Abstract
The stromal vascular fraction (SVF) in adipose tissue contains a pool of various stem and progenitor cells, but the existence of hematopoietic stem and progenitor cells (HSPCs) in the SVF has not been seriously considered. We detected the presence of HSPCs in the SVF by phenotypically probing with Lin−Sca-1+c-kit+ (LSK) and functionally confirming the presence using colony-forming cell assay and assessing the long-term multilineage reconstitution ability after SVF transplantation. The LSK population in the SVF was 0.004% plus or minus 0.001%, and 5 × 105 freshly isolated SVF cells gave rise to 13 plus or minus 4 multilineage colonies. In addition, 0.15% plus or minus 0.03% of SVF cells was home to bone marrow (BM), especially near vascular and endosteal regions, 24 hours after blood transplantation. SVF transplantation was capable of generating a long-term (> 16 weeks), but variable extent (2.1%-32.1%) multilineage reconstitution in primary recipients, which was subsequently transferred to the secondary recipients by BM transplantation. All HSPCs within the SVF originated from the BM. Furthermore, the granulocyte–colony-stimulating factor (G-CSF) mobilization of HSPCs from BM markedly elevated the number of phenotypic and functional HSPCs in the SVF, which induced a high efficiency long-term reconstitution in multilineage hematopoiesis in vivo. Our results provide compelling evidence that adipose tissue is a novel extramedullary tissue possessing phenotypic and functional HSPCs.
Introduction
Hematopoietic stem and progenitor cells (HSPCs) in the complicated hierarchy of the hematopoietic system maintain themselves and generate many kinds of blood cells throughout life. To maintain their proper functioning, HSPCs reside in a special microenvironment called a niche.1-3 Bone marrow (BM) is a dominant organ possessing a niche for HSPCs during adulthood. It is well known that a small fraction of HSPCs constantly circulate between the BM and peripheral blood without any stimulation.4,5 The exiting and re-entering of HSPCs is mediated by interactions between several surface molecules, such as selectins and integrins.1 In principle, on the way from peripheral blood back to the BM, HSPCs can settle down if the proper environment is provided. Supporting this concept, several extramedullary tissues were identified as HSPC-containing organs, including the liver,6 spleen,7 muscle,8,9 and thoracic duct.10 Among these organs, the liver and spleen are well-known locations for extramedullary hematopoiesis.1 In both tissues, HSPCs reside close to sinusoidal endothelial cells, which may provide a preferential microenvironment.6,11 The mechanism underlying HSPC maintenance in these extramedullary organs remains unclear, though the HSPCs still conserve their function properly.6-10
Adipose tissues contain many different types of adult stem cells that can be used for therapeutic purposes, especially for tissue regeneration.12 Adipose tissue is composed of lipid-filled mature adipocytes and other nonadipocyte cells, called the stromal vascular fraction (SVF).13 The SVF is not a fully defined cell population and is known to consist of various types of cells, including immune cells, fibroblasts, pericytes, endothelial cells, adipocyte progenitors, and stromal cells, as well as undefined stem cells.13 The stromal cells in the SVF share similarities with those of the BM, in various aspects. As for their cellular characteristics, both stromal cells contain a heterogeneous population of cells with a potential to differentiate into several lineages, including adipogenic, chondrogenic, and myogenic cells, depending on the culture conditions.12,14 The stromal cells in the BM are essential components for maintaining the niche environment for HSPCs to interact and regulate their stemness15-17 through several surface molecules. Among the molecules, CD13, CD29 (integrin-1), CD44 (hyaluronan receptor), CD90 (Thy-1), and Sca-1 (stem cell antigen-1) are expressed on SVF cells.14,18,19 Furthermore, one of the most critical molecules for the migration and retention of HSPCs, CXCL12, is highly expressed in both the SVF20 and BM.21,22 In addition, regional hypoxia in the BM has an important role in regulating HSPC function23 through the hypoxia-inducible factor-1α transcription factor modulating SDF-1α expression on endothelial cells.24 Morphologic analysis has revealed that quiescent HSPCs are located near hypoxic zones in BM.25 Adipose tissue is also highly hypoxic.26 Both organs are also supported by unique, but well-organized, vascular systems. The hypoxic environment and characteristic vascular system may play important roles in the recruitment and regulation of HSPCs.
Based on these facts, we hypothesized that adipose tissue is an extramedullary reservoir for HSPCs. Cousin et al reported that the SVF might have an hematopoietic function in vivo.27 However, it was suggested that transplanted SVF contributed to the rebound of endogenous hematopoiesis rather than its own hematopoietic functions in the recipients. Moreover, a recent report28 revealed that cultured SVF contains hemangioblasts, which have a bipotential capacity to differentiate into both endothelial cells and hematopoietic cells in vitro. However, the study focused on the potency of cultured SVF for hematopoiesis in vitro, instead of freshly isolated SVF. To address our hypothesis, we characterized presumable HSPCs in the SVF of mouse adipose tissue by phenotypic and functional analyses in vitro and in vivo. We also elucidated the origin of HSPCs in the SVF. Finally, we determined whether the number of SVF-HSPCs can be increased by promoting HSPC mobilization from the BM to the periphery. Our results provide compelling evidence that the SVF derived from adipose tissue contains phenotypic and functional HSPCs, despite their frequency being much less than HSPCs in the BM.
Methods
Animals
Specific pathogen–free C57BL/6J (B6) mice were purchased from The Jackson Laboratory. GFP+ mice (B6 genetic background) were a gift from Dr Masaru Okabe (Osaka University, Japan). The mice were bred in our pathogen-free animal facility, and 8- to 10-week-old male mice were used for this study. Animal care and experimental procedures were performed under approval from the Animal Care Committee of the Korea Advanced Institute of Science and Technology (KAIST). All mice were provided water and a standard diet (PMI LabDiet). All mice were anesthetized by an intramuscular injection of a combination of anesthetics (80 mg/kg ketamine and 12 mg/kg xylazine) before being sacrificed.
Isolation of the SVF, BM, and peripheral blood
Because epididymal adipose tissue has no adjacent tissues, we isolated the SVF from the epididymal adipose tissue of B6 and GFP+ mice to avoid contamination from nonadipose tissues as previously described.29 To exclude blood contamination in the adipose tissue, systemic perfusion with heparinized phosphate-buffered saline (PBS) was performed before harvesting or washing isolated adipose tissues with PBS. Then, the adipose tissues were incubated in Hanks balanced salt solution (HBSS; Sigma-Aldrich) containing 0.2% collagenase type 2 (Worthington) for 60 minutes at 37°C with constant shaking. After inactivating collagenase activity with 10% fetal bovine serum (FBS) containing Dulbecco modified eagle medium (DMEM), the cell suspension was filtered through a 40-μm nylon mesh (BD Biosciences), followed by centrifugation at 420g for 5 minutes. Floating adipocytes and supernatant were removed from the SVF pellet. The SVF pellet was washed and resuspended in sterilized PBS. The BM was harvested from B6 or GFP+ mice by flushing femurs and tibias with 10% FBS containing DMEM using a 21-gauge needle syringe. Peripheral blood from B6 mice was collected in a tube containing EDTA by cardiac puncture under anesthesia. Red blood cells (RBCs) were removed from the peripheral blood using an RBC lysis buffer (Sigma-Aldrich) before flow cytometric analysis. The single-cell suspension was filtered through a 40-μm nylon mesh. To count the cell number, we used a hemocytometer (Marienfeld). From the cell suspension, 10 μL was taken and mixed with 90 μL trypan blue solution (Sigma-Aldrich). A quantity of 10 μL of the mixture was added to the hemocytometer, and the number of viable cells was counted under inverted microscope (Zeiss).
Flow cytometry for HSPCs in the SVF
All antibodies used for cytometry were obtained from BD Biosciences, unless specifically indicated. To detect LSK cells in the SVF and BM, isolated SVF and BM cells were resuspended in 2% FBS containing HBSS and stained with anti–mouse stem cell antigen (Sca-1, clone D7; eBiosciences), anti–mouse c-Kit (clone 2B8), and lineage mixture containing anti–mouse CD3 (clone 145-2C11), anti–mouse CD4 (clone GK1.5), anti–mouse CD8 (clone 53-6.7), anti–mouse B220 (clone RA3-6B2), anti–mouse Gr-1 (clone RB6-8C5), anti–mouse Mac-1 (clone M1/70), and anti–mouse Ter-119 (clone Ter-119) for 20 minutes on ice. To detect long-term HSPCs with a long-term multilineage reconstitution capacity, anti-CD48 (clone HM48-1) and anti-CD150 (clone 9D1; eBiosciences) were included in the above staining mixture. To distinguish RBCs, anti–mouse Ter-119 antibody (clone Ter-119) and anti–mouse CD45 antibody (clone 30-F11) were used. Dead cells were excluded by 7-aminoactinomycin D (7-AAD; Invitrogen). Flow cytometric analyses for the stained cells were performed using the LSRII instrument (Becton Dickinson).
Colony-forming cell assay
The SVF and BM were suspended in 2% FBS containing Iscove modified Dubecco medium (IMDM) and then mixed with methylcellulose-based semisolid medium (M3434; StemCell Technologies) by vortexing. We plated 5 × 105 SVF and 1 × 104 BM cells per 1 mL media mixture on a 35-mm petri dish (BD Falcon), which was cultured in 5% CO2 humidified incubator at 37°C. The numbers of colonies were counted and types of colonies classified under inverted microscope (Ziess) by morphologic criteria 14 days after plating.
BM homing assays
B6 mice were lethally irradiated with a single dose of 12 Gy using a gamma irradiator (Gammacell 3000; MDS Nordion Inc). At 3 to 4 hours after irradiation, 5 × 106 GFP+ SVF or GFP+ BM cells were injected into recipients via the tail vein. Recipients were killed after anesthesia 24 hours after injection. The femur was processed for immunostaining and flow cytometry. For immunostaining, one side of the femur was fixed in 4% paraformaldehyde in PBS for 24 hours at 4°C. The fixed bone was decalcified with 10% EDTA in PBS for more than 48 hours at 4°C. The decalcified bone was infiltrated with 20% sucrose solution diluted in distilled water for 72 hours and then sectioned into a 20-μm thickness using a Cryotome (Leica). Sectioned samples were stained with hamster anti–mouse PECAM-1 (CD31) antibody (clone 2H8; Chemicon) for blood vessels and Hoechst (Invitrogen) for nuclei. For flow cytometry, BM cells were collected from the other side of the femur, and donor-derived GFP+ cells were detected.
Long-term reconstitution assay
B6 mice were sublethally irradiated with a single dose of 6 Gy using a gamma irradiator. At 12 to 16 hours after irradiation, 5 × 106 GFP+ SVF or GFP+ BM cells obtained from GFP+ mice were injected into recipients via the tail vein. Sixteen weeks after transplantation, GFP+ peripheral blood mononuclear cells (PBMCs) from the recipient mice were analyzed by flow cytometry. To examine multilineage reconstitution, the PBMCs were stained with CD3, B220, Gr-1, and Mac-1 antibodies after eliminating red blood cells (RBCs) using a RBC lysis buffer (Sigma-Aldrich), and the stained samples were analyzed. For the secondary transplantation, 16 to 20 weeks after the primary transplantation, 5 × 106 BM cells from the primary recipients were transplanted into lethally irradiated B6 mice via tail vein injection. Eight weeks after the secondary transplantation, PBMCs were analyzed by flow cytometry.
Immunohistologic detection of CD45+ and Sca-1+ cells in the adipose tissue
Epididymal adipose tissues from B6 mice were harvested, fixed with 1% paraformaldehyde in PBS for 24 hours, and whole-mounted. After blocking with 5% donkey serum in PBST (0.3% Triton X-100 in PBS) for 1 hour at room temperature, the whole-mounted tissues were incubated with anti-CD45 antibody, rat clone 30-F11, 1:500 (BD Biosciences), anti–Sca-1 antibody, goat polyclonal, 1:500 (R&D Systems), and anti–mouse CD31 antibody, hamster clone 2H8, 1:1000 (Chemicon International). After several PBST washes, the tissues were incubated for 2 hours at room temperature with the following secondary antibodies: Cy3-conjugated anti–hamster IgG antibody, 1:1000; fluorescein isothiocyanin (FITC)–anti–rat antibody, 1:1000; and Cy3-conjugated anti–goat IgG antibody, 1:1000 (all Jackson ImmunoResearch Laboratories).
Promotion of HSPC mobilization by cyclophosphamide/G-CSF treatment
B6 or GFP+ mice were injected intraperitoneally with 4 mg cyclophosphamide (Sigma-Aldrich) on the first day and then with 5 μg G-CSF (Choongwea Pharma Corporation) for 6 successive days. As a control, buffer (PBS) was treated in the same manner. Mice were killed on the day after the last G-CSF injection. The SVF was harvested and used for flow cytometric analysis, colony-forming cell (CFC) assay, and long-term reconstitution transplantation.
Statistics
Values presented are means plus or minus standard deviation (SD). Significant differences between means were determined by Student t test or analysis of variance followed by the Student-Newman-Keuls test. Significance was set at a value of P less than .05.
Results
The SVF contains phenotypic HSPCs
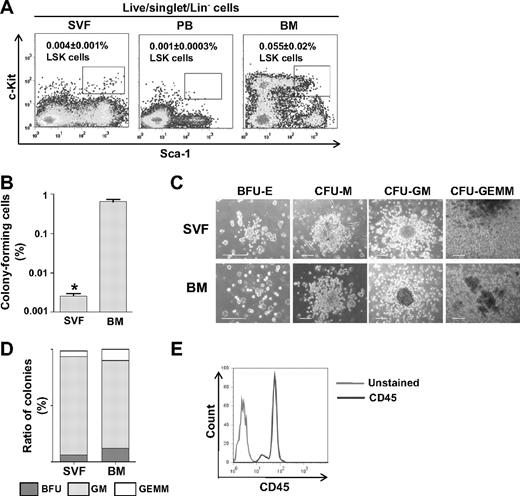
The procedures for SVF preparation are illustrated in supplemental Figure 1A (available on the Blood website; see the Supplemental Materials link at the top of the online article). To determine whether the SVF contains phenotypic HSPCs, flow cytometric analysis was performed for detection of LSK cells. Because the SVF contains heterogenic progenitor cells that express Sca-1 and c-Kit (data not shown), we carefully gated the forward scatter/side scatter (FSC-SSC) plot to exclude non–LSK Sca-1+ and c-Kit+ cells by comparing it to the FSC-SSC plot of BM-LSK cells. The LSK population in the SVF was 0.004% plus or minus 0.001% (n = 5), whereas in BM it was 0.055% plus or minus 0.02% (n = 6; Figure 1A). We further identified the HSPC subtypes common lymphoid progenitor cells (CLPs) and common myeloid progenitor cells (CMPs) in the LSK cells by probing for IL7Rα.30 The frequency of Lin−IL7Rα+Sca-1+c-Kit+ CLPs and Lin−IL7Rα−Sca-1−c-Kit+ CMPs was 0.003% plus or minus 0.001% (n = 5) and 0.008% plus or minus 0.002% (n = 5) in the SVF, respectively, and it was 0.01% plus or minus 0.001% and 1.0% plus or minus 0.2% in the BM (n = 3; supplemental Figure 2). These results provide compelling evidence that the SVF contains phenotypic HSPCs, though their frequency is approximately 14-fold less than in the BM. To ensure that the HSPCs in the SVF were not derived from residual blood cells inside the blood vessels of the adipose tissue after perfusion (supplemental Figure 1B), we determined whether RBCs were present in the SVF using flow cytometric analysis for Ter-119+/CD45− cells. The SVF after the perfusion of adipose tissues contained 3.7% plus or minus 0.6% (mean ± SD, n = 3) Ter-119+/CD45− cells, and the SVF without adipose tissue perfusion contained 46.1% plus or minus 1.6% (mean ± SD, n = 3) Ter-119+/CD45− cells (supplemental Figure 1C). Thus, most of the blood cells were adequately flushed from the adipose tissue. Moreover, the LSK population in the peripheral blood was 0.001% plus or minus 0.0003% (n = 5; Figure 1A). Therefore, it is unlikely that the SVF-LSK cells, if any, were derived from residual blood cells inside the blood vessels after perfusion.
The SVF contains phenotypic and functional HSPCs. (A) Representative flow cytometric profiles of LSK cells in the SVF, BM, and peripheral blood (PB). One million SVF, BM, and PB cells were pregated for live cells, and then Lin− cells were analyzed for Sca-1 and c-Kit expression. The percentage of LSK in the total SVF (n = 5) BM (n = 5) or PB (n = 5) is expressed as mean ± SD. (B-E) Freshly isolated SVF (n = 5) or BM (n = 5) was plated on methylcellulose-based medium and incubated for 14 days for CFC assay. (B) The frequency of colony-forming cells is shown. The bars represent mean ± SD (n = 5); *P < .05 vs BM. (C) Types of colonies are photographed. Scale bars, 100 μm. (D) The colonies are classified by morphologic criteria and calculated in percentages. (E) Flow cytometric analysis for CD45 expression in cells from SVF-derived colonies.
The SVF contains phenotypic and functional HSPCs. (A) Representative flow cytometric profiles of LSK cells in the SVF, BM, and peripheral blood (PB). One million SVF, BM, and PB cells were pregated for live cells, and then Lin− cells were analyzed for Sca-1 and c-Kit expression. The percentage of LSK in the total SVF (n = 5) BM (n = 5) or PB (n = 5) is expressed as mean ± SD. (B-E) Freshly isolated SVF (n = 5) or BM (n = 5) was plated on methylcellulose-based medium and incubated for 14 days for CFC assay. (B) The frequency of colony-forming cells is shown. The bars represent mean ± SD (n = 5); *P < .05 vs BM. (C) Types of colonies are photographed. Scale bars, 100 μm. (D) The colonies are classified by morphologic criteria and calculated in percentages. (E) Flow cytometric analysis for CD45 expression in cells from SVF-derived colonies.
The SVF contains HSPCs capable of forming hematopoietic colonies
To test whether the SVF has functional hematopoietic activity in vitro, the CFC assay31 was performed by plating freshly isolated SVF on methylcellulose-based medium. This plating gave rise to 13 plus or minus 4 (n = 5) colonies per 5 × 105 cells, whereas freshly isolated BM gave rise to 69 plus or minus 10 (n = 5) colonies per 1 × 104 cells (Figure 1B). Morphologic criteria analyses demonstrated that the SVF gave rise to variable types of colonies, such as erythroid burst-forming units (BFU-Es), macrophage colony-forming units (CFU-Ms), granulocyte macrophage–colony-forming units (CFU-GMs), and granulocyte, erythroid, macrophage, megakaryocyte–colony-forming units (CFU-GEMMs; Figure 1C). The size and appearance of each type of colony derived from the SVF were identical to the colonies derived from the BM (Figure 1C). Moreover, the proportion of each colony type from the SVF (BFU-E:GFU-GM:CFU-GEMM = 6.3%:87.6%:4.7%) was similar to that of BM (12.3%:78.0%:9.7%), but there was more CFU-GM and less CFU-GEMM and BFU-E than in BM (Figure 1C). To confirm that the cultured colonies contained hematopoietic cells, we harvested colonies and examined their surface expression of a pan-hematopoietic marker, CD45, by flow cytometry. Most cells (> 99%, n = 5) from SVF-derived colonies were CD45+ (Figure 1E), indicating that SVF possesses functional HSPCs that can give rise to several types of blood cells, although the frequency of HSPCs in the SVF is much less than in the BM.
Transplanted SVF can home to the BM of irradiated recipients
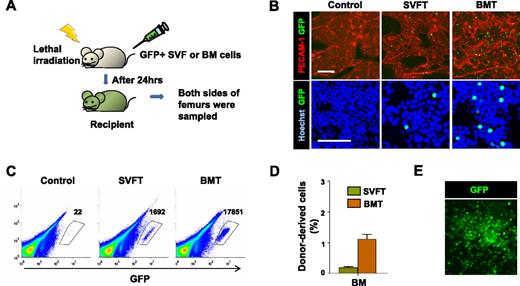
To examine the ability of the SVF to engraft conditioned BM, 5 × 106 SVF or BM cells isolated from GFP+ mice were transplanted into lethally irradiated syngenic B6 mice (Figure 2A). At 24 hours after transplantation, fewer, but detectable, GFP+ cells were detected in the BM of recipients who received GFP+ SVF than in the BM of recipients who received GFP+ BM (Figure 2B). Regardless of the origin difference between the SVF and BM, most of the donor-derived cells were located near the vasculature in the trabecular region, which were marked by PECAM-1 immunostaining (Figure 2B). In the diaphysis region of the bone, GFP+ cells derived from SVF or BM were both detected near the border between marrow and bone, rather than the central marrow region (supplemental Figure 3). Flow cytometric analysis confirmed that the transplanted GFP+ SVF cells were present in the BM of the irradiated recipients, and the number of these homing cells was roughly 10-fold less than the number of transplanted GFP+ BM cells homed to the BM (Figure 2C-D). The GFP+ SVF recipients contained 0.15% plus or minus 0.03% GFP+ cells in the total BM cells, whereas the GFP+ BM recipients had 1.10% plus or minus 0.29% GFP+ cells in the total BM cells (n = 4 for each group; Figure 2D). To confirm that the BM-homing GFP+ cells derived from the SVF were functional HSPCs, we seeded whole BM from GFP+ SVF recipients on methylcellulose-based medium. The BM gave rise to GFP+ hematopoietic colonies (Figure 2E), suggesting that BM-homing SVF cells have hematopoietic activity and participate in hematopoiesis.
The SVF contains HSPCs that can home to the BM of irradiated recipients. (A) Experimental scheme. After lethal irradiation, the mice underwent transplantation with control buffer (control), 5 × 106 GFP+ SVF (SVFT) cells, or 5 × 106 GFP+ BM (BMT) cells. At 24 hours after transplantation, femurs were sampled. (B) The femurs were cryosectioned, immunostained for PECAM-1 (for vasculature) and stained with Hoechst dye (for nuclei), and viewed by fluorescent microscopy. Scale bars, 100 μm. (C) Representative flow cytometric profiles of BM cells from control, SVFT, and BMT recipients. Numbers indicate the occurrence of GFP+ cells among 106 live BM cells. (D) Percentage of GFP+ cells in the BM is presented. Bars represent mean ± SD of 4 mice per group. *P < .05 vs BM. (E) The BM cells of SVFT recipients were plated on methylcellulose-based medium, and cultured cells gave rise to GFP+ colonies.
The SVF contains HSPCs that can home to the BM of irradiated recipients. (A) Experimental scheme. After lethal irradiation, the mice underwent transplantation with control buffer (control), 5 × 106 GFP+ SVF (SVFT) cells, or 5 × 106 GFP+ BM (BMT) cells. At 24 hours after transplantation, femurs were sampled. (B) The femurs were cryosectioned, immunostained for PECAM-1 (for vasculature) and stained with Hoechst dye (for nuclei), and viewed by fluorescent microscopy. Scale bars, 100 μm. (C) Representative flow cytometric profiles of BM cells from control, SVFT, and BMT recipients. Numbers indicate the occurrence of GFP+ cells among 106 live BM cells. (D) Percentage of GFP+ cells in the BM is presented. Bars represent mean ± SD of 4 mice per group. *P < .05 vs BM. (E) The BM cells of SVFT recipients were plated on methylcellulose-based medium, and cultured cells gave rise to GFP+ colonies.
HSPCs in the SVF are capable of long-term reconstitution in hematopoiesis in the recipient mice
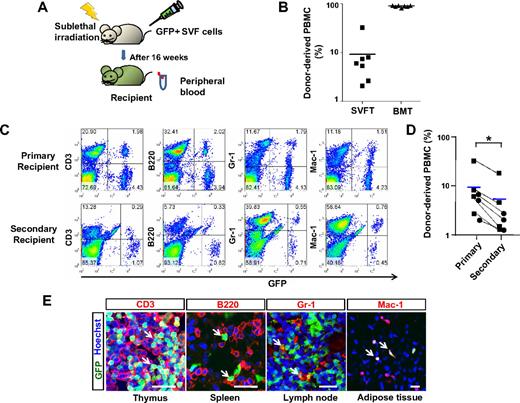
To examine whether HSPCs in the SVF are capable of long-term multilineage reconstitution in hematopoiesis in vivo, 5 × 106 GFP+ SVF cells were transplanted into sublethally irradiated syngenic recipients (Figure 3A). Flow cytometric analyses were performed 16 weeks after transplantation. All 7 recipients of GFP+ SVF transplantation contained a definite portion of GFP+ cells in the peripheral blood (Figure 3B). The percentage of GFP+ cells in the peripheral blood of the SVF transplantation recipients was 2.1% to 32.1%, whereas the percentage in BM transplantation recipients was 83.2% to 91.2% (Figure 3B). These data indicate that SVF transplantation is capable of generating long-term, but variable extents, of reconstitution in hematopoiesis. Among the 7 recipients, 4 recipients contained a definite population of GFP+ cells in each lineage, T lymphocytes, B lymphocytes, and myeloid cells (Figure 3C), and 3 recipients exhibited T lymphocyte–dominant reconstitution (supplemental Figure 4). The long-term multilineage reconstitution capacity of HSPCs in the SVF was confirmed by serial transplantation into secondary recipients. The percentage of GFP+ cells in the peripheral blood of the secondary recipients that received transplanted BM from the primary SVF recipients was 1.2% to 24.1% (n = 7) and it was less than the percentage of the primary SVF recipients (the secondary recipients versus the primary recipients; 6.1 ± 10.9 vs 9.1 ± 10.4, P < .05 by paired Student t test; Figure 3D). However, all secondary recipients showed the same pattern of reconstitution as the primary recipients (Figure 3C and supplemental Figure 4). Alternatively, immunohistochemical analyses revealed that donor SVF-derived CD3+ T lymphocytes, B220+ B lymphocytes, and Gr-1+/Mac-1+ myeloid cells were detected in various tissues, including the thymus, spleen, lymph node, and adipose tissue of primary recipients (Figure 3E). Together, these results indicate that SVF contains HSPCs capable of generating long-term multilineage reconstitution.
HSPCs in the SVF are capable of long-term reconstitution in hematopoiesis in recipient mice. (A) Experimental scheme. After sublethal irradiation, the mice underwent transplantation with control buffer (control), 5 × 106 of GFP+ SVF (SVFT) cells, or 5 × 106 GFP+ BM (BMT) cells. At 16 weeks after transplantation, blood and several organs were sampled. (B) The percentage of donor-derived GFP+ cells in PBMCs was analyzed by flow cytometry. Bars represent the mean (n = 7 per group), and each ■ (SVFT) and ▲ (BMT) represents 1 sample. (C) Representative flow cytometric profiles of PBMCs in primary (SVFT) and secondary recipients (BMT from the primary SVFT). (D) Changes of percentages of GFP+ cells in PBMCs from the primary recipients to the secondary recipients were plotted (dotted lines). ■ represents the multilineage-reconstitution recipients and ● represents T lymphocyte–dominant reconstitution recipients. Blue bars represent the mean (n = 7 per group). *P < .05 vs primary recipients. (E) Immunostaining for CD3 (T lymphocyte), B220 (B lymphocyte), Gr-1 (myeloid cell), and Mac-1 (myeloid cell) and the counter-staining of nuclei with Hoechst dye in the indicated organs. White arrows indicate blood cells derived from SVF. Scale bars, 20 μm.
HSPCs in the SVF are capable of long-term reconstitution in hematopoiesis in recipient mice. (A) Experimental scheme. After sublethal irradiation, the mice underwent transplantation with control buffer (control), 5 × 106 of GFP+ SVF (SVFT) cells, or 5 × 106 GFP+ BM (BMT) cells. At 16 weeks after transplantation, blood and several organs were sampled. (B) The percentage of donor-derived GFP+ cells in PBMCs was analyzed by flow cytometry. Bars represent the mean (n = 7 per group), and each ■ (SVFT) and ▲ (BMT) represents 1 sample. (C) Representative flow cytometric profiles of PBMCs in primary (SVFT) and secondary recipients (BMT from the primary SVFT). (D) Changes of percentages of GFP+ cells in PBMCs from the primary recipients to the secondary recipients were plotted (dotted lines). ■ represents the multilineage-reconstitution recipients and ● represents T lymphocyte–dominant reconstitution recipients. Blue bars represent the mean (n = 7 per group). *P < .05 vs primary recipients. (E) Immunostaining for CD3 (T lymphocyte), B220 (B lymphocyte), Gr-1 (myeloid cell), and Mac-1 (myeloid cell) and the counter-staining of nuclei with Hoechst dye in the indicated organs. White arrows indicate blood cells derived from SVF. Scale bars, 20 μm.
HSPCs in the SVF originated from the BM
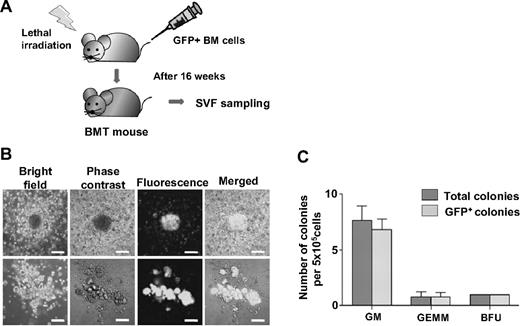
To elucidate whether HSPCs in the SVF originated from the BM, the CFC assay was performed with SVF obtained from the mice that underwent transplantation with 5 × 106 GFP+ BM cells (Figure 4A). All colonies were GFP+ (Figure 4B-C). These results indicate that all HSPCs within the SVF originated from the BM and properly maintain their function in the adipose tissue.
HSPCs in the SVF originated in the BM. (A) Experimental scheme. After lethal irradiation, the mice underwent transplantation with 5 × 106 GFP+ BM (BMT) cells. At 16 weeks after transplantation, the SVF was sampled and analyzed. (B) CFC assay of SVF from GFP+ BMT mice. Representative colonies were viewed by bright field, phase-contrast, and fluorescence, and then merged. Scale bars, 100 μm. (C) The colonies were classified and counted by morphologic criteria.
HSPCs in the SVF originated in the BM. (A) Experimental scheme. After lethal irradiation, the mice underwent transplantation with 5 × 106 GFP+ BM (BMT) cells. At 16 weeks after transplantation, the SVF was sampled and analyzed. (B) CFC assay of SVF from GFP+ BMT mice. Representative colonies were viewed by bright field, phase-contrast, and fluorescence, and then merged. Scale bars, 100 μm. (C) The colonies were classified and counted by morphologic criteria.
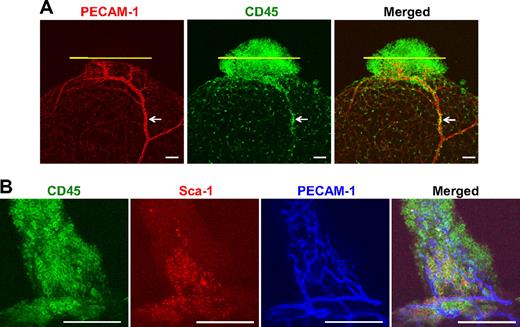
Detection of potential HSPCs in the adipose tissue by immunostaining for hematopoietic CD45+ cells
Immunostaining revealed that abundant CD45+ cells were clustered in the tip region of adipose tissue,26 but the CD45+ cells were sparsely distributed in the body region of adipose tissue (Figure 5A). Moreover, a significant portion of clustered CD45+ cells was localized in the avascular regions (Figure 5A) and approximately 12% to 18% of CD45+ cells located in the tip region of the adipose tissue were Sca-1+ cells (n = 4; Figure 5B). These data suggest that the HSPCs derived from the BM massively migrated to this region from the circulation and proliferated in this probable ischemic niche.26 Although further studies are mandatory to further characterize the CD45+/Sca-1+ cells, they could be the main source of the HSPC of the SVF.
Presence of hematopoietic CD45+ and CD45+/Sca-1+ cells in the adipose tissue as potential HSPCs. Epididymal adipose tissues (nonperfused) from 8-week-old mice were whole-mounted, and PECAM-1+ blood vessels (red or blue), CD45+ cells (green), and Sca-1+ cells (red) were visualized by double and triple immunofluorescence staining. Two representative examples are shown. (A) Abundant CD45+ cells are clustered in the tip region of the adipose tissue, and CD45+ cells are sparsely distributed in the body region of the adipose tissue. A portion of the clustered CD45+ cells is localized in the avascular regions, which are demarcated by yellow lines. Occasionally, a group of CD45+ cells is located inside the blood vessels (arrows). (B) Approximately 16% of CD45+ cells are Sca-1+ cells in the tip region of the adipose tissue. Scale bars, 100 μm.
Presence of hematopoietic CD45+ and CD45+/Sca-1+ cells in the adipose tissue as potential HSPCs. Epididymal adipose tissues (nonperfused) from 8-week-old mice were whole-mounted, and PECAM-1+ blood vessels (red or blue), CD45+ cells (green), and Sca-1+ cells (red) were visualized by double and triple immunofluorescence staining. Two representative examples are shown. (A) Abundant CD45+ cells are clustered in the tip region of the adipose tissue, and CD45+ cells are sparsely distributed in the body region of the adipose tissue. A portion of the clustered CD45+ cells is localized in the avascular regions, which are demarcated by yellow lines. Occasionally, a group of CD45+ cells is located inside the blood vessels (arrows). (B) Approximately 16% of CD45+ cells are Sca-1+ cells in the tip region of the adipose tissue. Scale bars, 100 μm.
Promotion of HSPC mobilization from the BM increases HSPCs in the SVF
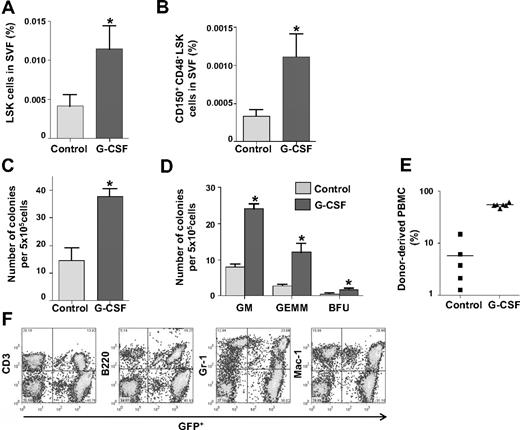
Because BM-originated HSPCs were detected in the SVF under homeostatic conditions, we investigated whether HSPCs in the SVF increased by enhanced mobilization from the BM. To enhance mobilization, sequential cyclophosphamide and G-CSF treatment was applied.7 After the cyclophosphamide/G-CSF treatment, the LSK population in the SVF (0.011% ± 0.003%, P = .002) was increased compared with the untreated control (0.004% ± 0.001%, Figure 6A). In addition, the long-term HSPC population32,33 was detectable (0.001% ± 0.0003%) in the SVF treated with cyclophosphamide/G-CSF, but it was hardly detectable (0.0003% ± 0.00008%) in SVF treated with control (Figure 6B). Moreover, the SVF treated with cyclophosphamide/G-CSF contained 3.5-fold more colony-forming cells compared with the SVF treated with control (Figure 6C). The proportion (BFU-E:GFU-GM:CFU-GEMM) of each colony type in SVF treated with cyclophosphamide/G-CSF was proportionally increased compared with SVF treated with control (Figure 6D). All 6 recipients that underwent transplantation with SVF and treated with cyclophosphamide/G-CSF exhibited relatively higher (31.8%-61.0%) long-term multilineage reconstitution compared with recipients that underwent transplantation with SVF treated with control (Figure 6E-F). These findings indicate that the mobilization of HSPCs from the BM could expand the number of phenotypic and functional HSPCs in the SVF.
HSPCs in the SVF increased upon promotion of HSPC mobilization from the BM. (A-B) Comparisons of the percentage of LSK cells and CD150+CD48−LSK cells in the SVF treated with control buffer (control) or cyclophosphamide/G-CSF (G-CSF). Bars indicate mean ± SD (n = 4 per each group). *P < .05 vs control. (C-D) CFC assay of 5 × 105 SVF cells treated with control or G-CSF. The number of total colonies and each type of colony was counted. Bars represent mean ± SD (n = 4). *P < .05 vs control. (E) Percentage of donor-derived GFP+ cells in PBMCs was analyzed by flow cytometry. Bars represent mean, and each ■ (control, n = 5) and ▲ (G-CSF, n = 6) represents 1 sample. (F) Representative flow cytometric profiles show long-term multilineage reconstitution in the blood of primary recipients that underwent transplantation with G-CSF–treated SVF.
HSPCs in the SVF increased upon promotion of HSPC mobilization from the BM. (A-B) Comparisons of the percentage of LSK cells and CD150+CD48−LSK cells in the SVF treated with control buffer (control) or cyclophosphamide/G-CSF (G-CSF). Bars indicate mean ± SD (n = 4 per each group). *P < .05 vs control. (C-D) CFC assay of 5 × 105 SVF cells treated with control or G-CSF. The number of total colonies and each type of colony was counted. Bars represent mean ± SD (n = 4). *P < .05 vs control. (E) Percentage of donor-derived GFP+ cells in PBMCs was analyzed by flow cytometry. Bars represent mean, and each ■ (control, n = 5) and ▲ (G-CSF, n = 6) represents 1 sample. (F) Representative flow cytometric profiles show long-term multilineage reconstitution in the blood of primary recipients that underwent transplantation with G-CSF–treated SVF.
Discussion
This study demonstrates for the first time that the SVF in adipose tissue contains phenotypic and functional HSPCs, which are capable of generating hematopoietic cells in an in vitro culture system as well as the in vivo long-term reconstruction of multilineage hematopoiesis. All HSPCs in the SVF originate from the BM. Enhanced mobilization of HSPCs from the BM increases the number of HSPCs in the SVF, which results in a high-efficiency, long-term reconstitution in multilineage hematopoiesis. We suggest that adipose tissue may be an alternative extramedullary tissue for HSPCs, and the SVF in adipose tissues may be a novel resource for obtaining functional and transplantable HSPCs.
Although the population of LSK cells and amount of colony formation was much less in the SVF than the BM, our results show that HSPCs in the SVF have typical surface phenotypes like HSPCs in the BM, and they form hematopoietic colonies, such as BFU-E–, CFU-M–, CFU-GM–, and CFU-GEMM–like HSPCs in the BM. Thus, SVF evidently contains phenotypic and functional HSPCs. These results led us to investigate whether transplanted SVF is able to home to the BM and reconstitute long-term multilineage hematopoiesis in vivo. When HSPCs derived from BM are transplanted via the circulatory system, they imperatively home to the BM for proper proliferation and maintenance.1-5 This homing capacity is one of the instinct properties of HSPCs.1-5 Bone marrow ablated by irradiation constitutes a homing site for transplanted HSPCs,34 especially near the vascular and endosteal regions in BM.1-3,35 Our results show that transplanted SVF cells, like transplanted BM cells, are found more in the trabecular region than the central compact region of bone. Furthermore, consistent with recent reports,36 in the trabecular region, transplanted SVF cells existed along the vasculature, whereas in the central compact region, transplanted SVF cells were mainly observed near the border between the bone and marrow. This characteristic localization of SVF cells in the BM may reflect a successful lodge on the part of SVF cells in the hematopoietic niche as definite HSPCs.1 Thus, the BM homing of SVF cells may represent a reliable cue for the in vivo functionality of HSPCs in the SVF. Our results also revealed that HSPCs in the SVF successfully engraft into the BM and achieve long-term (> 16 weeks) multilineage reconstitution of hematopoietic cells in sublethally irradiated recipients. This phenomenon is in marked contrast to transiently reconstituted hematopoietic cells, which are usually undetectable by 8 to 10 weeks after transplantation.37 Although the long-term multilineage reconstitution by SVF was less frequent and more variable than that of BM, the recipients clearly had donor-derived hematopoietic cells after primary transplantation, which transferred to the secondary recipient by BM transplantation. These results are compelling evidence that the SVF contains functional HSPCs.
We demonstrated that HSPCs in the SVF originate from the BM. The more HSPCs mobilized by G-CSF in the BM, the more HSPCs that are detected in the SVF. Although the SVF is known to contain several different stem cells,12 our results indicate that HSPCs in the SVF do not originate from resident adipose tissue cells. The HSPCs in the BM constantly move from the BM to systemic circulation and back to the BM, and they also migrate to extramedullary tissues, including adipose tissue, though by an unidentified mechanism. There are 3 possibilities for adipose tissue being a candidate extramedullary homing site for HSPCs: (1) adipose tissue constitutes an hypoxic environment,26 which can provide suitable oxygen tension for HSPCs; (2) adipose tissue has abundant unidentified stromal cells that resemble BM stromal cells19 ; and (3) the complex adipose tissue vasculature surrounded by stromal cells might compose an appropriate microenvironment for HSPCs. It would be reasonable to argue that HSPCs in the SVF can be passenger HSPCs floating in the peripheral blood in adipose tissues. However, in this study, we used adipose tissue after blood removal. To ensure that the HSPCs in the SVF were not derived from residual blood cells inside the blood vessels of the adipose tissue after perfusion, we performed several rigorous experiments. Flow cytometric analysis indicated that most blood cells were adequately flushed from the adipose tissue. Because the proportion of LSK cells in the peripheral blood is very low, the contribution of peripheral blood–LSK cells to the SVF-LSK cells is negligible. Moreover, we detected an abundant distribution of potential HSPCs in the probable niche26 of the adipose tissue by immunostaining for hematopoietic CD45+/Sca-1+ cells. Further studies are needed to fully characterize the CD45+/Sca-1+ cells, but the HSPCs of the SVF could mainly be derived from these CD45+/Sca-1+ cells. Based on these findings, we propose that HSPCs derived from BM could mobilize to adipose tissue, reside in the vascular niche after homing, and maintain their properties and function properly. Further studies are needed to determine what circumstances can affect homing and the mobilization of HSPCs within the SVF of adipose tissues.
Limitations of the ex vivo expansion of HSPCs is a challenging obstacle in treating patients with hematologic disorders. According to our present data and previous reports,38,39 the estimated amount of HSPCs in total adipose tissues would be equivalent to approximately 0.2% of that of HSPCs in the total BM. Can the SVF be used practically as an alternative resource of HSPCs? A vast amount of the SVF in adipose tissues can be easily obtained from patients using conventional liposuction and isolation methods.13 Adipose tissue is one of the most accessible tissues by mild operation and the only removable tissue in the human body without functional defect.13 Practically, it would be possible to obtain pure HSPCs if we sort out the HSPCs from the SVF. Moreover, our results suggest that more HSPCs could be obtained from the SVF if we promote the mobilization of HSPCs from BM using G-CSF. Therefore, our answer is that SVF could be practically used as an alternative resource of HSPCs for transplantation when we define a technology for the purification of HSPCs from SVF.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Jin Sun Hong and Eun Sun Lee for their technical assistance.
This research was supported by a grant (SC-5120, G.Y.K.) from the Stem Cell Research Center of the 21st Century Frontier Research Program.
Authorship
Contribution: J.H., I.K., and G.Y.K. designed and organized the experiments, analyzed the data, generated the figures, and wrote the manuscript; and J.H., Y.J.K., H.R.M., H.G.R., and C.-H.C. performed the experiments, CFC assay, and flow cytometric and histologic analyses.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gou Young Koh or Injune Kim, Department of Biological Sciences and Graduate School of Nanoscience and Technology (WCU), KAIST, 373-1, Guseong-dong, Daejeon, 305-701, Republic of Korea; e-mail: gykoh@kaist.ac.kr or injunek@kaist.ac.kr.