Abstract
We conducted a phase 2 study of ruxolitinib in patients with relapsed/refractory leukemias. Patients with acceptable performance status (0-2), adequate organ function, and no active infection, received ruxolitinib 25 mg orally twice a day for 4 weeks (1 cycle). Response was assessed after every 2 cycles of treatment, and patients who completed 2 cycles were allowed to continue treatment until disease progression. Dose escalation to 50 mg twice daily was permitted in patients demonstrating a benefit. Thirty-eight patients, with a median age of 69 years (range, 45-88), were treated. The median number of prior therapies was 2 (range, 1-6). Twelve patients had JAK2V617F mutation. Patients received a median of 2 cycles of therapy (range, 1-22). Three of 18 patients with postmyeloproliferative neoplasm (MPN) acute myeloid leukemia (AML) showed a significant response; 2 achieved complete remission (CR) and one achieved a CR with insufficient recovery of blood counts (CRi). The responding patients with palpable spleens also had significant reductions in spleen size. Overall, ruxolitinib was very well tolerated with only 4 patients having grade 3 or higher toxicity. Ruxolitinib has modest antileukemic activity as a single agent, particularly in patients with post-MPN AML. The study was registered at www.clinicaltrials.gov as NCT00674479.
Introduction
The development and function of hematopoietic cells depend on complex signaling pathways that are triggered by numerous cytokines and their receptors. The roles of these intracellular signaling pathways in cell-cycle regulation, apoptosis, and leukemogenesis have been extensively evaluated.
The JAK/STAT signaling pathway has a significant impact on how hematopoietic cells respond to diverse cytokines and growth factors. Signaling through STAT proteins mediates cell growth, differentiation, apoptosis, transformation, and other fundamental cell functions.1 For example, STAT1 mediates the growth-inhibitory effects of IFN-γ, through induction of the cyclin-dependent kinase inhibitor p21waf1, whereas STAT5 mediates the proliferative effects of IL-3 and GM-CSF.2,3 Similarly, phosphorylation of STAT3 can mediate both IL-6– and IL-10–induced growth arrest and result in GM-CSF– and IL-3–induced proliferation.3-5 Abnormalities of the JAK/STAT pathway and constitutive STAT activation have been well characterized in a variety of leukemias.6
The JAK enzymes (JAK1, JAK2, JAK3, and tyrosine kinase 2 [TYK2]) transduce extracellular signals to the nucleus via STAT proteins. The JAKs play important roles in myeloid and lymphoid cell proliferation and differentiation. Somatic JAK3 mutations (eg, JAK3A572V, JAK3V722I, and JAK3P132T) and fusion transcripts involving JAK2 (eg, ETV6-JAK2, PCM1-JAK2, and PCR-JAK2) have been described in acute and chronic myeloid malignancies.7 Constitutive activation of STAT-1, -3, and -5 in AML cell lines has been demonstrated by immunoprecipitation of the tyrosine-phosphorylated proteins. These STAT proteins are believed to contribute to the autonomous proliferation of AML blasts.8
A proportion of patients with myeloproliferative neoplasm (MPN) progresses to develop acute myeloid leukemia (AML). The STAT5 protein, a major factor in BCR-ABL1–negative MPN pathology, plays a key role in the malignant transformation from MPN to AML (secondary AML [sAML]) when it is constitutively activated.9-11 In the majority of patients with JAK2-mutated MPN who progress to AML, the mutant clone is lost and TET2 mutations may be present in a clone distinct from that harboring a JAK2 mutation, suggesting that the leukemia arises in a JAK2 wild-type cell.12,13 Although a JAK2 allele burden of > 50% represents a risk factor for progression to myelofibrosis or polycythemia vera, it is not related to transformation to acute leukemia.14
JAK activation can lead to resistance to targeted therapies. BCR-ABL–positive acute lymphoblastic leukemia (ALL) cells were sensitized to imatinib by the inhibition of the JAK pathway, suggesting that the combination of a JAK inhibitor and imatinib might be beneficial in refractory Philadelphia chromosome–positive disease.15 Up-regulation of JAK signaling is associated with resistance to tyrosine kinase inhibitors in chronic myelocytic leukemia (CML). Inhibition of JAK2 overcomes this resistance, providing a rationale for the use of JAK2 inhibitors to eradicate residual disease in CML.16,17 Ruxolitinib is a potent selective ATP-competitive inhibitor of JAK1 and JAK2 kinases, with an IC50 of 3.3nM and 2.8nM, respectively, and no significant inhibition of 25 other important intracellular kinases at concentrations of 100 times these IC50s. Ruxolitinib inhibits JAK signaling by the cytokines that support transformed cells and are responsible for constitutional symptoms. It arrests growth factor signaling by inhibiting downstream STAT phosphorylation. It is active in cellular assays driven by JAK2V617F and, at the same time, is not selective for mutated JAK2V627F. Clinical benefit of ruxolitinib has been shown in MPN, as well as in pilot studies in psoriasis and rheumatoid arthritis; furthermore, ruxolitinib has been very well tolerated by patients in these studies.18,19
The primary objectives of this study were to determine the activity of ruxolitinib in patients with various refractory or relapsed hematologic malignancies and to evaluate its safety.
Methods
Eligibility criteria
Eligible patients included adults with relapsed and/or refractory AML (de novo or secondary to MPN), ALL, myelodysplastic syndromes (MDS; including chronic myelomonocytic leukemia [CMML]), or CML patients whose disease was either in blast phase or had failed prior treatment with 2 tyrosine kinase inhibitors. Eastern Cooperative Oncology Group (ECOG) performance status of 0-2 and adequate organ functions, including creatinine ≤ 2.0 mg/dL, total bilirubin ≤ 1.5 × institutional upper limit of normal (ULN), and liver transaminases ≤ 3 × ULN, were required. Patients with active HIV infection or infectious hepatitis were excluded. Patients had to wait at least 2 weeks after their last therapy to begin treatment. Hydroxyurea was allowed up to 5 g per day for the first 14 days during the first cycle of the study.
This study was conducted according to the Declaration of Helsinki. The University of Texas MD Anderson Cancer Center Institutional Review Board approved the protocol. All patients gave written informed consent before participation in the study.
STAT phosphorylation measurement
To show the activity of ruxolitinib on the JAK/STAT pathway, we measured the levels of phosphorylated STAT3 (pSTAT3) at baseline and after the therapy with ruxolitinib in limited number of patients (n = 8). Whole-blood samples were collected into heparinized tubes and analyzed within 24 hours of collection. Blood samples were aliquoted into 96-well plates (Corning Incorporated; 0.3 mL/well). For each subject at each time point, samples of blood were left unstimulated or stimulated with human IL-6 (R&D Systems, 100 ng/mL, final concentration) for 15 minutes at 37°C. After incubation, RBCs were lysed using hypotonic conditions (QIAGEN RBC lysis buffer). White blood cells were then quickly pelleted and lysed to make total cell extracts (Biosource lysis buffer with 1mM PMSF and 1× Sigma-Aldrich protease inhibitor solution added). The extracts were stored at −20°C until analysis by ELISA (Biosource STAT3 [pY705] ELISA kit) for levels of pSTAT3. Data from individual patients were assayed in duplicate and averaged.
Treatment regimen
The primary objective of this study was to observe the antitumor effects of this medication in patients with relapsed and/or refractory AML, ALL, MDS, and CML in blast phase or refractory to tyrosine kinase inhibitors. Ruxolitinib was administered orally at a dose of 25 mg twice daily for a 28-day cycle. The cycle was repeated in the absence of intolerable toxicity or disease progression. This dose and schedule was selected based on the available early data from the phase 1/2 study of the drug in patients with myelofibrosis.19 As thrombocytopenia was a significant toxicity in the myelofibrosis study, further dose escalation of the drug beyond what is discussed below was not allowed. A separate ongoing phase 1 dose-escalation study is evaluating whether the dose of ruxolitinib can be increased further significantly.
BM and cytogenetic analysis were performed after every 2 cycles of therapy. JAK2V617F allele burden was measured by real-time PCR before and after therapy and correlated to response.
The revised NCI Common Terminology Criteria for Adverse Events (CTCAE; Version 3.0) were used for adverse event reporting. In the event of grade 3 or 4 nonhematologic toxicity or grade 3 and 4 hematologic toxicity directly attributable to the drug, ruxolitinib was withheld until resolution of the toxicity to grade 1 or lower and then restarted at 15 mg twice daily. If the patient did not recover from grade 3 or 4 toxicity within 2 weeks, the treatment was discontinued. Treatment was also stopped if there was no benefit after 2 cycles of therapy. Evaluation of BM aspirate was done every 2 cycles. In patients without disease progression who tolerated ruxolitinib well without significant toxicity, a dose increase of up to 50 mg twice a day was allowed.
Statistical analysis
Summary statistics and frequency tables were used to summarize baseline patient characteristics and adverse event rates. We used a Gehen design for the statistical analysis of the data. Assuming a null response rate of 0%, targeting a 15% response rate and power of 80%, the trial initially was designed to enroll 10 patients in each disease category: (1) AML; (2) ALL; (3) MDS; and (4) CML. If at least one response was achieved in the 10 patients in a disease category, then a total of 30 patients would be enrolled in this disease category. Thirty patients would provide a confidence interval for response rate with half-width = 0.13, assuming the true response rate is 15%. The potential total sample size was therefore 120 patients. However, because of the availability of alternative clinical trials with potentially effective agents, accrual to the ALL, MDS, and CML categories was limited; the study was closed after an adequate number of patients with AML were treated.
Response criteria
For patients with AML, response and progression were evaluated according to the International Working Group (IWG) criteria.20 Complete remission (CR) was characterized as achieving an absolute neutrophil count > 1.0 × 109/L and platelet count > 100 × 109/L, with normal marrow differential with < 5% blasts in a normo- or hypercellular marrow. CR with insufficient recovery of the peripheral blood counts count (CRi) was characterized as having achieved all the above criteria for CR but with absolute neutrophil count < 1.0 × 109/L and platelet count < 100 × 109/L. Similarly, response and progression assessment for patients with ALL, CML, and MDS was based on previously published criteria.21 An increase in BM or peripheral blood myeloblasts from baseline at the initiation of therapy, worsening cytopenia (therapy-related cytopenia excluded), and/or becoming transfusion dependent were considered disease progression at the discretion of treating physician.
Results
Response and outcome
From May 2008 through April 2010, 38 patients were enrolled in the study. The median age of the patients was 69 years (range, 45-88 years). The median number of prior therapies was 2 (range, 1-6). Twelve (31%) patients carried a JAK2V617F mutation at the time of start of therapy. The baseline characteristics of patients are summarized in Table 1.
Overall, the median number of cycles of therapy received by the patients was 2 (range, 1-22). Three patients demonstrated a significant and persistent decline in the number of BM blasts (Table 2), with 2 achieving CR and 1 CRi. Of note, 1 patient with refractory MDS who received 3 cycles of treatment underwent a successful allogeneic stem cell transplantation. Symptoms like fatigue and weight loss improved significantly in the responsive patients and their energy level improved; this may be potentially attributable to the JAK1-inhibitory effect of ruxolitinib.19 The number of myeloblasts in both the peripheral blood and BM declined or stayed stable in 15 patients for > 2 cycles. Nine (24%) patients including 1 (3%) responder with CRi had dose escalation. There was no overall increase in toxicity among these patients.
All 3 patients who achieved CR or CRi had sAML (after MPN). Two of them had JAK2V617F mutation which was detected before and after the therapy. (Table 2) In all 3, response occurred in the absence of preceding myelosuppression and disappearance of blasts from the marrow occurred slowly over varying number of cycles. Two of these 3 patients had received prior decitabine (1 cycle in 1 and 3 cycles in the other) and neither had responded.
Phosphorylated STAT3 levels before and after treatment
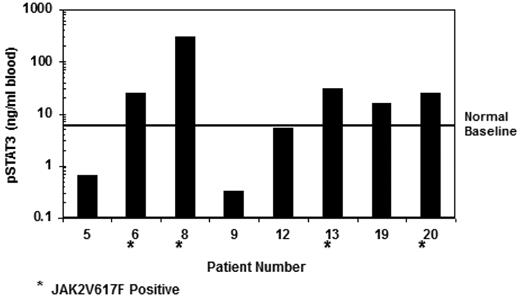
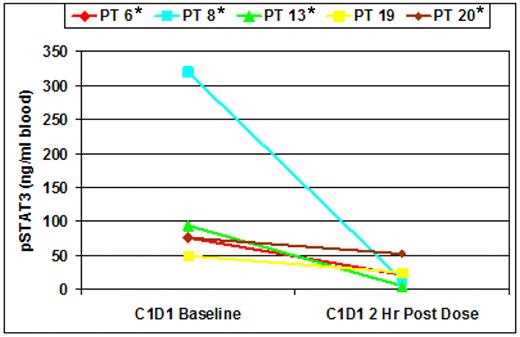
Blood samples were available from a limited number of patients for the purpose of STAT phosphorylation measurements. An examination of the baseline level of phosphorylated STAT3 (pSTAT3) revealed that several subjects contained cells that exhibited activation of STAT3 without the addition of cytokine stimulation (Figure 1). With the limited sample set it could not be determined whether this was attributable to low levels of cytokine present in the plasma or endogenous activation of the JAK/STAT pathway in the cells. However, 4 of the 5 patients with elevated baseline pSTAT (notably, the 4 with the highest level of activation) had the JAK2V617F mutation. To address whether the pSTAT3 signal could be modulated by JAK inhibition, samples were obtained before dose and at 2 hours after dosing with 25 mg of ruxolitinib. These data (Figure 2) demonstrate that, for each patient, elevated levels of pSTAT3 could be significantly decreased by administration of ruxolitinib, suggesting that the activation of the STAT3 signal was accomplished through JAK activation. This modulation of increased pSTAT3 occurred irrespective of the presence of JAK2V617F mutation.
Baseline levels of pSTAT3 in a subset of patients obtained before dosing with ruxolitinib. Patients marked with an asterisk were positive for the presence of JAK2V617F.
Baseline levels of pSTAT3 in a subset of patients obtained before dosing with ruxolitinib. Patients marked with an asterisk were positive for the presence of JAK2V617F.
IL-6 (100 ng/mL) stimulated levels of pSTAT3 at baseline and 2 hours after administration of 25 mg of ruxolitinib. Cells stimulated and providing the signal are monocytes and AML blasts. Patients marked with an asterisk were positive for the presence of JAK2V617F.
IL-6 (100 ng/mL) stimulated levels of pSTAT3 at baseline and 2 hours after administration of 25 mg of ruxolitinib. Cells stimulated and providing the signal are monocytes and AML blasts. Patients marked with an asterisk were positive for the presence of JAK2V617F.
JAK2V617F allele burden
Twelve (31%) patients were positive for JAK2V617F and among them 8 were evaluated for the allele burden both before and after therapy with ruxolitinib as shown in Figure 3. There was no significant difference between the levels of JAK2V617F before and after therapy in any patient (Figure 3).
Eight patients who benefited from the therapy with ruxolitinib including 2 responders had positive JAK2V617F mutation both before and after the therapy. There is no clear association between the therapy with ruxolitinib and the level of allele burden in the refractory leukemias.
Eight patients who benefited from the therapy with ruxolitinib including 2 responders had positive JAK2V617F mutation both before and after the therapy. There is no clear association between the therapy with ruxolitinib and the level of allele burden in the refractory leukemias.
Toxicity
Overall, ruxolitinib was well tolerated by the majority of patients. Only 4 patients developed grade 3 or greater toxicities that were possibly or probably attributable to the study drug. One patient, who had severe thrombocytopenia at the time of study enrollment, died of an intracranial hemorrhage while on study. Three patients had grade 1 or 2 toxicity, and the remaining patients did not have any side effects that were judged to be related to the study drug. Most patients had significant cytopenias before the initiation of therapy and in none were these significantly worsened while on the study. Three patients had reduction in their dose to 15 mg twice a day, in 2 related to grade 3 elevation of liver enzymes, and in one related to grade 3 thrombocytopenia. Further dose reductions to 10 mg twice a day occurred in 2 of the same 3 patients.
Discussion
Developing a successful therapeutic approach for patients with relapsed and refractory hematologic malignancies continues to be challenging. In particular, patients with AML developing after an antecedent hematologic disorder and especially those progressing from prior MPNs have a poor response to treatment with cytotoxic chemotherapy regimens designed for AML, even as their initial therapy.22 Few other agents have shown significant activity in this setting.
The potential efficacy of the hypomethylating agents, decitabine and 5-azacitidine, have been studied in patients with MPN.23,24 In a recently published study, 54 patients with Philadelphia-negative MPN (including 21 essential thrombocythemia [ET], 21 polycythemia vera [PV], 7 primary myelofibrosis, and 5 unclassified MPN) who had progressed to AML (n = 26) or MDS (n = 28) were treated with azacitidine.25 The overall response rate was 52% (24% CR, and 11% partial response [PR], 8% marrow CR or CRi, 9% hematologic improvement) and median response duration was 9 months. A recurrence of chronic-phase features of the initial MPN was observed in 39% of the responders. The median overall survival was 11 months. The authors concluded that azacitidine provided encouraging results in Ph-negative MPN having progressed to AML or MDS, but response duration is short, and consolidation treatments have to be evaluated.25
In another study from Mount Sinai School of Medicine, 4 (57%) of 7 patients with AML with an antecedent history of MF treated with decitabine reported subjective improvement in the symptoms of fatigue/global weakness and left upper quadrant abdominal fullness/pain. All 7 patients experienced an objective reduction in spleen size on physical examination with a mean 35% reduction of palpable splenomegaly (range 11%-61%). Four (57%) of 7 patients had a decrease in RBC transfusion requirements and a single patient achieved transfusion independence after 3 cycles of therapy. The 3 patients achieving CRi remained alive at the last follow-up in the report.26
Verstovsek and colleagues have demonstrated the activity of ruxolitinib in patients with symptomatic advanced MF.19 In their study, achievement of clinical benefit with this agent was clearly demonstrated. The circulating inflammatory cytokines were diminished significantly after the therapy with ruxolitinib leading to durable resolution of debilitating constitutional symptoms. According to this study involving 153 patients with a median follow-up of ∼ 15 months, only 3 cases of transformation to AML were observed, compared with an expected incidence of 11 cases, considering the expected historical leukemic transformation frequency of 0.06 per patient-year.19
We used ruxolitinib as salvage therapy in a group of 38 patients with refractory leukemias to target the inhibition of the constitutive JAK-STAT signaling pathway. All 3 responders (2 CR and 1 CRi) had sAML. There was no correlation between response and the presence of the JAK2V617F mutation, suggesting that other signaling pathways activated in these cells may play an important or ancillary role in driving the proliferation of these cells.
There are several limitations in this study. First, all patients treated in this study had received other therapies before ruxolitinib, and it is conceivable that the drug may be more beneficial as a frontline therapy. Second, the JAK2V617F allelic burden before and after therapy was only available in a limited group of the patients treated, limiting any interpretation on the role of the drug in suppressing malignant clones that are specifically driven by this mutation. In future studies, it would be important to monitor the presence of the mutation in the transformed cells and its response to treatment to better define the dynamics of the drug and its potential mechanism of action and to elucidate whether the JAK2V617F mutation is a driving mutation in the malignant cells. In addition, the dose of ruxolitinib was not further escalated in this study and it may be possible that higher doses of the drug may be more effective. Finally, the potential role of a more selective JAK2 inhibitor in achieving responses will need to be evaluated future studies.
It is highly likely that combinations of drugs that target different pathways will be required to develop successful therapeutic regimens in this disease. As hypomethylating agents have demonstrated significant clinical benefits in the subgroup of AML patients secondary to MPNs, it is highly plausible that combination of these agents with JAK1/JAK2 inhibitors such as ruxolitinib may have significant clinical activity. Preclinical and clinical studies examining the role of such combination regimens should be undertaken in view of the lack of an effective regimen with survival benefit and given the aggressive nature of this type of leukemia. Furthermore, both the hypomethylating agents and JAK inhibitors such as ruxolitinib have relatively limited and nonoverlapping side-effect profiles with good tolerability, an important property particularly in the older age group.
In conclusion, ruxolitinib has modest antileukemia activity and an acceptable toxicity profile particularly in post-MPN AML. Future trials combining ruxolitinib with other drugs, including hypomethylating agents are planned.
This study was presented in part at the American Society of Hematology annual meeting, December 2010, Orlando, FL.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was partly supported by a research grant from the Incyte Corporation.
Authorship
Contribution: A.E. analyzed the data and wrote the manuscript; S.V., P.A.S., and R.C.N. analyzed the data, and wrote and approved the manuscript; Z.E., J.B., J.C., S.F., A.F., G.B., and H.M.K. treated the patients on the study, and reviewed and approved the manuscript; C.B. and S.G. collected the data; and F.R. designed the study, treated the patients on the study, analyzed the data, and wrote and approved the final manuscript.
Conflict-of-interest disclosure: S.V. received research funding from Incyte Corporation. J.C. received research funding from Novartis. P.A.S. and R.C.N. have been employed by Incyte Corporation as senior director and vice president, respectively. H.M.K. received research funding from Novartis. F.R. received honoraria from Novartis and research funding from Incyte Corporation. The remaining authors declare no competing financial interests.
Correspondence: Farhad Ravandi, MD, Department of Leukemia, MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 428, Houston, TX 77030; e-mail: fravandi@mdanderson.org.