significantly over the past decade. Monotherapy with chlorambucil or fludarabine have both been shown to be inferior to the combination of fludarabine and cyclophosphamide (FC) with respect to overall response rate (ORR), complete response (CR) rate, and progression-free survival (PFS) in younger patients with CLL.10-12 One uncontrolled phase 2 study of the combination of fludarabine, cyclophosphamide, and rituximab (FCR) in previously untreated patients noted a higher ORR (95%) and CR rate (70%) compared with that seen in historical control patients treated with FC at the same institution. This response rate was also higher than that seen in FC-treated patients in the above-mentioned studies.13 At the same time, a parallel study administered FCR to previously treated patients with CLL.14 This study reported a 73% ORR but only a 25% CR rate.14 However, in both these trials with FCR, patients exhibiting a CR had an extended PFS and overall survival compared with patients with a partial response (PR), similar to other previously reported trials in CLL.15 The complications of FCR as initial and salvage therapy were manageable in patients less than 70 years of age and included myelosuppression and infection. Although CR and PFS in subsequent phase 3 studies are less than that observed in the pilot phase 2 studies of FCR, the benefit of this 3-drug combination has been confirmed in both the front-line16 and relapse17 settings.
Lumiliximab is a genetically engineered (macaque variable regions, human constant regions) monoclonal antibody targeting CD23, a transmembrane glycoprotein expressed on the majority of CLL cells.18,19 Lumiliximab induces similar levels of apoptosis to rituximab in CD23-bearing lymphoid cell lines and CLL cells after secondary cross-linking, and prolongs survival of severe combined immune deficiency mice inoculated with CD23-bearing lymphoblastic cell lines.20 In preclinical studies, lumiliximab was shown to enhance the effects of fludarabine and rituximab, providing a rationale for combining lumiliximab with regimens containing fludarabine and rituximab in clinical trials in CLL.20 As CD23 is expressed on a high proportion of CLL cells but is only minimally expressed on other cells, targeting this molecule provides a treatment modality that is specific to CLL with the potential to minimize additional toxicity. In a 46-patient, phase 1, dose-escalation trial performed in patients with relapsed and refractory CLL, lumiliximab monotherapy was well tolerated at doses of up to 500 mg/m2 given 3 times per week for 4 weeks.21 Although no CRs or PRs were noted in this trial, evidence of disease reduction was observed in a subset of patients.21 Seventeen of 33 patients (52%) had a decrease in lymph node bulk, and 42 of 46 patients (91%) had modest reduction in lymphocytosis. However, these effects were transient, with most patients progressing by 2 months after therapy. No additional benefit was observed with the more frequent dosing regimens at 500 mg/m2. The recommended dose for future studies of lumiliximab in combination with other agents was 500 mg/m2.
Based on this favorable safety profile and preclinical enhancement of the antitumor effect of both rituximab and fludarabine,20 we sought to perform a phase 1/2 study adding lumiliximab to the FCR regimen for patients with previously treated CLL. We hypothesized that lumiliximab might enhance the effectiveness of FCR-based therapy without exacerbating the toxicity observed with FCR.
Methods
Patients
Enrollment occurred from June 2004 to December 2005. Patients with symptomatic, previously treated CLL were considered eligible if they (1) provided written informed consent in accordance with the Declaration of Helsinki, (2) were at least 18 years of age, (3) had CD23+ CLL by 1996 National Cancer Institute Working Group criteria (NCIWG 96),22 (4) had progressive disease defined by the NCIWG 96 criteria after at least one prior course of therapy, (5) had a prestudy World Health Organization performance status less than or equal to 2, (6) had an expected survival of at least 6 months, (7) had a 4-week interval of no radiotherapy, radioimmunotherapy, biologic therapy, or chemotherapy before study enrollment, (8) had adequate liver and renal function (bilirubin ≤ 2.0 mg/dL, aspartate aminotransferase or alanine aminotransferase ≤ 2× institutional upper limit of normal, and serum creatinine ≤ 1.5× institutional upper limit of normal), (9) had platelet counts greater than 50 × 109/L, (10) had an absolute neutrophil count greater than 109/L, and (11) were not refractory to FCR therapy. FCR-refractory disease was defined as no response to this regimen or progression within 6 months of completion of therapy. The study as outlined herein was reviewed and approved by the institutional review boards of all participating institutions.
Pretreatment and serial laboratory assessments
Baseline laboratory assessments included complete blood count with differential, platelet count, and absolute lymphocyte count; serum chemistries, including liver functions; urinalysis; direct and indirect antibody tests; β-2-microglobulin; interphase cytogenetics23 ; and an electrocardiogram. In the absence of disease progression, patient samples for complete blood count and serum chemistry measurements were collected biweekly during the treatment period, monthly during the posttreatment follow-up period up to month 12, every 3 months after month 12 to the end of year 2, then every 6 months after year 2 to the end of year 4. Computed tomographic scans were not performed as part of the response evaluation criteria.
Treatment
Patients were assigned sequentially to cohorts 1, 2, or phase 2 expansion, and received up to 6 cycles (according to tolerability/disease progression) of lumiliximab plus FCR at intervals of 28 days. Stepped-up dosing of rituximab and lumiliximab was given for all patients in cycle 1. Patients in cohort 1 received intravenous (IV) rituximab 50 mg/m2 on day 1 of cycle 1 and 325 mg/m2 IV on day 3; lumiliximab 50 mg/m2 IV on day 2, and 325 mg/m2 IV on day 4; fludarabine 25 mg/m2 IV on days 2, 3, and 4; and cyclophosphamide 250 mg/m2 IV on days 2, 3, and 4. In cycles 2 to 6, cohort 1 received rituximab 500 mg/m2 IV and lumiliximab 375 mg/m2 IV on day 1; fludarabine 25 mg/m2 IV on days 1, 2, and 3; and cyclophosphamide 250 mg/m2 IV on days 1, 2, and 3. Patients in cohort 2 received rituximab 50 mg/m2 IV on day 1 of cycle 1 and 325 mg/m2 IV on day 3; lumiliximab 50 mg/m2 IV on day 2 and 450 mg/m2 on day 4; fludarabine 25 mg/m2 IV on days 2, 3, and 4; and cyclophosphamide 250 mg/m2 IV on days 2, 3, and 4. In cycles 2 to 6, cohort 2 received rituximab 500 mg/m2 IV and lumiliximab 500 mg/m2 IV on day 1; fludarabine 25 mg/m2 IV on days 1, 2, and 3; and cyclophosphamide 250 mg/m2 IV on days 1, 2, and 3. The first infusion of lumiliximab was given over a 4-hour period with the infusion rate in subsequent infusions gradually increased such that infusions were completed in an approximately 2 hours, unless infusion reactions necessitated a rate reduction. The rituximab infusion was given according to the package insert. Granisetron hydrochloride (or equivalent) was provided for all patients on each day of therapy and as clinically indicated. All patients received allopurinol for the first 10 days of therapy. Other supportive care was administered at the discretion of the treating physician. All patients received oral acetaminophen and diphenhydramine hydrochloride before each dose of antibody therapy.
Toxicity assessment and dose-limiting toxicity
Toxicity assessments were based on the National Cancer Institute Common Toxicity Criteria version 3. Dose-limiting toxicity (DLT) was defined as occurrence of any of the following adverse events with possible, probable, or unknown relationship to lumiliximab occurring up to day 28: grade 3 or greater nonhematologic toxicity (excluding electrolyte disorders, headache, fatigue, nausea, vomiting, and diarrhea, which are expected with fludarabine treatment); grade 2 acute allergic infusion reactions, consisting of urticaria and/or asymptomatic bronchospasm; and grade 4 hematologic toxicity persisting for 14 days or more.
Criteria for dose escalation
A standard 3 + 3 dose-escalation schema was followed with the plan to escalate from the cohort-1 to the cohort-2 dose if 0 of the first 3 patients or 1 of 6 patients experienced a DLT. Similar criteria were used in cohort 2 such that the 375 mg/m2 lumiliximab dose would be defined as the recommended phase 2 dose if one or more of the first 6 patients in this cohort experienced a DLT. After enrollment of the 6 patients in cohort 2, expansion of this cohort by a further 22 patients occurred to further assess safety and preliminary efficacy of this regimen.
Response assessments
Patients were assessed for response at weeks 13 and 25. The primary efficacy variable in this study was ORR, defined as the percentage of patients with response classified as CR or PR, using the NCIWG 96 criteria for CLL.
Pharmacokinetics
Serum for lumiliximab and rituximab pharmacokinetics were collected preinfusion, 10 minutes, and 1, 2, and 24 hours after the completion of the infusion on study days 1, 3, and 4 of cycle 1, and on day 1 of cycles 3 and 6. Additional samples were collected before the treatment on day 3 of cycles 3 and 6, and on day 1 of weeks 3, 5, 11, 13, 23, and 25. Total serum concentrations of lumiliximab were determined using a validated enzyme-linked immunosorbent assay (ELISA) developed by Biogen Idec, in which a monoclonal anti-lumiliximab antibody was used as the capture reagent, followed by a blocking step and incubation with standards, controls, and patient samples. Lumiliximab in patient samples was detected by the addition of antihuman IgG-horseradish peroxidase (Southern Biotech); color was developed with tetramethylbenzidine substrate, and lumiliximab concentrations were calculated by extrapolation from a 4-parameter standard curve. The assay was validated according to International Conference on Harmonization guidelines and had a lower limit of quantitation of 450 ng/mL. Total serum concentrations of rituximab were determined using a validated ELISA developed by Biogen Idec, in which a polyclonal anti-rituximab antibody was used as the capture reagent, followed by a blocking step and incubation with standards, controls, and patient samples. Rituximab in patient samples was detected by the addition of antihuman IgG-horseradish peroxidase (Southern Biotech); color was developed with ABTS (2,2′-Azinobis[3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt) substrate, and rituximab concentrations were calculated by extrapolation from a 4-parameter standard curve.
Noncompartmental pharmacokinetic methods were used to estimate the disposition of both lumiliximab and rituximab. The program WinNonlin 5.2 (Pharsight) was used to estimate the pharmacokinetic parameters from cycles 1, 3, and 6. Parameters estimated included the maximum concentration (Cmax), the area under the curve from time of dosing to infinity, half-life, clearance, and volume of distribution. Estimates were generated separately for each cycle.
Pharmacodynamic studies
CD23 receptor occupancy by lumiliximab was assessed using flow cytometry. Peripheral blood samples for flow cytometry were collected in EDTA tubes at baseline and after treatment with lumiliximab and FCR. 2 noncompetitive anti-CD23 antibodies (anti-CD23 PE and lumiliximab–fluorescein isothiocyanate [FITC]) were used in a flow cytometric assay to assess the receptor occupancy of lumiliximab on the surface of B-CLL cells. Lymphocytes were gated on CD45 and side-scatter followed by gating on CD5+CD19+ cells to identify B-CLL cells.
Anti-lumiliximab antibodies
A validated anti-lumiliximab ELISA developed by Biogen Idec was used to determine the concentration of human antibody to lumiliximab in serum. Lumiliximab was used as a capture reagent. After overnight incubation, plates were blocked then incubated with standards, controls, and patient samples. Anti-lumiliximab antibodies were detected using lumiliximab conjugated to horseradish peroxidase and tetramethylbenzidine as the substrate. Samples were tested at baseline, at weeks 13, 25, 41, and at month 12 after the first dose of lumiliximab. The lower limit of quantitation for this assay was 400 ng/mL of anti-lumiliximab antibody.
Anti-rituximab antibodies
A validated human anti-chimeric antibody (HACA)–specific ELISA developed by Biogen Idec was used to determine the concentration of anti-rituximab antibody in serum. A chimeric monoclonal antibody was used as a capture reagent. After overnight incubation, plates were blocked and then incubated with standards, controls, and patient samples. Human anti-chimeric antibodies were detected using a biotinylated monoclonal chimeric antibody, streptavidin-horseradish peroxidase, and tetramethylbenzidine substrate. Samples were tested at baseline, at weeks 13, 25, 41, and at month 12 after the first dose of rituximab.
Interphase cytogenetic analysis
Peripheral blood or bone marrow samples were cultured for 3 days with Pokeweed mitogen (final concentration 10 μL/mL; Sigma Aldrich) and phorbol 12-myristic 13-acetate (final concentration 40 ng PMA/mL; Sigma Aldrich) to stimulate the B cells. Harvest and slide making were by standard laboratory procedures. Probes for FISH were D12Z1 (12 centromere), TP53 (17p13.1), ATM (11q22.3), and D13S319 (13q14), all from Abbott Molecular, and were used according to the manufacturer's directions.
Statistical methods
Statistical analyses were performed using SAS statistical software (SAS Institute Inc), version 9.0. Adverse events were coded using MedDRA Version 11.0 (Northrop Grumman). Response rates were summarized using frequencies and percentages with 95% confidence intervals by exact binomial methods. Median time-to-event measures and graphs for time-to-event variables were generated using Kaplan-Meier methodology, with 95% confidence intervals by the sign test. PFS was measured from the day of registration until progression, relapse, or death due to any cause. Statistical testing was not performed.
Results
Patient baseline characteristics
Thirty-one patients gave consent and were treated at 5 clinical sites. Patient baseline characteristics are summarized in Table 1. The median age was 58 years with 8 patients being at least 65 years old. The majority of patients (71%) had Rai stage I/II disease, and the median β-2 microglobulin level was 3.3 μg/mL. The median number of therapies was 2, with 61% of patients having received fludarabine. Three subjects were refractory to fludarabine, as defined by failure to achieve a complete or partial response of at least 6 months duration after their last fludarabine-containing treatment regimen.
Toxicity assessments
Eight patients (26%) experienced grade 3 and 12 patients (39%) experienced grade 4 toxicities considered to be related to the study treatment (Table 2). These toxicities were those typically expected with the chemoimmunotherapy regimen. There were no DLTs and, as CD23 saturation was complete and sustained at the 500 mg/m2 lumiliximab dose,21 no escalation above this dose was performed.
Infections are common in active CLL and, not unexpectedly, were observed in patients participating in this trial. During treatment, three grade 3 infections (a clostridial infection, a cytomegalovirus infection, and a bacterial wound infection) were observed. All other infections were grade 1 or 2, with upper respiratory sites being most common.
The proportion of cycles completed was 1 to 3 cycles in 11 patients (35%), 4 cycles in 3 patients (10%), and 5 cycles in 2 patients (6%). Fifteen patients (48%) completed the intended 6 cycles of therapy. Nine patients (29%) discontinued therapy due to cytopenia, 2 of whom had neutropenic fever.
Response and response duration
Of the 31 patients enrolled, 20 (65%) exhibited a confirmed CR or PR by NCIWG 96 criteria. Sixteen of 31 patients (52%) attained a CR, including normocellular marrow, and normal neutrophil and platelet counts as prescribed by these guidelines. The kinetics of response in patients receiving this regimen were quick, with 14 of 20 responders (70%) having a normal lymphocyte count by the beginning of cycle 2, whereas a further 1 of 20 responders (5%) had normalization by the beginning of cycle 3.
Based on a median follow-up of 17.1 months (range, 1.5-47.7 months), the median PFS was 30.4 months (range, 9.8-47.7 months) for complete responders, 28.7 months (range, 6.9-47.7 months) for all responders, and 19.3 months (range, 1.5-47.7 months) for all patients (Figure 1A). The median duration of response (DR) was 27.5 months (range, 7.0-44.9 months) for patients with CR (Figure 1B) and 8.1 months (range, 4.2-33.1 months) for patients with PR. However, 8 of the responding patients (40%) had not yet died or demonstrated progression at the time of this analysis (January 9, 2009) and continue to be followed up for PFS and DR. These patients were censored for these analyses. Twenty-six of the 31 patients discontinued the study before the end of the 4-year follow-up period: 24 due to disease progression requiring subsequent CLL therapy, 1 for personal reasons, and 1 due to death. Twelve patients died during long-term follow-up; 9 deaths were due to disease progression.
Kaplan-Meier analyses of response. PFS for all patients (A) and DR (B) for all responders (PR and CR) treated with fludarabine, cyclophosphamide, rituximab, and lumiliximab.
Kaplan-Meier analyses of response. PFS for all patients (A) and DR (B) for all responders (PR and CR) treated with fludarabine, cyclophosphamide, rituximab, and lumiliximab.
Response by interphase cytogenetics
Several studies have demonstrated the importance of interphase cytogenetics in predicting response and response duration of combined chemoimmunotherapy and have recently gained acceptance in the community. Therefore, we assessed the status of the most common abnormalities observed in CLL. Using the Döhner prioritization schema,3 the proportion of patients with each abnormality included 13% (4 of 31) with del(17p13.1), 26% (8 of 31) with del(11q22.3), 19% (6 of 31) with trisomy 12, and 42% (13 of 31) with del(13q14). One of 4 patients (25%) with del(17p13.1) had a transient response to therapy, whereas 5 of 8 (63%) patients with del(11q22.3) attained a CR.
Pharmacokinetics
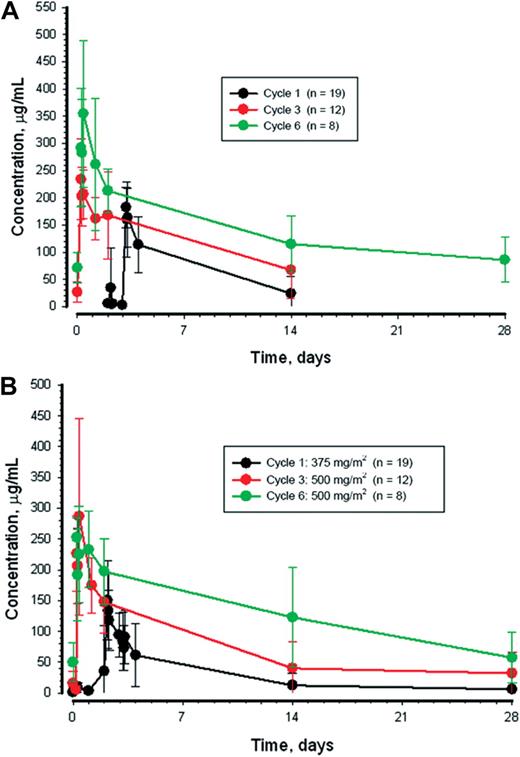
Across both lumiliximab dose cohorts, 19 (61%), 15 (48%), and 10 (32%) of 31 patients at cycles 1, 3, and 6, respectively, had measurable concentrations that permitted the calculation of pharmacokinetic variables for lumiliximab. Similarly, 22 (71%), 15 (48%), and 10 (32%) patients had measurable concentrations of rituximab that permitted pharmacokinetic calculations from cycles 1, 3, and 6, respectively. Mean plus or minus standard deviation serum concentrations over time for patients treated with 500 mg/m2 of lumiliximab are shown in Figure 2, and pharmacokinetic parameters are provided in Table 3. Volume of distribution for both lumiliximab and rituximab were similar to plasma volume. Clearance of both antibodies was reduced with continued treatment resulting in increases in the half-life and area under the curve values. Cmax roughly doubled for both lumiliximab and rituximab from cycle 1 to 6.
Plasma concentrations. Mean ± SD plasma concentrations of lumiliximab (A) and rituximab (B) during cycles 1, 3, and 6 of treatment for patients who received 500 mg/m2 of lumiliximab.
Plasma concentrations. Mean ± SD plasma concentrations of lumiliximab (A) and rituximab (B) during cycles 1, 3, and 6 of treatment for patients who received 500 mg/m2 of lumiliximab.
Pharmacodynamics
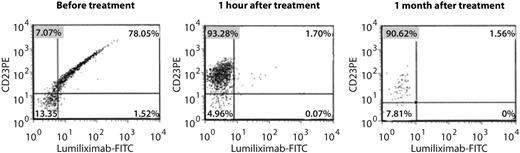
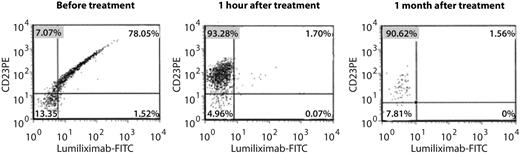
Flow cytometric assessment of CD23 receptor occupancy showed that a high percentage of cells were anti-CD23-PE positive and lumiliximab-FITC negative 1 hour after dosing with lumiliximab, indicating a high level of receptor occupancy by lumiliximab, and receptor occupancy was sustained at 1 month postdose (Figure 3).
Receptor occupancy by lumiliximab in the presence of sCD23. Flow cytometric antibody assay used to measure the relative binding of lumiliximab to CD23. CD23-expressing cells are labeled with anti–CD23-PE antibodies that recognize an epitope different from that of lumiliximab. Cells are subsequently stained with lumiliximab-FITC. If the B-CLL cells have lumiliximab bound to CD23, lumiliximab-FITC, which is added during the assay period, will not bind to CD23, indicating that lumiliximab administered to the patient is still bound. FITC indicates fluorescein isothiocyanate.
Receptor occupancy by lumiliximab in the presence of sCD23. Flow cytometric antibody assay used to measure the relative binding of lumiliximab to CD23. CD23-expressing cells are labeled with anti–CD23-PE antibodies that recognize an epitope different from that of lumiliximab. Cells are subsequently stained with lumiliximab-FITC. If the B-CLL cells have lumiliximab bound to CD23, lumiliximab-FITC, which is added during the assay period, will not bind to CD23, indicating that lumiliximab administered to the patient is still bound. FITC indicates fluorescein isothiocyanate.
Anti-lumiliximab and anti-rituximab antibodies
Thirteen of the 31 treated patients had evaluations of serum anti-lumiliximab antibodies and anti-rituximab antibodies measured at both baseline and at least one postdose time point (weeks 13, 25, 41, or month 12). All measured values were undetectable.
Discussion
This is the first study of lumiliximab in combination with FCR in patients with relapsed or refractory CLL. Treatment with lumiliximab combined with FCR was well tolerated, resulted in a high number of patients with a durable CR, and was not associated with increased infectious risk or prolonged cytopenias compared with historical controls.14 No notable toxicities outside of those typically expected with FCR were noted. The phase 2 expansion study demonstrated a favorable ORR and CR rate. Specifically, 20 patients (65%) exhibited an NCIWG 96 confirmed PR or CR, with 16 of these patients (52%) having a CR. These responses were durable, with a median DR of 27.5 months for patients exhibiting a CR and 8.1 months for patients with PR.
Although comparison with a historical control phase 2 trial of FCR has significant limitations, it is notable that the CR rate of 52% observed in this trial is double that of a large reported single institution trial by Wierda and colleagues14 in which 25% exhibited a CR. ORR was not significantly different in these 2 trials. Additional analysis indicates that the number of completed cycles of therapy is similar between this trial and the trial by Wierda et al (1-3 cycles, 35% vs 32%; 4 cycles, 10% vs 9%; 5 cycles, 6% vs 8%; and 6 cycles, 48% vs 46%). Given that attainment of CR seems to be a predictor of long-term remission and treatment-free interval, these results suggest that lumiliximab may represent an exciting new therapy for CLL that warrants further study. However, it must also be considered that differences in the eligibility criteria, such as the exclusion of patients with preexisting neutropenia or thrombocytopenia, and/or differences in baseline characteristics between the previously reported FCR study and this trial relative to median platelet count, mean β-2 microglobulin, and proportion of patients with Rai stage III/IV disease could explain this. Several trials in relapsed CLL14,24 have demonstrated that pretreatment thrombocytopenia and Rai stage disease may influence treatment response. Therefore, determining the influence of the addition of lumiliximab to FCR-based chemoimmunotherapy in previously treated CLL will require a randomized trial.
The clearance of both lumiliximab and rituximab was observed to decrease throughout the treatment period, resulting in increased half-life and increased exposure to the antibodies. Cmax was also observed to increase. Both of these factors are likely to be related to reduction in circulating CLL cells, with treatment resulting in fewer binding sites for the antibodies. This has previously been reported for the monoclonal antibodies rituximab25 and alemtuzumab.26
CD23 is highly expressed on the membrane of CLL B-cells and can be cleaved from the cell surface via an unknown mechanism, resulting in a several hundred-fold increase of the concentration of free serum CD23 compared with individuals without B-CLL. An increase in serum CD23 parallels the clinical stage of the disease, and serum CD23 has also been observed to increase substantially shortly after lumiliximab dosing in patients with CLL.21 Increased serum CD23 could, theoretically, interfere with the efficacy of lumiliximab by binding in the circulation. We therefore assessed receptor occupancy by lumiliximab in the presence of serum CD23. Receptor occupancy by lumiliximab was maintained at high levels at 1 month after dosing, which suggests that the serum CD23 increase does not affect lumiliximab binding at a lumiliximab dose of 500 mg/m2.
Overall, these data combined with the preclinical data provide a rationale for further investigation of lumiliximab in combination with FCR, as part of a randomized trial to determine the true added benefit of lumiliximab combined with FCR as a combination therapy for patients with CLL.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients for participating in this trial and the research staff at each site for making this study possible.
The clinical costs of this study were sponsored by Biogen Idec Inc. This work was also supported by the D. Warren Brown Foundation, Specialized Center of Research from the Leukemia & Lymphoma Society, and P01 CA 095426 from the National Cancer Institute.
Authorship
Contribution: J.C.B. assisted in designing the research, accrued patients to the study, reviewed/interpreted the data, drafted the first version of the paper, reviewed multiple revisions, and accepts responsibility for the accuracy of the paper as the corresponding author; T.J.K., I.W.F., J.C., T.S.L., and W.W. contributed to study design, accruing patients and reviewing the manuscript; N.H. contributed to the design of the research, performed the cytogenetic analysis and interpretation, and reviewed the manuscript; J.W., S. Hughes, and S. Harris contributed to study design, data collection and analysis, interpretation of data, and writing of the paper; S.T. contributed to data collection, analysis and interpretation of data, writing of the paper, contributed to study design, data collection, analyzed and interpreted the data, and reviewed the manuscript; D.W. contributed to the study design, data collection, and reviewed the manuscript; A.M. contributed to study design, data collection, analysis and interpretation of data, and editing of the paper; B.L. contributed to study design, data collection, and editing of the paper; and S.O. contributed to study design, accruing patients, and reviewing the manuscript.
Conflict-of-interest disclosure: J.W., S. Hughes, S.T., S. Harris, D.W., A.M., and B.L. are current or ex-employees of Biogen Idec. The remaining authors declare no competing financial interests.
Correspondence: John C. Byrd, B302 Starling Loving Hall, The Ohio State University, 320 West 10th Ave, Columbus, OH 43210; e-mail: john.byrd@osumc.edu.