Key Points
For patients in developing countries with APL, a clinical network of institutions made it possible to reduce significantly the early mortality and improve the OS.
Abstract
Thanks to modern treatment with all-trans retinoic acid and chemotherapy, acute promyelocytic leukemia (APL) is now the most curable type of leukemia. However, this progress has not yielded equivalent benefit in developing countries. The International Consortium on Acute Promyelocytic Leukemia (IC-APL) was established to create a network of institutions in developing countries that would exchange experience and data and receive support from well-established US and European cooperative groups. The IC-APL formulated expeditious diagnostic, treatment, and supportive guidelines that were adapted to local circumstances. APL was chosen as a model disease because of the potential impact on improved diagnosis and treatment. The project included 4 national coordinators and reference laboratories, common clinical record forms, 5 subcommittees, and laboratory and data management training programs. In addition, participating institutions held regular virtual and face-to-face meetings. Complete hematological remission was achieved in 153/180 (85%) patients and 27 (15%) died during induction. After a median follow-up of 28 months, the 2-year cumulative incidence of relapse, overall survival (OS), and disease-free survival (DFS) were 4.5%, 80%, and 91%, respectively. The establishment of the IC-APL network resulted in a decrease of almost 50% in early mortality and an improvement in OS of almost 30% compared with historical controls, resulting in OS and DFS similar to those reported in developed countries.
Introduction
All-trans retinoic acid (ATRA) in combination with anthracycline-based chemotherapy constitutes the mainstay of modern strategies for the treatment of patients with newly diagnosed acute promyelocytic leukemia (APL).1-3 Contemporary clinical trials have reported complete remission (CR) and long-term disease-free survival (DFS) rates of approximately 90% and 85%, respectively.3-11 The major cause of treatment failure is death during induction therapy. This ranged from 5% to 10% in recent multicenter trials;3,4,6,8,11,12 most deaths were the result of hemorrhage, infection, and differentiation syndrome (DS).
Unfortunately, the treatment outcome for patients in developing countries with APL is significantly inferior compared with that reported in Europe and United States. A retrospective analysis of 134 Brazilian patients with APL reported a death rate of 32% during induction and 10% during consolidation.13 This is in contrast with results from trials conducted in developed countries, where these rates of death during induction ranged from 5% to 10% and were < 5% during consolidation, respectively.3,4,6,8,12 Consequently, the reported long-term overall survival (OS) is <60% in developing countries.13 An important feature is that most of the deaths that occur during induction are caused by hemorrhage. Therefore, APL-associated coagulopathy is a particularly relevant target for medical education programs because it may be counteracted by early administration of ATRA and by rapid initiation of supportive measures.1,14
The International Consortium on Acute Promyelocytic Leukemia (IC-APL) was established in 2005 as an initiative of the International Members Committee of the American Society of Hematology. The purpose was to create a network of institutions in developing countries that would exchange experience and data and receive support from well-established cooperative groups in the United States and Europe. The IC-APL formulated treatment guidelines based on protocols from successful clinical trials3 in developing countries and adapted them to local conditions and resources. These guidelines covered standardized approaches for specific and prompt diagnosis, treatment, supportive care, and disease follow-up measures. Here we demonstrate that by using limited resources, it is possible to reduce the gap between developed and developing countries regarding the quality of care and treatment outcome in APL.
Methods
Eligibility of countries to participate in the IC-APL
In order to participate in the IC-APL, countries had to fulfill minimum requirements. These included availability of principle drugs scheduled in the IC-APL protocol, preexisting transfusion medicine services that could guarantee adequate availability of platelets and other blood derivatives, a hematology laboratory capable of performing basic fluorescence microscopy, the ability to report data and participate in meetings using web-based tools, and identification of 1 national coordinator and 1 person responsible for molecular studies. Brazil, Mexico, Chile, and Uruguay were selected as participating countries.
Eligibility of patients
Adult patients (age 15 to 75 years) in whom the diagnosis of APL was suspected underwent bone marrow (BM) aspiration followed by immunofluorescence staining using the anti-promyelocytic leukemia (anti-PML) antibody (PG-M3).15 ATRA treatment was initiated immediately in all cases in which the diagnosis of APL was suspected based on morphology. The provisional diagnosis of APL was confirmed using the anti-PML immunofluorescence technique, upon which daunorubicin was combined with ATRA. Cytogenetic analysis for t(15;17) and/or polymerase chain reaction (PCR) analyses for PML/RARA rearrangements were then performed on BM samples; the detection of 1 or the other was required for confirmation of the diagnosis of APL and enrollment in the protocol. Patients with de novo APL, normal hepatic and renal function, no cardiac contraindications to anthracycline chemotherapy, and an Eastern Cooperative Oncology Group performance status of less than 4 were eligible. Informed consent was obtained from all patients. According to the Declaration of Helsinki, the research ethics board of each participating hospital approved the protocol.
Induction therapy
Induction therapy was identical to that adopted in the Programa Español de Tratamiento en Hematologia/Dutch-Belgian Hemato-Oncology Cooperative Group Leucemia Promielocítica Aguda (PETHEMA/HOVON LPA) 2005 trial,3 except that idarubicin (12 mg/m2/d) was replaced by daunorubicin (60 mg/m2/d) because daunorubicin was more readily available and cost less than idarubicin in the participating countries. Supplemental Table 1 shows the IC-APL treatment schedule. Oral ATRA (45 mg/m2/d) was administered in 2 daily divided doses until complete hematologic remission was achieved. Daunorubicin was given as an intravenous bolus on days 2, 4, 6, and 8, except for patients >70 years who received only the 3 first doses of daunorubicin. For patients ≤20 years, the ATRA dose was adjusted to 25 mg/m2/d.
Postinduction therapy
Patients who achieved complete hematological remission (CR) received 3 monthly consolidation courses with ATRA and anthracycline-based chemotherapy that was identical to that adopted in the PETHEMA/HOVON LPA2005 trial,3 except that, as for induction, idarubicin was replaced by daunorubicin. Consolidation therapy followed a risk-adapted strategy according to the risk groups, as defined by the PETHEMA/GIMEMA groups.3,16 For low-risk patients, ATRA was combined with daunorubicin (25 mg/m2 on days 1 to 4) in the first cycle, mitoxantrone (10 mg/m2 on days 1 to 3) in the second cycle, and daunorubicin (60 mg/m2 on day 1) in the third cycle. For intermediate-risk patients, consolidation was intensified by increasing the dose of daunorubicin to 35 mg/m2 in the first cycle and by repeating the infusion of 60 mg/m2 on the second day of the third cycle. For high-risk patients, consolidation was further intensified by adding cytarabine in cycles 1 (1000 mg/m2 on days 1 to 4) and 3 (150mg/m2 q8h days 1 to 4). In addition, mitoxantrone (10 mg/m2/d) was administered for 5 days in the second cycle; the anthracyclines dosage in cycles 1 and 3 was as described for low-risk patients. High-risk patients >60 years did not receive cytarabine and were treated as intermediate-risk patients. The use of ATRA and anthracycline monochemotherapy for the treatment of elderly patients was based on the previously reported results of the PETHEMA studies LPA96 and LPA99.17 Maintenance therapy was identical to that used in the LPA2005 trial, as described elsewhere.3
Anti-PML immunofluorescence staining
BM aspirates were stained with PG-M3 and analyzed as described previously.15
Genetic confirmation of the diagnosis and monitoring of treatment response
Pretreatment BM samples were cultured and analyzed by standard cytogenetic methods. Samples were also tested for the presence of PML/RARA rearrangements by reverse-transcription polymerase chain reaction (RT-PCR) according to the BIOMED-1 guidelines in centralized laboratories in Ribeirão Preto (Brazil, E.M.R.), Puebla (Mexico, J.G.-E.), Santiago (Chile, M.S.U.), and Montevideo (Uruguay, M.d.R.U.).18 Treatment response was confirmed by morphological analysis and by RT-PCR at the end of the 3 consolidation cycles. Minimal residual disease (MRD) was monitored at national reference laboratories by RT-PCR of BM samples obtained every 3 months during maintenance and for 2 years after completion of treatment. Additional samples from 71 patients were also evaluated for MRD at the end of induction and at the end of the second consolidation cycle. RT-PCR assays were set up with a sensitivity level of 1 leukemic cell in 10−4 cells.
Supportive measures
We followed the guidelines of the European LeukemiaNet for supportive measures for APL patients.1 Platelets were transfused to maintain the platelet count above 30 000 to 50 000/uL, and cryoprecipitate was administered to maintain the fibrinogen level above 150 mg/dL. DS was diagnosed according to the presence of the following signs or symptoms, as described by Frankel et al:19 unexplained fever, dyspnea, pleural and/or pericardial effusion, pulmonary infiltrates, renal failure, hypotension, and unexplained weight gain of >5 kg. No single sign or symptom was considered sufficient to make a diagnosis of the syndrome. Patients with 4 or more of these signs or symptoms were classified as having severe DS; those with 2 or 3 signs or symptoms were considered to have moderate DS. Regardless of the severity, treatment with dexamethasone at a dose of 10 mg twice daily by intravenous injection was started at the earliest symptom or sign of DS and maintained until the resolution of the syndrome. ATRA was temporarily discontinued only in the setting of severe DS that led to respiratory or renal dysfunction requiring admission to the intensive care unit.
Network structure and operation
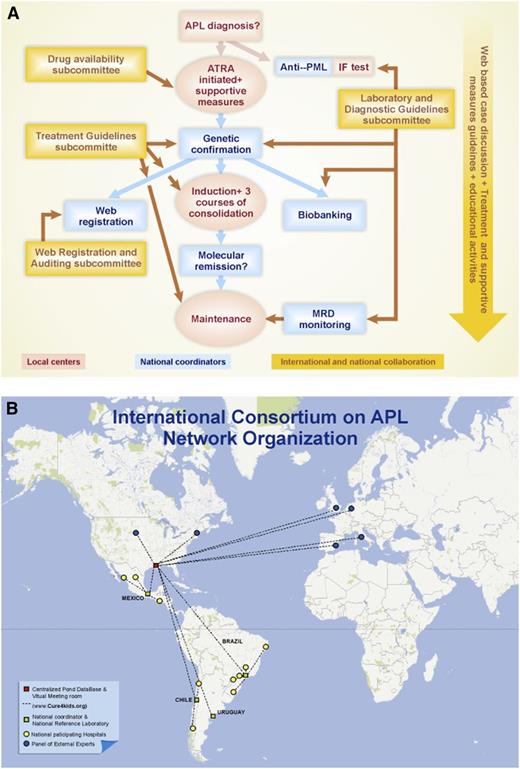
Figure 1 illustrates how the network operated in a given case and the geographic distribution of the centers. All cases were registered using common clinical record forms (CRFs) in the Pediatric Oncology Network Database (POND), which was used for online collection of clinical and laboratory data. The 4 national coordinators periodically accessed and analyzed the integrity of the data and the essential treatment response and toxicity parameters of the patients enrolled in their respective countries. Bimonthly discussions were held via virtual meetings in each country at which a summary of the clinical evolution and laboratory and MRD results of each case were discussed in a chat room at www.cure4kids.org. When necessary, international experts participated in the meeting, and face-to-face meetings with all participants were held at least twice a year. The Treatment Guidelines Subcommittee was responsible for the study design and CRF elaboration. To ensure that drugs were available at all times, even at the most remote centers, the Drug Availability Subcommittee was created. Examples of its activities include establishing a small stock of ATRA in the emergency rooms of a few centers and submitting letters to health authorities asking for their support. The Laboratory and Diagnostic Guidelines Subcommittee was responsible for the standardization of diagnostic and MRD methods as well as for the control of biobanking. The Web Registration and Auditing Subcommittee oversaw the quality of the data in the web database and the infrastructure for the web meetings. Finally, a separate committee was responsible for obtaining financial support from private and public sources. Training on anti-PML immunofluorescence staining and data management was organized in each country, and educational material and presentations for different audiences were created in order to increase the awareness of APL by the medical community.
Organization of the IC-APL network for the diagnosis and treatment of a given case of APL. Actions taken at the local centers are indicated in red and those taken at the national reference laboratories are indicated in blue. (A) The interaction with the panel of international experts and the educational activities are shown in yellow. (B) Geographical distribution of the IC-APL institutions.
Organization of the IC-APL network for the diagnosis and treatment of a given case of APL. Actions taken at the local centers are indicated in red and those taken at the national reference laboratories are indicated in blue. (A) The interaction with the panel of international experts and the educational activities are shown in yellow. (B) Geographical distribution of the IC-APL institutions.
Definitions and statistical analysis
Here we report on patients who were entered into the study between June 2006 and September 2010. Patient follow-up was updated in November 2011. Molecular remission was defined as the disappearance on an ethidium bromide gel of the PML/RARA–specific band visualized at diagnosis with the use of RT-PCR assays. Molecular persistence was defined as PCR positivity in 2 consecutive BM samples collected at the end of consolidation therapy; molecular relapse was defined as reappearance of the PML/RARA–specific band in 2 consecutive BM samples collected 15 days apart at any time after consolidation therapy. Patients whose partial thromboplastin time and prothrombin time test results were abnormal were deemed to have a coagulopathy, regardless of whether they presented with bleeding symptoms or not. Patients were categorized according to the risk of relapse as described elsewhere.3,16
Univariable and multivariable logistic regression analysis was performed in order to identify prognostic factors for CR. OS, disease–free survival, and event–free survival (EFS) were calculated using the Kaplan–Meier method. OS was defined as the time from the initiation of induction therapy to death from any cause; those alive or lost to follow-up were censored on the date when last known to be alive. DFS was defined as the time from CR to disease relapse or death from any cause, whichever occurred first. Patients who were alive without disease relapse were censored at the time last seen alive and disease free. EFS was defined as the time from the initiation of induction therapy to disease relapse, development of secondary malignancy, or death from any cause, whichever occurred first. The log–rank test was used to compare Kaplan–Meier curves. Cumulative incidence curves for nonrelapse death and relapse with or without death were constructed reflecting time to relapse and time to nonrelapse death as competing risks.20 Time to relapse and time to nonrelapse death were measured from the date of CR. Univariable and multivariable proportional hazards (PHs) regression analysis was performed for potential prognostic factors for overall survival. Potential prognostic factors examined in multivariate analysis were age at diagnosis, patient sex, white blood cell (WBC) counts, platelet counts, hemoglobin levels, coagulopathy, French-American-British classification, PML breakpoint, creatinine, albumin, uric acid, fibrinogen, and BM blasts. Due to the large number of potential prognostic factors relative to the number of events, multivariable analysis for OS was performed using the penalized PH model.21-23 The PH assumption for each variable of interest was tested. Linearity assumption for all continuous variables was examined in logistic and PH models using restricted cubic spline estimates of the relationship between the continuous variable and log relative hazard/risk. Multivariable regression analysis for relapse, nonrelapse death, and DFS was not performed due to the small number of events. The multiple imputation technique24-26 was used to reduce bias due to missing data in multivariable regression analysis. All P values are 2-sided at the significance level of 0.05. All calculations were performed using SAS 9.2 (SAS Institute, Cary, NC) and R 2.10.1 (the CRAN Project).
Results
Patient baseline characteristics
IC-APL–participating institutions evaluated 215 consecutive patients in whom a diagnosis of APL was suspected were evaluated in. Thirty-two were considered ineligible: in 15 patients, the diagnosis of APL was not confirmed by anti-PML immunofluorescence and later by (cyto)genetic testing; 4 had poor performance status; and 3 had received previous chemotherapy. In addition, for 2 patients, ATRA or daunorubicin was not available at the institution at the time of diagnosis, 4 patients refused to sign the informed consent, 2 patients were >75 years, and 2 patients were pregnant. Table 1 shows the baseline characteristics of the 183 patients enrolled in the study. In Brazil, 122 patients were enrolled, along with 30 in Mexico, 23 in Chile, and 8 in Uruguay. More patients were enrolled in Brazilians because of the larger population at risk as well as the larger number of participating institutions and the longer accrual period; Chile and Uruguay joined the study 8 months after Brazil and Mexico joined.
Approximately one-third of patients had >10 × 109/L leukocytes at diagnosis and thus were classified as high risk for relapse; whereas only 30 (16%) patients presented with ≤10 × 109/L leukocytes and >40 × 109/L platelets and were considered low risk. Thus, the majority (52%) of patients were classified as intermediate risk. Coagulopathy was present in 114 (62%) of the patients at diagnosis, the median plasma level of fibrinogen was 156.9 mg/dL (range: 10 to 605), and in 107 patients these levels were ≤170 mg/dL. There were no significant differences in the distribution of patients according to risk categories or to the subtype of PML breakpoint among the 4 countries (data not shown).
Diagnosis using anti-PML immunofluorescence technique
Immunofluorescence staining of BM aspirates using PG-M3 was performed at local institutions for 130 cases in which the diagnosis of APL was suspected based on the morphology. For 115 patients, this technique allowed for the rapid diagnosis of APL based on the detection of the characteristic microspeckled pattern of PML delocalization from nuclear bodies (supplemental Figure 1). In the 15 negative cases, the presence of the PML/RARA rearrangement was not confirmed by RT-PCR and/or cytogenetics, and the patients were considered ineligible. Among the 115 cases with positive PML antibody results, the presence of the PML/RARA rearrangement was confirmed by RT-PCR (n = 59), cytogenetics (n = 5), or both (n = 51). In the remaining 53 cases, APL diagnosis was based only on cytogenetics. In 1 case, the immunofluorescence pattern was equivocal. RT-PCR showed a PLZF-RARA fusion predicting retinoid resistance. Consequently, this patient was not eligible for the study protocol. No false-negative results were observed with the PML antibody test. On average, results were available within 4 hours; results of RT-PCR lagged by 2 or 3 days.
Induction therapy
Of the 183 patients enrolled, 3 patients who started the induction treatment were lost to follow-up without assessment for CR and thus were excluded in the analysis of response to induction. Of the 180 evaluable patients, 153 (85%) achieved complete hematological remission (Table 2). The average time to achieve CR was 38 days. Twenty-seven patients (15%) died between diagnosis and the first morphologic evaluation of BM, which was on the thirtieth day of treatment (early death), with death occurring in 9 cases within the first 7 days. The early death rate according to the risk stratification was 20.7%, 11.6%, and 6.7% in the high-, intermediate-, and low-risk groups (P = .01), respectively. The major causes of death were hemorrhage (13 patients, 48.1%), infection (7, 25.9%), and DS (5, 18.5%; Table 3). Among the 13 deaths caused by hemorrhage, 4 occurred within 7 days of diagnosis. DS was diagnosed in 42 (23%) patients, with an associated mortality rate of 11.9%. It should be noted that in spite of the relatively high frequency of deaths due to infection during induction, the median time to neutrophil recovery (absolute neutrophil counts [ANC] > 1 × 109/L) was 25 days (Table 2). One patient showed a considerable decrease of the left ventricular ejection fraction (LVEF; 61% at diagnosis and 25% at day 23) and received a modified consolidation treatment. The LVEF recovered and was maintained at 50% after consolidation and maintenance with a 15-month follow-up.
Seven of the 17 patients with APL who were deemed ineligible died during induction with ATRA and anthracyclines (n = 15) or monochemotherapy with anthracycline (n = 2), and all deaths were caused by hemorrhage. Ten patients achieved hematological CR, of whom 2 were lost to follow-up after consolidation, 1 died of central nervous system bleeding during consolidation, 1 relapsed after 1.8 months, and 6 survived and were in hematological remission.
Table 4 shows the univariable and multivariable logistic regression analyses for CR. The analyses of the complete list of variables are presented in supplemental Table 2. WBC counts and albumin levels were significantly associated with CR in both the univariable and multivariable analyses. The presence of coagulopathy was significant only in the multivariable analysis, and the abnormal levels of creatinine were significant only in the univariable analysis.
Consolidation and maintenance therapy
Data regarding consolidation toxicities were available for 148 first, 147 second, and 144 third consolidation cycles (supplemental Table 3). Five patients did not have consolidation toxicity information: 2 died immediately after achieving hematologic remission and 3 had missing data. The most frequent hematologic toxicity was neutropenia, which was classified as grade 4 according to the National Comprehensive Cancer Network classification; this lasted >15 days in 9%, 21%, and 11% of patients in the first, second, and third consolidation cycles, respectively (supplemental Table 3). Hospitalization lasting >10 days was required in 5% to 11% of the episodes of hematologic toxicity. The frequency of hospitalization was higher for patients in the intermediate- and high-risk groups compared with those in the low-risk group (5% to 11%, 8% to 18%, and 34% to 51% in low-, intermediate-, and high-risk groups, respectively; P < .001). Ten patients died in CR (Table 3), all during consolidation; the main cause of death was infection (8, 80%). In 1 case, the cause of death was respiratory failure, but there was >1 cause leading to this complication. No significant toxicities were reported during maintenance.
Treatment outcome
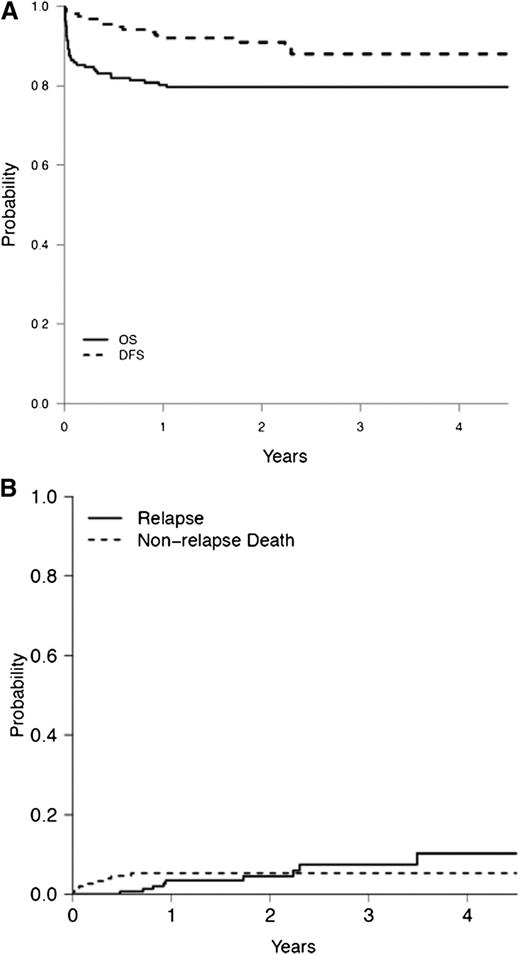
At the time of analysis, 75.4% of patients enrolled and 90.2% of those achieving CR were alive; 5 (2.6%) were lost to follow-up and 10 (5.2%) died while in CR. There was 1 case of molecular persistence of the PML/RARA rearrangement after consolidation and 9 relapses, of which 4 were molecular relapses. Two of the 9 relapsed patients died during salvage therapy, whereas all others achieved a second remission using alternative therapy. No secondary malignancies were reported. The median follow-up among survivors was 28 months (range: 7 months to 62 months). The 2-year OS and DFS were 80% (95% confidence interval [CI]: 73% to 85%] and 91% (95% CI: 86% to 95%), respectively (Figure 2A). The 2-year cumulative incidence of relapse (CIR) was 4.5% (95% CI: 1.8% to 9.2%). The 2-year cumulative incidence of nonrelapse mortality (CI of NRM) was 5.3% (95% CI: 2.4% to 9.6%; Figure 2B). None of the patients classified as low risk relapsed (Table 5). In contrast, the 2-year CIR and CI of NRM among high-risk patients was 14.1% ([95% CI: 4.6% to 28.7%] and 10.9% [95% CI: 3.9% to 21.8%], respectively; P = .08 for CIR and P = .04 for CI of NRM; Table 5). Furthermore, the EFS of high-risk patients was 59% (95% CI: 45% to 72%; P = .005), resulting in a significantly lower rate of 2-year OS of 67% (P = .01; Table 5). Supplemental Table 4 shows the Cox proportional hazards analysis for OS, age, WBC counts, hemoglobin levels, the presence of coagulopathy, creatinine levels (abnormal vs normal), and albumin levels, which were significantly associated with OS. Due to the small number of events, a multivariable model was not explored for DFS analysis. In univariable analysis of DFS, only WBC counts demonstrated a significant association (supplemental Table 5).
(A) OS and DSF and (B) cumulative incidence of relapse and cumulative incidence of nonrelapse mortality rates among patients with acute promyelocytic leukemia treated in the IC-APL study.
(A) OS and DSF and (B) cumulative incidence of relapse and cumulative incidence of nonrelapse mortality rates among patients with acute promyelocytic leukemia treated in the IC-APL study.
Discussion
Using APL as a model disease, we demonstrate that clinical networking may result in significant improvement in patient outcome. We also show the potential to achieve treatment results in developing countries that are comparable to those obtained in the United States and Europe. Table 6 shows some of the problems faced and the solutions encountered by the IC-APL network before and during development of the study.
APL has a high cure rate in developed countries and a high prevalence in young patients. It is therefore an ideal disease in which to test the impact of educational measures and cooperative networks on short- and long-term outcomes of patients in developing countries, especially in light of its high mortality if left untreated.1 In addition, therapy is based on relatively inexpensive drugs, and the adoption of standard supportive measures reduces the risk of severe complications related to the disease and its treatment.1
Prior to establishment of the IC-APL, the 2-year OS of adult patients with APL in Brazil was about 50% and the induction mortality rate was >30%.13 Unfavorable results were also reported among children from 7 Latin American countries.27 As a result of the IC-APL, the 2-year OS increased to 80% and the induction mortality rate was reduced by half. Mortality associated with DS and infection were also reduced compared with the retrospective analysis previously reported.13,28 However, although the observed mortality rate for DS was similar to that reported by de Botton et al29 in the APL93 trial, it was greater than that reported in more recent studies using ATRA and anthracyclines.3,5,6,12 Therefore, we believe that the need to improve the early diagnosis and treatment of DS in the context of the IC-APL still exists.
With regard to the clinical and laboratory features at presentation, we observed no relevant differences in the present study compared with those reported in developed countries, except for the increased frequency of patients in the high relapse-risk group.4,6,9 It is possible that this higher frequency reflects a late diagnosis of APL due to poorer or delayed access to tertiary specialized centers. It may also be more representative of the true risk-group distribution. High-risk patients are less likely to be treated in clinical trials due to the urgent need to deliver the first dose of anthracycline. The frequency of the breakpoint cluster region 1 (bcr1) PML/RARA isoform was not increased compared with that reported in Europe, which contrasts with previous studies analyzing patients from Latin America.30-32 This difference, however, likely reflects the distinct geographical and ethnic origin of patients in the 2 reported series (eg, Mexican mestizos by Santos et al 28 and mostly Brazilian in this study).
Immunofluorescence staining using the anti-PML antibody was adopted as a rapid diagnostic method that could be performed at all centers and did not require a sophisticated infrastructure. A central laboratory in each participating country performed the genetic confirmation using RT-PCR, and, in all cases, both techniques yielded concordant results. Our results corroborate those of Dimov et al33 who estimated the sensitivity and specificity of the immunofluorescence staining test to be 98.9% and 98.7%, respectively. More importantly, the results demonstrate the feasibility and reliability of the immunofluorescence staining technique in the context of developing countries. Due to the short time required to confirm diagnosis, use of the immunofluorescence technique allowed the rapid initiation of daunorubicin and supportive measures, most likely contributing to reduced early mortality.
There is a disparity between reported outcomes, particularly in the hemorrhagic death rates in clinical trials as compared with population-based studies. Park et al34 analyzed the outcomes of 1400 patients with APL recorded in 13 cancer registries in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program between 1992 and 2007. They reported a death rate within 1 month of APL diagnosis of 17.3% and 3-year OS of 65.7%. This rate did not change when different time periods were compared. Lehmann et al35 studied 105 patients with APL registered in the population-based Swedish Adult Acute Leukemia Registry between 1997 and 2006 and reported death rates of 29% and 26% within 30 and 14 days of diagnosis, respectively. At 6.4 years median follow-up, 62% of patients were alive. Both American and Swedish studies indicate that the incidence of early death is higher in the “real world” than in clinical trials because the latter fail to account for patients who die of hemorrhagic complications before registration. Comparison between the 2 aforementioned studies and the IC-APL study is hampered by the small number of patients accrued in the latter study relative to the large population and the lack of population-based data in the 4 involved countries. However, the reduction in deaths caused by hemorrhage during induction after the implementation of IC-APL suggests that medical education and rapid diagnosis with prompt measures to counteract APL-associated coagulopathy are important mainstays in decreasing early mortality.
The 2-year DFS and OS of patients enrolled in the IC-APL study were comparable to those from trials conducted in developed countries, despite the relatively high proportion of high-risk patients.3-5,7,8,36 Although the CIR was significantly higher in patients presenting with WBC >10 × 109/L compared with the low- and intermediate-risk groups, the overall rates were comparable to those reported in trials conducted in developed countries (supplemental Table 6).3,5,8,9,11 However, the NRM in the high-risk group (10.9%) was higher than that reported in recent trials.3,8
In the present study, arsenic trioxide (ATO) was not included in the treatment protocol because of its high cost in the involved countries. ATO is not reimbursed by the public health systems in Brazil, Uruguay, and Mexico as front-line therapy for APL. The experience with ATO as a single agent reported in India, China, and Iran demonstrates that this agent is highly effective and treatment has acceptable toxicity.37-40 Moreover, in the North American Leukemia Intergroup Study C9710, the addition of ATO to standard therapy with ATRA plus daunorubicin as consolidation resulted in significant improvement in the DSF and EFS of adult patients in all risk groups.11
Monitoring of minimal residual disease using RT-PCR for the PML/RARA rearrangement showed that the IC-APL treatment schedule achieved molecular remission in the majority of patients. This is in accordance with results obtained with ATRA and anthracycline-based protocols used in developed countries.41-43 Four of the 9 relapses (44.4%) were molecular, which is in agreement with studies that used this technique.44 However, a higher sensitivity of test has been reported in trials using real-time quantitative polymerase chain reaction assays.42,43 Although this frequency is low, we consider it worthwhile to perform molecular monitoring based on the results reported with the preemptive treatment with ATO of these patients.43 The 4 patients who had molecular relapses (all during maintenance) were treated with alternative regimens (2 with ATO and 2 with polychemotherapy and ATRA) and achieved a second remission; however, our sample size was too small to draw any conclusions. We opted to perform the molecular tests exclusively on BM aspirates, following the recommendation of the European LeukemiaNet.1 Importantly, our experience demonstrates the feasibility of molecular monitoring in developing countries through the establishment of national reference laboratories.
The treatment of acute leukemia and APL requires specific expertise and skills. The results presented here demonstrate that it is possible to improve the prognosis of severe and rapidly fatal malignancies, such as APL, in developing countries by international collaboration at relatively low cost. The dissemination of essential clinical management guidelines and increased awareness of APL, coupled with a simple method for rapid diagnosis and the implementation of Internet tools for the exchange of clinical expertise, was effective in abolishing differences in treatment outcomes between developed and developing countries. With the aim of transferring the experience gained with clinical networking in the IC-APL to another more challenging leukemia subset, a consortium for the treatment of acute myeloid leukemia has recently been initiated.
Presented at the Presidential Symposium at the 51st annual meeting of the American Society of Hematology, New Orleans, LA, 2009.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood commentary on this article in this issue.
Acknowledgments
The authors thank Dr Brunangelo Falini at the University of Perugia, Italy, who provided the anti-PML antibody (PG-M3) free of charge.
This study was supported by the American Society of Hematology, Fondazione Umberto Veronesi, Roche Saudi Arabia, Fundação de Apoio à Pesquisa do Estado de São Paulo (grant 1998/14247-6), Fundacion Mexicana para la Salud, St. Jude Children’s Research Hospital, and Cephalon Europe.
Authorship
Contribution: E.M.R., G.J.R.-A., M.S.U., and L.M. were the national coordinators in Brazil, Mexico, Chile, and Uruguay, respectively. They designed and performed research and analyzed data. E.M.R., J.G.-E., M.S.U., and M.d.R.U. were responsible for the national reference laboratories in Brazil, Mexico, Chile, and Uruguay, respectively. They performed research and analyzed data. M.A.S., H.T.K., and the national coordinators were responsible for the Treatment Guidelines Subcommittee. They designed research and analyzed data. F.L.-C., R.G., and H.C.K. were responsible for the Laboratory Guidelines Subcommittee. They designed research and analyzed data. R.C.R., M.T., and H.T.K. were responsible for the Web Registration and Auditing Subcommittee. They designed research and analyzed data. M.T., R.C.R., and R.G. were responsible for the Funding Subcommittee. G.J.R.-A. and R.C.R. were responsible for the Drug Availability Subcommittee. C.M.N., S.L.S., D.G., A.G., N.B., and B.L. designed research and analyzed data. H.T.K. performed statistical analysis. R.H.J., H.G.-A., R.A.M.M., R.B., R.P., K.P., E.M.F., M.d.L.C., C.S.C., L.A.M., and D.G.-A. performed research. E.M.R., M.A.S., N.B., F.L.-C., D.G., and B.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eduardo M. Rego, Hematology/Oncology Division, Department of Internal Medicine, Medical School of Ribeirão Preto and Center for Cell Based Therapy, University of São Paulo, Av Bandeirantes 3900, CEP 14048-900, Ribeirão Preto, Brazil; e-mail: emrego@hcrp.fmrp.usp.br.