Abstract
Natural killer (NK) cells develop in the bone marrow and are known to gradually acquire the ability to eliminate infected and malignant cells, yet the cellular stages of NK lineage commitment and maturation are incompletely understood. Using 12-color flow cytometry, we identified a novel NK-committed progenitor (pre-NKP) that is a developmental intermediate between the upstream common lymphoid progenitor and the downstream NKP, previously assumed to represent the first stage of NK lineage commitment. Our analysis also refined the purity of NKPs (rNKP) by 6-fold such that 50% of both pre-NKP and rNKP cells gave rise to NKp46+ NK cells at the single-cell level. On transplantation into unconditioned Rag2−/−Il2rγc−/− recipients, both pre-NKPs and rNKPs generated mature NK cells expressing a repertoire of Ly49 family members that degranulated on stimulation ex vivo. Intrathymic injection of these progenitors, however, yielded no NK cells, suggesting a separate origin of thymic NK cells. Unlike the rNKP, the pre-NKP does not express IL-2Rβ (CD122), yet it is lineage committed toward the NK cell fate, adding support to the theory that IL-15 signaling is not required for NK commitment. Taken together, our data provide a high-resolution in vivo analysis of the earliest steps of NK cell commitment and maturation.
Introduction
Natural killer (NK) cells are lymphocytes that are part of the innate immune system and provide a wide variety of protective functions against malignancies and viral infections.1-4 Unlike T or B cells, NK cells do not undergo receptor rearrangement but instead use a repertoire of germ line-encoded receptors, including inhibitory and activating receptors: killer cell immunoglobulin-like receptor in humans and the Ly49 family in mice.5 These receptors allow for the sensing of stress ligands on affected cells as well as decreased expression of major histocompatibility complex class I molecules.6-8 Once an NK cell has come in contact with a target cell, it uses effector molecules such as granzyme B and perforin to induce apoptosis.9,10 NK cells also can secrete cytokines such as IFNγ that can activate other innate immune cells as well as regulate the adaptive immune system.11,12
As with all blood lineages, NK cells develop from hematopoietic stem cells.13 The current understanding of hematopoietic differentiation has in large part come from the prospective isolation and functional characterization of phenotypically defined populations of cells from the bone marrow (BM). One of the first critical choices to be made in hematopoietic development is the myeloid versus lymphoid fate decision. The evidence that such a fate decision is made was provided by the identification of the clonogenic common lymphoid progenitor (CLP), which on transplantation can give rise to T cells, B cells, dendritic cells (DCs), and NK cells but not to myeloid cells in vivo.14-19 Recent genetic tracing studies have confirmed this pathway of lymphoid differentiation in vivo.20 The cellular stages of both B- and T-cell differentiation downstream of the CLP have been extensively studied,21,22 and the ability to prospectively isolate these developmental intermediates has proven invaluable for determining the sequence of the molecular events that drive maturation. Importantly, mutations in genes essential for lymphocyte maturation have been linked to many human immunodeficiency disorders.23
In contrast to the extensive characterization of the earliest steps of B- and T-cell differentiation,24,25 relatively little is known about the initial stages of NK commitment and development. Evidence suggests that the first stage en route to the generation of mature NK cells occurs when a CLP differentiates into a natural killer progenitor (NKP) and loses the ability to differentiate into T cells, B cells, and DCs.26 This is manifested by the acquisition of the IL-15 receptor complex, composed of IL-15 receptor α (IL-15Rα), IL-2Rβ (CD122), and IL-2 receptor common γ (IL-2Rγc)27 chains, making the progenitor responsive to IL-15, a cytokine necessary for NK development and survival.28-30 These cells subsequently progress to an immature NK cell stage defined by acquisition of NK1.1 and NKG2D expression, but they are not yet functionally mature.31,32 Concurrent with the acquisition of other NK lineage markers such as DX5 and Ly49 family members, they become fully mature and functional.32,33
Past studies have shown the importance of IL-15 signaling on NK development, because mice deficient in any component of the IL-15R complex or IL-15 are unable to generate mature NK cells. However, the role of IL-15 in mediating NK lineage commitment is still unclear. Previous studies by our laboratory demonstrated that induction of human IL-2Rβ signaling in CLPs suppressed the development of B cells and instead led to the development of myeloid cells,34 indicating that the developmental stage at which this signaling pathway is engaged may well be important for lineage commitment. In contrast, other studies have found normal numbers of NKPs are formed in Il2rγc−/− mice,35 suggesting that IL-15 may be more important for survival and proliferation of NK cells than for their initial commitment. However, because IL-2Rγc also is required for the development of B and T cells,36,37 it is not clear whether the NKPs that are formed in Il2rγc−/− mice are truly NK committed. As an example of this caveat, CLPs with the proper cell surface markers can clearly be formed in the absence of IL-7 signals, but they show altered lineage potentials.38 Finally, because of the apparent paucity of NKPs,26 experiments to demonstrate NK lineage commitment in vivo have not yet been performed. Thus, the role of IL-15 in NK lineage commitment is still unclear.
We hypothesized that if NK lineage commitment truly occurs in the absence of IL-15 signaling, then very early NK lineage-committed progenitors immediately downstream of CLPs that lack surface IL-2Rβ should be identifiable. Here, we report the characterization of Lin-c-kit-Flk2-CD27+CD244+IL-7Rα+CD122− cells in the mouse bone marrow that, on a clonal level in vitro and as a population in vivo, give rise to mature NKp46+ NK cells in the bone marrow, spleen, and blood but no other mature cell lineages. These progenitor cells that we termed the pre-NKP thus define a rare NK progenitor that is downstream of the CLP and upstream of the NKP.
Methods
Animals
All animal procedures were approved by the International Animal Care and Use Committee and the Stanford Administrative Panel on Laboratory Animal Care. C57Bl/Ka-Thy1.2 Ly5.1 (B/Ka), C57Bl/Ka-Thy1.1 Ly5.1 (BA), C57Bl/6-Thy1.2 Ly5.2 (Ly5.2), and Rag2−/− C57Bl/KA−Thy1.1 Ly5.1 (DKO) strains were derived and maintained in our laboratory. Peripheral blood was sampled from the tail vein, and all intravenous cell transplants were performed by injection into the retro-orbital sinus of isoflurane-anesthetized mice. Intrathymic injections were performed as described previously.17 Donor mice were 6 to 12 weeks old, and recipient mice ranged from 6 to12 weeks of age.
Antibodies
A complete list of all antibodies used in the study is shown in supplemental Table 2 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Bone marrow preparation and staining
Bone marrow was harvested from donor mice by crushing bones and removing debris on density gradient using Histopaque 1119 or 1077 (Sigma-Aldrich). Where indicated, bone marrow was lineage-depleted by adding lineage antibodies (Mac-1, Gr-1, Ter119, and CD19) and then adding sheep anti–rat Dynabeads (Invitrogen) and removing bound cells via magnetic field according to the manufacturer's instructions.
Fluorescence-activated cell sorting
All cells were sorted, and data were collected on an FACSAria II cell sorter (BD Biosciences). FlowJo software (TreeStar) was used for flow cytometric data analysis. Cells were sorted into ice-cold PBS with 2% FCS, or into tissue culture medium.
Cell cultures
Cells were cultured in Iscove modified Dulbecco medium (Invitrogen) with 10% FCS (Omega Scientific), 50mM 2-mercaptoethanol, sodium pyruvate, l-glutamine, and nonessential amino acids for the indicated time in the presence of 10 ng/mL recombinant mouse each Flt3L (R&D Systems), stem cell factor (SCF; R&D Systems), IL-7 (eBioscience), and IL-15 (eBioscience) and in the presence of OP9 or OP9-DL1 stromal cells when indicated.
Engraftment analysis
Mature thymocytes were depleted for host mature T cells using an anti-Thy1.1 (19XE5) antibody toxic to Thy1.1+ cells as described in Serwold et al.39 In brief, single-cell suspensions of thymuses were incubated with 40 mg of anti-Thy1.1 for 1 hour on ice. Dead cells and debris were separated by density gradient using Histopaque 1119. Spleens were harvested and made into single-cell suspensions and then treated with ACK lysis buffer (150mM NH4Cl, 1mM KHCO3, and 0.1mM EDTA) to remove red blood cells.
Quantitative PCR analysis
Total RNA was isolated by directly sorting progenitors into TRIzol (Invitrogen) and reverse transcribed using SuperScript III (Invitrogen). PCR reactions were set up with first-strand cDNA, gene-specific primers, passive reference dye, and SYBR Green QPCR Master Mix (Bio-Rad Laboratories) according to the manufacturer's instructions. Real-time PCR was performed in triplicate, and fluorometric data were collected at the annealing step of each cycle. A dissociation curve was performed at the end of 40 cycles to confirm specificity of amplification. The primers used for real-time PCR analysis were designed to avoid amplification of genomic DNA. The primers used in this study include Id2-R, 5′-CACAGAGTACT-TTGCTATCATTCG-3′; Id2-L, 5′-CCTGAACACGGACATCAGC-3′; B-actin-R, 5′-TCTGGCACCACACCTTCTA-3′; and B-actin-L, 5′-AGGCATACAGGGACAGCAC-3′.
Results
Identification of a pre-NKP in adult mouse bone marrow cells
Previous studies had identified a putative NKP in the adult bone marrow of mice.26 This population was identified as being negative for all mature lineage markers (Lin−) including the pan-NK markers DX5 and NK1.1 and positive for CD122 (IL-2Rβ). This NKP was lineage restricted, yet probably heterogeneous, because only 1/12 of single cells plated on OP9 stromal cells gave rise to mature NK cells in vitro.26 We used 12-color flow cytometry to identify other putative NK progenitors, to further refine the NKP, and to identify novel markers that are common in the NK developmental pathway. To this end, we examined markers such as CD27 and CD244 (2B4) that are expressed not only in early hematopoietic progenitors (including multipotent progenitors [MPPs] and CLP; supplemental Figure 1) but that also are expressed on immature and mature NK cells (supplemental Figure 2A).31
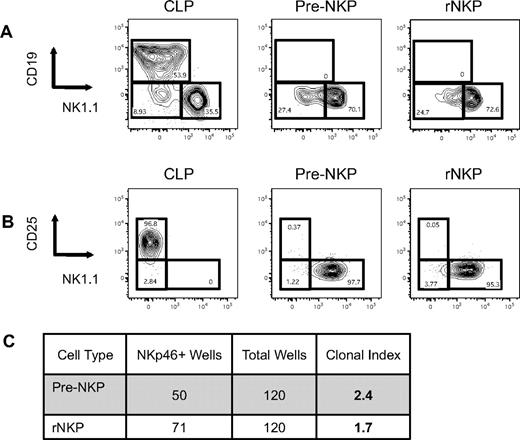
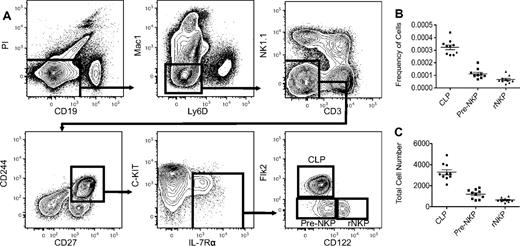
The Lin−CD27+CD244+ cell population in the bone marrow includes most early hematopoietic progenitors, including the CLP (defined as Lin-Flk2+IL-7Rα+Ly6D−) and some of the NKP (supplemental Figures 1 and 3A). To show that both the CD27- and CD244-positive populations contain all the NK potential in murine bone marrow, we transplanted Lin−CD27+, Lin−CD27−, Lin−CD244+, and Lin−CD244− populations from CD45.1 wild-type mice into congenic CD45.2 RAG2−/−IL2rγc−/− immunocompromised mice (DKO) and observed that only the CD27- and CD244-positive fractions gave rise to NK cells in the spleen after 2 weeks (supplemental Figure 5). Flow cytometric analysis using these 2 markers demonstrated that the NKPs as originally defined were highly heterogeneous and that only the CD27+CD244+ population in the previously defined NKP gave rise to NK cells when grown in vitro (supplemental Figure 2B; supplemental Table 1). We next examined the expression of the cytokine receptors Flk2 and IL-7Rα that are required for the optimal differentiation of lymphoid progenitors and mature lineages, to further refine the NKP.16 We observed that all NKP are Flk2− (supplemental Figure 3B). A majority of CD27+CD244+ NKPs were IL-7Rα+; however, both the IL-7Rα+ and IL-7Rα− fractions gave rise to NK cells in vitro (supplemental Figure 3B; supplemental Table 1). These data are consistent with the recent demonstration that all NK cells progress through an IL-7Rα+ intermediate.20 Thus, using known hematopoietic progenitor markers, along with a complement of hematopoietic lineage markers, we were able to obtain a highly purified homogenous NKP population that accounted for < 0.001% of the bone marrow, ∼ 1/10 the number of CLP, consistent with the physiologic ratios of T and B cells to NK cells (Figure 1B-C). Thus, we refined the definition of the NKP to be Lin−CD27+CD244+CD122+Flk2− and are referred to as “rNKP” throughout this paper.
Pre-NKPs and rNKPs can be highly purified from BM using 12-color flow cytometry. (A) Gating strategy for CLP, pre-NKP, and rNKP. (B) Frequency of CLP, pre-NKP, and rNKP in BM isolated from 2 femurs. (C) Absolute number of CLP, pre-NKP, and rNKP from 2 femurs. Shown are the individual data from 10 mice with the mean and standard deviation (B-C).
Pre-NKPs and rNKPs can be highly purified from BM using 12-color flow cytometry. (A) Gating strategy for CLP, pre-NKP, and rNKP. (B) Frequency of CLP, pre-NKP, and rNKP in BM isolated from 2 femurs. (C) Absolute number of CLP, pre-NKP, and rNKP from 2 femurs. Shown are the individual data from 10 mice with the mean and standard deviation (B-C).
Intriguingly, we also identified a rare population that showed almost identical cell surface phenotype as the rNKP except that the cells lacked the expression of CD122 (Figure 1A; supplemental Figure 4A). We have termed this cell type the “pre-NKP,” defined as Lin−CD27+CD244+CD122−IL-7Rα+Flk2−, and we propose that it is developmentally positioned downstream of CLPs and upstream of NKPs.
In vitro differentiation of pre-NKP and rNKP
We evaluated the NK developmental potential of the rNKP, the newly defined pre-NKP, and for comparison, CLP in permissive in vitro OP9 stromal cells culture assays. When Lin− bone marrow cells are grown in this system with the proper cytokines and growth factors, they reliably and reproducibly allow for the development of mature B cells, DCs, and Ly49+ NK cells.40 Also, OP9-delta1 (OP9-DL1) stromal cells that have been engineered to express the Notch ligand Delta-like 1 allow for the development of immature T cells.41
We sorted 10 progenitor cells per well onto confluent OP9 stroma in media supplemented with Flt3L, SCF, IL-7, and IL-15. Cultures were grown for 10 days with a spike of IL-15 on day 5. Cultures were than analyzed for the presence of mature hematopoietic cells. Similar to the previous report with the NKP, our rNKP gave rise to only NK cells, showing that it is indeed NK lineage committed in vitro26 . Importantly, the pre-NKP also gave rise to only NK cells, and not B, T, DC, or myeloid cells. The control CLP gave rise to B cells, NK cells, and DCs but no myeloid cells (Figure 2A, DC data not shown). To allow for the development of T cells, we repeated the same assay with OP9-DL1 cells. Again, the rNKP and pre-NKP gave rise to only NK cells with no T-cell development observed, whereas the CLP was able to give rise to high numbers of CD25+ T-cell progenitors (Figure 2B).
Pre-NKP and rNKP are lineage restricted and give rise only to NK cells in vitro. (A) Pre-NKP and rNKP lack B-cell potential in vitro. Individual wells with OP9 cells were seeded with 10 indicated progenitor cells and cultured in the presence of SCF, IL-7, Flt3 ligand, and IL-15. Wells were analyzed by flow cytometry 10 days later. No wells were positive for Gr-1+CD11b+ myeloid cells (data not shown). (B) Pre-NKP and rNKP lack in vitro T-cell potential. Flow cytometry analysis of the lineage output of individual wells seeded on OP9-DL1 cells with 10 indicated progenitor cells. No wells analyzed were positive for myeloid cells (data not shown). Data are representative of 1 of 4 experiments. (C) Pre-NKP and rNKP generate NK cells at a high clonal frequency. Number of wells plated with single cell and the number of wells positive for NKp46+ cells after 10 days. Data are representative of 1 of 3 experiments.
Pre-NKP and rNKP are lineage restricted and give rise only to NK cells in vitro. (A) Pre-NKP and rNKP lack B-cell potential in vitro. Individual wells with OP9 cells were seeded with 10 indicated progenitor cells and cultured in the presence of SCF, IL-7, Flt3 ligand, and IL-15. Wells were analyzed by flow cytometry 10 days later. No wells were positive for Gr-1+CD11b+ myeloid cells (data not shown). (B) Pre-NKP and rNKP lack in vitro T-cell potential. Flow cytometry analysis of the lineage output of individual wells seeded on OP9-DL1 cells with 10 indicated progenitor cells. No wells analyzed were positive for myeloid cells (data not shown). Data are representative of 1 of 4 experiments. (C) Pre-NKP and rNKP generate NK cells at a high clonal frequency. Number of wells plated with single cell and the number of wells positive for NKp46+ cells after 10 days. Data are representative of 1 of 3 experiments.
We next tested the NK potential of single cells in the rNKP and pre-NKP populations to verify homogeneity of each population. Using the same cultures conditions, we clone sorted rNKP and pre-NKP into 96-well plates and analyzed the wells on day 10 for mature NK cells. We found that ∼ 54% of each single rNKP (± 5%) and 40% of each single pre-NKP (± 2%) plated gave rise to mature NKp46+ NK cells (Figure 2C; supplemental Figure 6A-B). Once again, no other cell types were observed, demonstrating both the rNKP and pre-NKP were NK cell lineage restricted.
The developmental origin of NK cells has been a contentious issue, with some studies claiming that they are derived from MPPs and bypass CLPs, whereas others say they progress through a CLP intermediate.42 Using in vitro OP9 cultures, we showed that addition of IL-15 alone to cultures of highly purified CLP or pre-NKP gave rise to Lin−CD122+ progenitors after 4 days in culture, as well as more developed NK1.1+CD122+ cells, suggesting that CLP and pre-NKP can give rise to NKP (supplemental Figure 6C-D). Coupled with recent genetic tracing studies demonstrating that all NK cells arise from an IL-7Rα–expressing intermediate,20 we propose that progenitors indeed progress through the CLP stage en route to NK maturity.
In vivo differentiation of pre-NKP and NKP
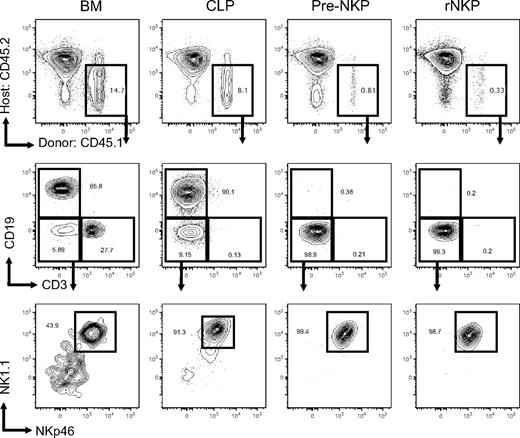
Previous studies had demonstrated that NKPs were lineage committed in vitro, but they did not test whether this was also true in vivo.26,43 Because in vitro conditions do not necessarily mimic physiologic conditions in vivo, we sought to develop assays that would allow us to test the in vivo lineage potential of pre-NKPs and rNKPs. We injected as few as 300 rNKP or pre-NKP, 1000 CLP, or 10 000 unfractionated bone marrow cells from wild-type CD45.1 mice into unconditioned RAG2−/−IL2rγc−/− CD45.2 congenic hosts, and then we analyzed tissues at 21 days after transplantation for NK reconstitution by CD45.1 donor cells. Unfractionated bone marrow gave rise to all cell types analyzed, even CD3+ T cells, which homeostatically proliferate from mature donor CD3+ cells (Figures 3 and 4A). At 3 weeks, CLP gave rise to an average of 14.4% of all nucleated spleen cells (± 3.5%), with the majority being CD19+ B cells (Figures 3 and 4A). Presumably because of the lack of proper thymic microenvironment in immunocompromised host mice, and the early time point at which mice were killed, we observed no peripheral CD3+ cells.39 However, a large number of mature NKp46+ NK cells developed from donor CLP (Figure 4C). No myeloid cells were detected in any of the CLP-transplanted mice. Similar results were obtained with sublethally irradiated wild-type recipients, except that a detectable number of DCs arose from donor CLP in this setting (data not shown). We decided to use unconditioned animals in subsequent studies because irradiated animals are known to up-regulate factors that can alter the homing of progenitors and hematopoietic differentiation.44 Thus, animals that are devoid of any NK, T, or B cells are suitable hosts in that they readily accept transplanted progenitors that reconstitute lymphoid lineages.
Pre-NKP and rNKP are lineage restricted to the NK cell fate in vivo. CD45.1 unfractionated BM, CLP, pre-NKP, and rNKP were transplanted into unconditioned congenic CD45.2 Rag2−/−IL2rγc−/− recipients. Spleens 3 weeks after transplantation were isolated and analyzed by flow cytometry for lineage potential of donor cells. Each progenitor type was injected into 5 individual mice per experiment. Data are representative of 1 of 5 experiments.
Pre-NKP and rNKP are lineage restricted to the NK cell fate in vivo. CD45.1 unfractionated BM, CLP, pre-NKP, and rNKP were transplanted into unconditioned congenic CD45.2 Rag2−/−IL2rγc−/− recipients. Spleens 3 weeks after transplantation were isolated and analyzed by flow cytometry for lineage potential of donor cells. Each progenitor type was injected into 5 individual mice per experiment. Data are representative of 1 of 5 experiments.
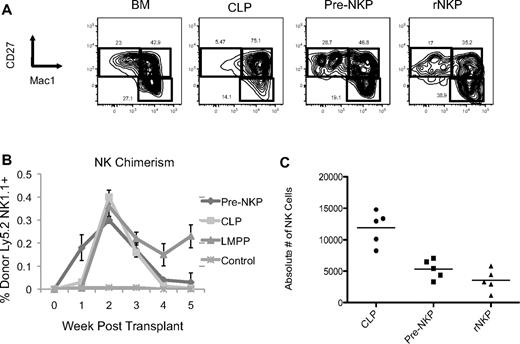
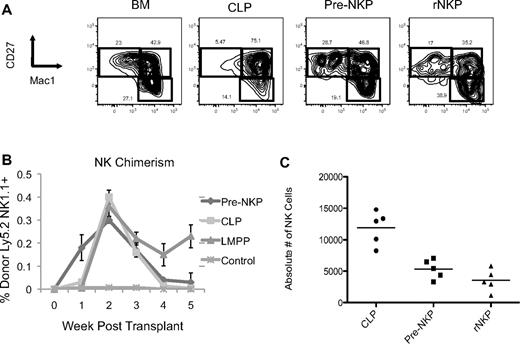
Pre-NKP and rNKP in vivo–derived NK cells are phenotypically mature and give rise to NK cells on transplantation faster than CLP or LMPP. (A) CD45.1 unfractionated BM, CLP, pre-NKP, and rNKP were transplanted into unconditioned congenic CD45.2 Rag2−/−IL2rγc−/− recipients. Spleens 3 weeks after transplantation were isolated and analyzed by flow cytometry for mature NK cell markers CD27 and Mac1. Each progenitor type was injected into 5 individual mice per experiment. Data are representative of 1 of 2 experiments. (B) Ly5.2+ progenitors where transplanted into unconditioned Ly5.1+ DKO mice. Mice where bled every week and analyzed by flow cytometry for donor NK cell reconstitution. Pre-NKP gave rise to NK cells as early as 1 week and were exhausted after 3 weeks. CLP gave rise to few, but detectable NK cells at 1 week and also became exhausted by 4 weeks. We observed no NK cells at 1 week in the LMPP transplants, but by 2 weeks they where comparable to both pre-NKP and CLP. Each progenitor type was injected into 5 individual mice per experiment. Data are representative of 1 of 2 experiments. (C) Absolute number of donor-derived NK cells from the spleens of mice transplanted with different progenitors. Data are representative of 1 of 2 experiments.
Pre-NKP and rNKP in vivo–derived NK cells are phenotypically mature and give rise to NK cells on transplantation faster than CLP or LMPP. (A) CD45.1 unfractionated BM, CLP, pre-NKP, and rNKP were transplanted into unconditioned congenic CD45.2 Rag2−/−IL2rγc−/− recipients. Spleens 3 weeks after transplantation were isolated and analyzed by flow cytometry for mature NK cell markers CD27 and Mac1. Each progenitor type was injected into 5 individual mice per experiment. Data are representative of 1 of 2 experiments. (B) Ly5.2+ progenitors where transplanted into unconditioned Ly5.1+ DKO mice. Mice where bled every week and analyzed by flow cytometry for donor NK cell reconstitution. Pre-NKP gave rise to NK cells as early as 1 week and were exhausted after 3 weeks. CLP gave rise to few, but detectable NK cells at 1 week and also became exhausted by 4 weeks. We observed no NK cells at 1 week in the LMPP transplants, but by 2 weeks they where comparable to both pre-NKP and CLP. Each progenitor type was injected into 5 individual mice per experiment. Data are representative of 1 of 2 experiments. (C) Absolute number of donor-derived NK cells from the spleens of mice transplanted with different progenitors. Data are representative of 1 of 2 experiments.
Both pre-NKP and rNKP gave rise to a significant number of NKp46+ NK cells, with no other lymphoid or myeloid cell types observed, demonstrating that the pre-NKP as well as the rNKP are both lineage restricted to the NK cell fate in vivo (Figures 3 and 4A,C). Chimerism levels ranged from 3.0% to 0.5% for both progenitor progeny with the average pre-NKP chimerism being 0.87% (± 0.5%) and average rNKP chimerism being 0.65% (± 0.4%). Because of the rarity of these cells, low chimerism levels for the NK progenitors were expected because only ∼ 300 progenitors were injected per recipient. All other tissues tested, including bone marrow, liver, and blood, had variable but detectable numbers of donor-derived NK cells (data not shown). The pre-NKP donor-derived NK cells were first detectable in the blood at day 5, peaked between days 14 and 21, and were largely undetectable by 5 weeks after transplantation (Figure 4B).
Recent studies have identified thymic NK cells that are believed to originate in the thymus and migrate to lymph nodes and spleen.45 To discern whether the rNKP or pre-NKP may give rise to this specific subset, we performed intrathymic injections of pre-NKP, rNKP, or CLP cells, or sham injections (PBS alone). At day 10 after transplantation, we analyzed the thymus and did not observe any NK output from the 3 progenitors (supplemental Figure 7). CLP intrathymic injections yielded robust thymocyte populations, including DN1 and DN2 (ckit+CD25+), as well as a small number of B cells. We have been unable to observe NK output from intrathymic injections of adult bone marrow progenitors or through analysis of fluorescently labeled thymic emigrants (Serwold et al39 ; D.B., unpublished data, 2008). Thus, thymic-NK cells may come from remnant fetal bipotent T/NK cell progenitors.46,47 Importantly, the pre-NKP and rNKP did not give rise to any thymocytes or T cells, further demonstrating their NK lineage restriction.
Phenotypic characterization of in vivo–derived NK cells
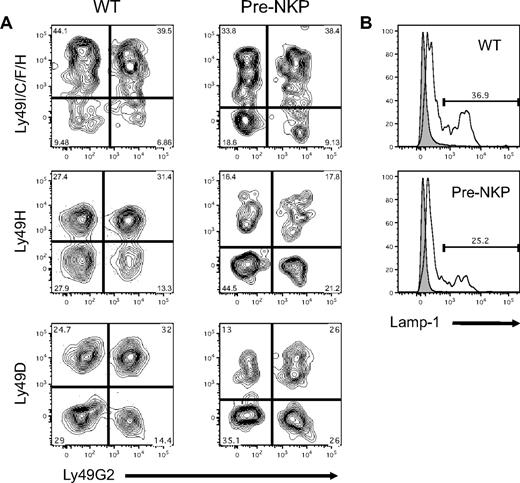
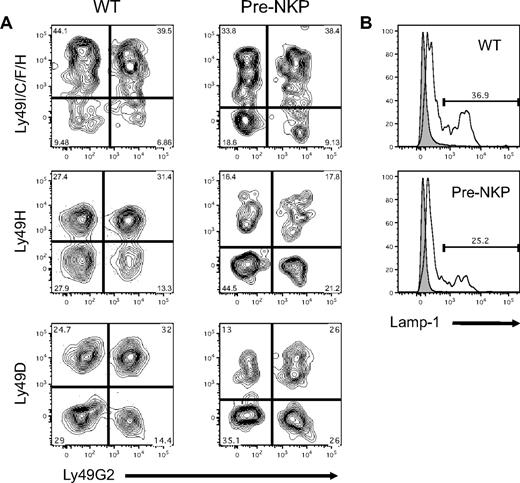
Unlike T and B cells, NK cells do not undergo receptor rearrangement and instead rely on a repertoire of activating and inhibitory receptors. To assess whether NK cells derived from pre-NKP gave rise to a mature NK repertoire, we used flow cytometry to analyze the expression patterns of a wide variety of Ly49 family members. At day 21, mice that had been transplanted with pre-NKP were analyzed for NK reconstitution and Ly49 expression on donor NKp46+ cells. These reconstituted NK cells showed a variety of combinations of Ly49 receptor expression patterns suggesting proper maturation and diversification, with frequency similar to those of wild-type mice (Figure 5A; supplemental Figure 8B). Similar results were seen for both the rNKP- and CLP-derived NK cells (supplemental Figure 8A).
Pre-NKP–derived NK cells have mature functional phenotype. (A) Pre-NKP–derived splenic NK cells express diverse combinations of Ly49 receptors. Wild-type NK cells and donor NKp46+ cells from pre-NKP transplanted Rag2−/−IL2rγc−/− mice were stained with a panel of anti-Ly49 family antibodies. Data represents 1 of 2 experiments. (B) Pre-NKP–derived NK cells degranulate on activation. Wild-type NK cells and NKp46+ cells derived in vivo from pre-NKP were isolated and stimulated with plate bound anti-NK1.1 for 4 hours in the presence of lysosomal-associated membrane protein 1 (Lamp-1) antibody. Data are representative of 2 independent experiments.
Pre-NKP–derived NK cells have mature functional phenotype. (A) Pre-NKP–derived splenic NK cells express diverse combinations of Ly49 receptors. Wild-type NK cells and donor NKp46+ cells from pre-NKP transplanted Rag2−/−IL2rγc−/− mice were stained with a panel of anti-Ly49 family antibodies. Data represents 1 of 2 experiments. (B) Pre-NKP–derived NK cells degranulate on activation. Wild-type NK cells and NKp46+ cells derived in vivo from pre-NKP were isolated and stimulated with plate bound anti-NK1.1 for 4 hours in the presence of lysosomal-associated membrane protein 1 (Lamp-1) antibody. Data are representative of 2 independent experiments.
One of the hallmarks of NK cell function is the ability to degranulate when they become activated. To test whether NK cells derived in vivo from the pre-NKP can degranulate when stimulated, we incubated splenic cells ex vivo with plate bound anti-NK1.1 or isotype control and measured outer cell membrane levels of lysosomal-associated membrane protein 1 by flow cytometry. After 4 hours of stimulation our in vivo pre-NKP–derived NK cells degranulated to a similar extent as wild-type controls, and significantly more than the isotype treated (Figure 5B). Because of the relatively small number of donor derived NK cells isolatable per transplantation (< 5000), we were unable to perform in vitro killing assays and had difficulty detecting significant production of IFNγ via intracellular flow cytometry.
Discussion
Here, we have identified the earliest committed NK cell progenitor in the adult murine bone marrow, which resides downstream of the CLP and can give rise to the NKP. Through the use of 12-color flow cytometry, we showed that these progenitors express CD27 and CD244, markers known to be expressed by early hematopoietic progenitors as well as by mature NK cells. Interestingly these cells are fate committed even though they lack detectable expression of CD122, a critical subunit of the receptor for IL-15, a cytokine known to be necessary for NK development. Using in vitro population and clonal assays, we showed that these cells represent a very rare and homogenous population that are able to give rise to NKp46+ NK cells and no other hematopoietic cell types. Also, in the more physiologic environment of in vivo transplantation, these cells can reconstitute the NK compartment in immunocompromised mice with mature NKp46+ cells that express a repertoire of Ly49 receptors and can degranulate on stimulation.
Previous studies that sought to identify early hematopoietic progenitors have often used the expression of growth factor/cytokine receptors to isolate prospective populations. For example, because genetic studies clearly demonstrated a critical role for IL-7 in T- and B-cell development,48 initial efforts to identify the CLP focused on the developmentally earliest population in the bone marrow that expressed the IL-7R.15 This led to the discovery of the phenotypic CLP that was negative for all mature lineage markers and expressed IL-7Rα.15 Similar studies using the IL-2Rβ were used to identify a population enriched for NKPs.26 Subsequent studies demonstrated that phenotypic NKPs and NK cells were still able to develop in IL-15–deficient mice,35 albeit at reduced numbers. This led to the hypothesis that IL-15 was not necessary for commitment but rather acted as a prosurvival and proliferative signal. Our data add support to this hypothesis, because the pre-NKP does not express detectable levels of the IL-15R, yet it is lineage committed to the NK lineage both in vitro and in vivo. Future studies using these discrete NK progenitors will help clarify this hypothesis.
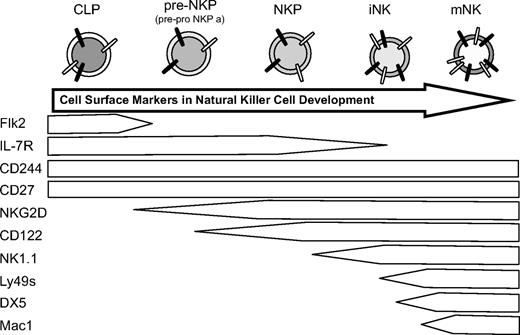
Early hematopoietic progenitors are phenotypically defined by their cell surface markers. They dynamically lose and acquire specific sets of markers during differentiation in a sequential yet overlapping manner. This is also true during NK development.31 Schlenner et al demonstrated in a recent study on fate mapping of lymphocytes that all mature NK cells progress through an IL-7Rα–expressing progenitor.20 Although this study did not comment on the role of CLPs in NK development, our data demonstrates that CLPs can give rise to IL-7Rα–expressing pre-NKP en route to NKPs (Figure 6) and thus almost certainly contribute to NK development in vivo. These in vivo findings refute earlier in vitro studies suggesting that NK cells do not develop through an IL-7Rα+ intermediate.42
Proposed model for NK bone marrow development. CLP, immature NK cells (iNK), and mature NK cells in the bone marrow, not terminally differentiated splenic NK cells that lack expression of CD27 (mNK).
Proposed model for NK bone marrow development. CLP, immature NK cells (iNK), and mature NK cells in the bone marrow, not terminally differentiated splenic NK cells that lack expression of CD27 (mNK).
Recent studies have suggested the existence of a separate thymic pathway of NK cell development, leading to a distinct subset of mature NK cells in the periphery.45 Recent data from our laboratory and others have shown that the CLP, rather than earlier progenitors with myeloid potential such as the MPP or lymphoid primed multipotent progenitor (LMPP), seeds the thymus and gives rise to the majority of T-cell progenitors.20,39 Given our data that CLPs generate NK cells in vivo, a logical prediction is that CLPs that seed the thymus are also the main source of thymic-derived NK cells. Surprisingly, however, no donor-derived NK cells were detected from intravenous or intrathymic injections of adult bone marrow CLP, pre-NKP, or rNKP. It is possible that the thymic NK cells observed by others may be derived from resident thymic bipotent T/NK progenitors formed during fetal development or from a distinct adult bone marrow progenitor that seeds the thymus. Recently, Charoudeh et al,49 using the previously identified NKP population defined as Lin−CD122+DX5−NK1.1−, suggested that the NKP has both NK- and T-cell potential in contradiction to our data presented here and in previous studies. In our hands, neither T cell–permissive assays in vitro nor intrathymic injections allowed for the development of any T cells derived from either the rNKP or pre-NKP. The basis for these differences is unknown; however, it is possible that our refined gating strategies more effectively eliminate contaminating mature T cells and T-cell progenitors than the aforementioned studies. Indeed, we have found that the marker CD244 is vital to deplete T cells with low CD3 expression from the Lin−CD122+DX5−NK1.1− population. This is supported by data from Rag2-deficient mice, in which the entire NKP population expresses CD244 positive (data not shown).
Another recent study by Carotta et al43 identified 2 novel early NK progenitors termed pre-pro NKP a and b. These populations were identified with the use of an ID2 reporter mouse. Similar to the pre-NKP described here, the pre-pro NKPa cells also express IL-7R and CD244, and they lack expression of Flk2. Based on similarities of cell surface marker expression, expression of Id2, and in vitro behavior, we believe the pre-pro NKPa is highly similar to the pre-NKP we have identified here. Our study complements the in vitro findings of the Carotta et al42 by demonstrating that the pre-NKPs are also NK restricted in vivo and by providing additional cell surface markers that can be used to identify pre-NKPs without the use of the Id2 reporter.
NK cells have significant clinical potential, given that they can lyse transformed and virally infected cells. Indeed, the rapid generation of NK cells by transplanted CLPs after allogeneic bone marrow transplantation may well be capable of providing protection against CMV infections.50 Importantly, pre-NKPs seem to be capable of generating mature NK cells more rapidly than do CLPs. It will be interesting to see whether NKP transplantation provides more rapid and NK-specific protection against CMV infection in myeloablated recipients than do CLPs.
Overall, on the basis of frequencies, clonal output, and direct comparison with other progenitor populations during transplantations, we conclude that the pre-NKP is the developmentally earliest reported cell committed to the NK pathway and that it represents an intermediate progenitor that links the CLP and the NKP.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank L. Jerabek for laboratory management; C. Richter and T. Naik for antibody production; A. Mosley, J. Dollaga, and D. Escoto for animal care; and W. Yokoyama for helpful comments. All experiments were conducted in the laboratory of I.L.W.
This study was supported by National Institutes on Health grants F31AG032854, R01CA86065, and T32AI07290 (J.W.F.); K01DK078318 (D.B.); CA09151 (M.A.I.); and 5R01AI047457 and 5R01AI047458 (I.L.W.); California Institute for Regenerative Medicine fellowship T1-00001 (M.A.I.); and the Thomas and Stacey Siebel Foundation (I.L.W).
National Institutes of Health
Authorship
Contribution: J.W.F., D.B., and M.A.I. designed and conducted experiments and collected data; J.W.F. wrote the manuscript; J.S. and H.K. provided advice and preliminary data; and I.L.W. directed the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: John W. Fathman, Institute of Stem Cell Biology and Regenerative Medicine, Stanford University School of Medicine, Stanford, CA 94305-5323; e-mail: jfathman@stanford.edu.