Key Points
Sickle RBC ROS production is mediated in part by NADPH oxidase activity.
Sickle RBC ROS production can be induced by plasma signaling molecules.
Abstract
Chronic inflammation has emerged as an important pathogenic mechanism in sickle cell disease (SCD). One component of this inflammatory response is oxidant stress mediated by reactive oxygen species (ROS) generated by leukocytes, endothelial cells, plasma enzymes, and sickle red blood cells (RBC). Sickle RBC ROS generation has been attributed to sickle hemoglobin auto-oxidation and Fenton chemistry reactions catalyzed by denatured heme moieties bound to the RBC membrane. In this study, we demonstrate that a significant part of ROS production in sickle cells is mediated enzymatically by NADPH oxidase, which is regulated by protein kinase C, Rac GTPase, and intracellular Ca2+ signaling within the sickle RBC. Moreover, plasma from patients with SCD and isolated cytokines, such as transforming growth factor β1 and endothelin-1, enhance RBC NADPH oxidase activity and increase ROS generation. ROS-mediated damage to RBC membrane components is known to contribute to erythrocyte rigidity and fragility in SCD. Erythrocyte ROS generation, hemolysis, vaso-occlusion, and the inflammatory response to tissue damage may therefore act in a positive-feedback loop to drive the pathophysiology of sickle cell disease. These findings suggest a novel pathogenic mechanism in SCD and may offer new therapeutic targets to counteract inflammation and RBC rigidity and fragility in SCD.
Introduction
Vaso-occlusion and hemolysis from the rigid and concurrently fragile red blood cells (RBC) in patients with sickle cell disease (SCD) cause a variety of acute and chronic manifestations ranging from frequent and severe painful crises to stroke and chronic organ failure. Chronic inflammation has emerged as an important pathogenic mechanism in SCD, and oxidative stress is increasingly recognized as a component of this chronic inflammatory state, inducing damage to a variety of subcellular and tissue structures.1,2 Patients with SCD have decreased plasma levels of glutathione, vitamin C, and vitamin E, presumably due to consumption by increased oxidant production.3-5 RBC and other cell types show evidence of lipid peroxidation and oxidative damage to structural proteins.6-8 Additionally, plasma from SCD patients has elevated levels of advanced glycation end products9,10 and products of lipid peroxidation (F-2 isoprostanes, malonaldehyde, and 4-hydroxynonenal),11-13 all of which are markers of oxidative stress.
There are several postulated mechanisms for the increased oxidative stress in patients with SCD. Sickle (SS) RBC reactive oxygen species (ROS) generation has been attributed to sickle hemoglobin auto-oxidation and iron-mediated Fenton chemistry reactions catalyzed by denatured heme moieties bound to the RBC membrane.14 Plasma hemoglobin and free heme resulting from chronic hemolysis generate superoxide radicals via the same nonenzymatic mechanisms.15 In patients on chronic transfusion regimens, the accumulation of free iron in hepatocytes and other cell types can also contribute to oxidative stress. Repeated cycles of tissue ischemia and reperfusion result in the release of xanthine oxidase (XO) from hepatic and other tissues16 as well as the upregulation of NADPH oxidase in polymorphonuclear cells, monocytes, and endothelial cells.17-19 The chronic inflammatory state associated with SCD has been shown to activate the NADPH oxidase-mediated oxidative burst in phagocytic cells.20,21 Arginase released into plasma from lysed RBC,22 as well as endothelial cell arginase23 induced and activated by proinflammatory signals, deplete the nitric oxide (NO) synthase substrate arginine. This leads to uncoupling of plasma, blood cell, and endothelial NO synthase (eNOS) and results in the production of oxygen radicals instead of NO and decreased NO availability.24 Creating another vicious cycle, eNOS also uncouples to produce superoxide due to oxidation of the eNOS cofactor tetrahydrobiopterin.18
Sickle erythrocytes have been shown to have elevated levels of ROS generation relative to normal (AA) RBC,25,26 but the exact mechanisms of sickle RBC ROS production have not been examined in detail. Hemoglobin S (HbS) has an increased rate of auto-oxidation and superoxide production relative to normal hemoglobin (HbA), but detailed estimates of this auto-oxidative tendency reveal a rate of ROS generation lower than that observed in SS RBC,27 suggesting that other, as-yet-unexplored mechanisms must be at play. We present evidence that NADPH oxidase is a source of ROS in human SS RBC and that the activation of NADPH oxidase is mediated by protein kinase C (PKC) and Rac GTPase signaling within the sickle erythrocyte. We also demonstrate that RBC NADPH oxidase activity can be induced by plasma inflammatory cytokines. These findings suggest a novel pathogenic mechanism in SCD, namely that systemic inflammation and enzymatically derived ROS within the sickle erythrocyte act in a positive-feedback loop to contribute to acute and chronic organ damage of SCD.
Methods
RBC collection and density fractionation
Leftover blood samples from pediatric patients with SCD who had not been transfused in the past 3 months and from normal controls were obtained through Institutional Review Board–approved human subject sample repositories from the Repository of Non-Malignant Hematological Disorders and the Normal Donor Repository at Cincinnati Children's Hospital Medical Center (CCHMC). In all cases, samples were collected in K2EDTA tubes and stored at 4°C until use within 72 hours of collection. Samples used for comparative experiments were batched by date of collection to minimize confounding caused by storage. Prior to use, samples were allowed to settle by gravity and the overlying plasma and buffy coat were removed by aspiration. RBC were then further washed twice with phosphate-buffered saline (PBS) followed by centrifugation at 500 g to remove residual plasma and white blood cells (WBC) with the top cell layer.
For the experiments using fractionated RBC, whole blood was layered onto Optiprep density gradient medium (Sigma-Aldrich, St. Louis, MO) diluted from stock solution in PBS following the manufacturer’s instructions to obtain gradient layers with densities of 1.075, 1.085, 1.090, 1.095, 1.010, and 1.015 g/mL (fractions 1 to 6, respectively). Samples were then centrifuged at 1875g for 30 minutes at room temperature in a swinging-bucket bench-top centrifuge. The top layer, consisting of WBC, was removed by aspiration and successive layers, labeled fractions 1 through 6, were then collected, washed twice with PBS, and used for subsequent experiments.
RBC ROS detection by flow cytometry and inhibition/stimulation experiments
Total or fractionated RBC were resuspended at 0.5% hematocrit in PBS and incubated with CM-H2-DCFDA (Invitrogen, Carlsbad, CA) at 4 µM for 30 minutes at 37°C. Samples were then washed once with warm PBS and analyzed for fluorescence in the fluorescein isothiocyanate channel on a BD FACS-Canto cytometer using BD FacsDiva software. Where indicated, blood samples were costained with phycoerythrin (PE)-labeled antibody against CD71, PE-labeled antibody against glycophorin A (GPA), or PECy7-labeled antibody against CD45 (BD Biosciences, San Jose, CA) to identify reticulocytes, RBC, or WBC, respectively. In inhibition/stimulation experiments, RBC were preincubated for 1 hour at 37°C with the following reagents: the Rac GTP-specific inhibitor NSC23766 (500 µM); the PKC activator PMA (2 or 10 µM; EMD Chemicals, Billerica, MA); the PKC inhibitor calphostin (500 nM, Sigma-Aldrich); the calcium chelator BAPTA-AM (50 µM, Invitrogen); the NADPH oxidase inhibitors diphenyleneiodonium (DPI; 10 or 50 µM, Sigma-Aldrich), apocynin (100 µM, Sigma-Aldrich), and gp91-dsTat (50 µM, Anaspec, Fremont, CA); the xanthine oxidase inhibitor oxypurinol (500 µM, Sigma-Aldrich); and the mitochondrial electron transport inhibitor rotenone (50 µM, Sigma-Aldrich). CM-H2-DCFDA was then added to each sample for an additional 30-minute incubation as described above prior to quantitation of the ROS signal.
In the plasma-switch experiments, fraction 1 RBC from SS patients or AA controls were resuspended at 0.5% hematocrit in ABO blood group–matched plasma that had been depleted of residual cells by centrifugation. Samples were then incubated for 4 to 24 hours at 37°C, as noted in the corresponding figure legends. CM-H2-DCFDA was then added and the samples were incubated for an additional 30 minutes at 37°C, washed, and evaluated by flow cytometry.
Immunoblotting
RBC were washed with PBS twice after removal of the buffy coat to minimize WBC contamination and resuspended in lysis buffer (20 mM Tris-HCl [pH 7.6], 100 mM NaCl, 10 mM MgCl2, 2 mM PMSF, 0.5 mM DTT, 2 mg/mL Lysozyme, 1% Triton X-100, 0.2% deoxycholic acid) with protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN) and lysed by sonication. Lysates were cleared of debris by centrifugation at 16 000g for 10 minutes at 4°C, and supernatant from each sample was used for subsequent analysis. A total of 100 µg protein from each sample was subjected to electrophoresis on 4% to 15% gradient acrylamide gels (Bio-Rad, Hercules, CA), western blotting, and immunoblotting for NADPH oxidase subunits (NOX isoforms) detection. Rabbit polyclonal antibodies against human NOX1, NOX3, NOX4, and NOX5 (Santa Cruz Biotechnology, Santa Cruz, CA) and NOX2 (Abcam, Cambridge, MA) were used at 1:200 dilution, mouse anti–human CD45 (BD Transduction Laboratories, San Jose, CA) was used at 1:1000 dilution, followed by horseradish peroxidase–conjugated secondary antibodies and chemiluminescent reagent (GE Life Sciences, Pittsburgh, PA). Blot images were captured and analyzed using a FujiFilm Intelligent Dark Box II and manufacturer-supplied Image Reader LAS-1000 software.
For analysis of membrane-associated receptor proteins, packed RBC from AA and SS blood samples were lysed by suspension in 20 volumes of hypotonic lysis buffer (5 mM sodium phosphate [pH 7.4], 10 mM NaCl, 10 mM EGTA, 10 mM MgCl2, 2 mM PMSF, 0.5 mM DTT, 1 mM sodium orthovanadate, 0.1 mM okadaic acid, 50 mM NaF) with protease inhibitors (Roche Applied Science). Samples were incubated on ice for 5 minutes with occasional inversion to ensure complete lysis and then centrifuged at 16 000 g, at 4°C, for 20 minutes to pellet the RBC membranes. The membrane pellets were washed twice with lysis buffer, resuspended in 0.5 mL lysis buffer with 1% Triton X-100, and sonicated to solubilize them. Protein samples were used for western blotting as described above. Rabbit anti–human endothelin receptor 1B (Sigma-Aldrich) and goat anti–human transforming growth factor β1 (TGFβ1) receptor antibody (R&D Systems, Minneapolis, MN) were used at 1:200 dilution. Standardization of protein loading between wells was confirmed by probing the blots with monoclonal antibody against GAPDH (Fitzgerald Scientific, Acton, MA) at 1:5000 dilution.
Rac-GTP pull-down assays
Fraction 1 AA RBC were resuspended in endogenous plasma at 0.5% hematocrit. Recombinant human endothelin 1 (ET-1 [2.5 µM]; Sigma-Aldrich) or TGFβ1 (0.5 µg/mL; R&D Systems) were added and the samples were incubated at 37°C for 4 hours in a standard tissue culture incubator. The RBC were then lysed as described above, and active GTP-bound Rac was isolated from total lysates by using the p21-binding domain of the p21-activated kinase 1 (PAK1) fused with glutathione S-transferase bound to glutathione-agarose (Sigma-Aldrich), as previously described.28 Pull-down samples and aliquots of the original lysate were then analyzed by western blotting to quantitate active and total Rac content for each sample. GAPDH was detected in the aliquots of the original lysate as loading control.
High-speed cell imaging analysis in flow
The distribution of NOX1 and NOX2 within WBC and RBC in patient samples was studied by ImagestreamX (Amnis Corporation, Seattle, WA), which combines flow cytometry and microscopy (×60/numerical aperture 0.9 objective lens) capabilities. Within 24 hours after collection, blood cells were washed once with PBS and pelleted for 20 seconds at 2000g in a bench-top centrifuge and then fixed in PBS containing 4% formaldehyde for 15 minutes at room temperature. Formaldehyde was removed by centrifugation (20 seconds at 2000g) and aspiration of the supernatant, and the cell pellet was cooled on ice for 15 minutes before permeabilization by consecutive suspensions in ice-cold (kept at −20°C) 50% acetone, then 100% acetone, and then again 50% acetone solution, as previously described,29 followed by 1 wash with fluorescence-activated cell soring (FACS) buffer (PBS plus 0.5% bovine serum albumin). Cells were resuspended in FACS buffer and incubated with rabbit polyclonal antibody against human NOX1 (Sigma-Aldrich) or NOX2 (Abcam) at 1:100 dilution. After washing twice with FACS buffer, the samples were incubated with AlexaFluor-488 anti–rabbit immunoglobulin G antibody (Invitrogen) at 1:500 dilution and the nuclear stain Draq5 (Cell Signaling Technology) at a concentration of 40 µM in FACS buffer and then processed by ImagestreamX. Approximately 10 000 events per experiment were collected and analyzed with the associated Image Data Exploration and Analysis software (IDEAS; Amnis).
Data processing and statistical analysis
Mean fluorescence intensity (MFI) values obtained by flow cytometry were normalized within each experiment to a baseline of 100 arbitrary fluorescence units for the control samples. Standard error of the mean was calculated using raw data and is depicted as a percentage of the normalized MFI for each group of samples.
Statistical analysis was performed on raw data using KaleidaGraph software (Synergy Software, Reading, PA). To identify statistically significant differences between means, we compared groups of unrelated samples (eg, AA and SS samples) using the unpaired 2-tailed Student t test and groups of samples tested under 2 different conditions using the paired 2-tailed Student t test. Comparisons between multiple groups of samples within an experiment were performed by analysis of variance. Significant differences (P < .05) are denoted by asterisks above the relevant bars on histogram plots.
Results
Elevated ROS generation in sickle erythrocytes is not exclusively due to increased reticulocyte percentage or younger cell age
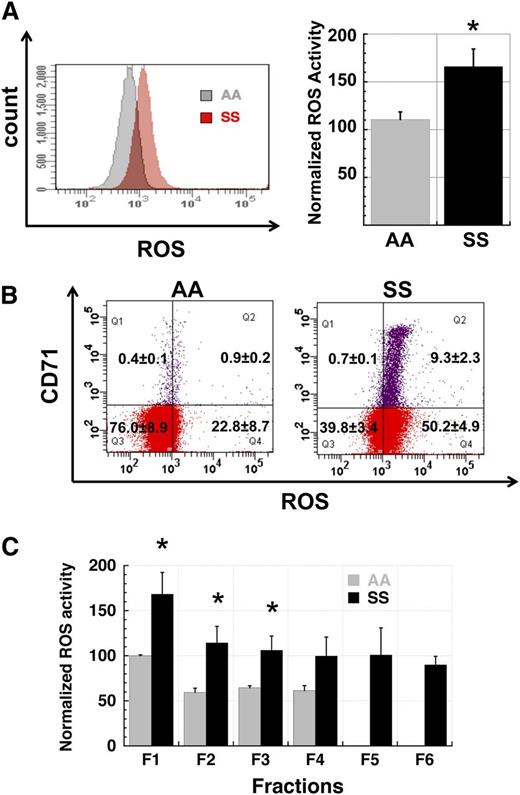
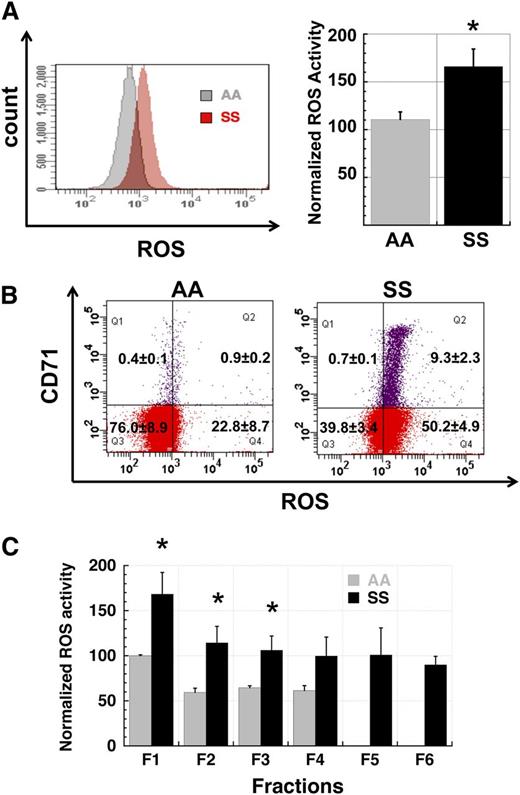
We first analyzed levels of ROS generation in total RBC populations from untransfused SCD patients and normal control subjects to confirm and extend prior reports of elevated ROS production in SS RBC. SS RBC have a distinctly elevated profile of ROS generation when compared with AA RBC (Figure 1A). Because SCD patients commonly manifest a significant reticulocytosis due to ongoing hemolysis, we differentiated reticulocyte and mature RBC ROS signals in AA and SS whole-blood samples by simultaneously staining with CM-H2-DCFDA for ROS levels and with CD71-PE to distinguish CD71+ young reticulocytes from CD71- RBC. In both AA and SS samples, the reticulocyte ROS signal was at the high end of that seen in nonreticulocyte RBC but the overall SS RBC ROS signal was higher than the corresponding AA signal (Figure 1B), indicating that the increased ROS production in SS RBC samples is not exclusively due to the reticulocytosis seen in these samples. However, the overall younger age of SS RBC in circulation relative to AA RBC may contribute to their higher level of ROS production.
Production of reactive oxygen species is elevated in SS RBC. (A) Comparison of ROS levels in total populations of AA and SS RBC. SS RBC manifest a 1.5- to 2.5-fold higher signal than AA RBC by flow cytometric measurement of CM-H2-DCFDA-derived signal (P < .05). The left panel shows fluorescence histograms of representative AA and SS samples while the right panel shows normalized MFI data of AA (n = 3) and SS samples (n = 5). (B) Elevated ROS production in SS RBC is not confined to the reticulocyte subpopulation. Total AA and SS RBC samples were simultaneously stained for ROS levels with CM-H2-DCFDA and for the reticulocyte marker CD71. While SS reticulocytes have a higher average signal than non-reticulocyte SS RBC (right panel), the whole population of non-reticulocyte SS RBC demonstrates a right shift relative to AA non-reticulocyte RBC (left panel). Flow cytograms are representative of 3 AA and 3 SS samples. (C) Elevated ROS production in SS RBC relative to AA RBC is preserved across all fractions (F1 - F6) of density-fractionated erythrocytes. Fractions 5 and 6 cells represent sickle “dense cells” and are not typically found in AA samples. n = 3 AA and 3 SS samples and P < .05 where designated by asterisks.
Production of reactive oxygen species is elevated in SS RBC. (A) Comparison of ROS levels in total populations of AA and SS RBC. SS RBC manifest a 1.5- to 2.5-fold higher signal than AA RBC by flow cytometric measurement of CM-H2-DCFDA-derived signal (P < .05). The left panel shows fluorescence histograms of representative AA and SS samples while the right panel shows normalized MFI data of AA (n = 3) and SS samples (n = 5). (B) Elevated ROS production in SS RBC is not confined to the reticulocyte subpopulation. Total AA and SS RBC samples were simultaneously stained for ROS levels with CM-H2-DCFDA and for the reticulocyte marker CD71. While SS reticulocytes have a higher average signal than non-reticulocyte SS RBC (right panel), the whole population of non-reticulocyte SS RBC demonstrates a right shift relative to AA non-reticulocyte RBC (left panel). Flow cytograms are representative of 3 AA and 3 SS samples. (C) Elevated ROS production in SS RBC relative to AA RBC is preserved across all fractions (F1 - F6) of density-fractionated erythrocytes. Fractions 5 and 6 cells represent sickle “dense cells” and are not typically found in AA samples. n = 3 AA and 3 SS samples and P < .05 where designated by asterisks.
In addition to increased reticulocytosis, RBC from SS patients exhibit significant cellular dehydration relative to AA RBC. To evaluate how RBC hydration status correlated with levels of ROS generation, we fractionated the erythrocytes from whole-blood samples from AA and SS patients by centrifugation in discontinuous density gradients. Well-hydrated cells segregate in the top fractions of the density gradient whereas denser dehydrated cells concentrate in the bottom fractions.30 When cells from AA and SS patients were compared across these density fractions, cells from the lighter fractions had higher levels of ROS production than those from denser fractions, but in all cases SS cells had higher levels of ROS production than comparable AA cells (Figure 1C).
RBC ROS production is mediated in part by NADPH oxidase activity and is a significant component of blood cell–derived ROS
To test the hypothesis that ROS production in SS RBC is mediated in part by enzymatic activity, we preincubated the reticulocyte-rich density fraction 1 RBC from SS subjects with the NADPH oxidase inhibitor DPI, the xanthine oxidase inhibitor oxypurinol, and the mitochondrial electron transport inhibitor rotenone for 1 hour prior to analysis of ROS production (Figure 2B). DPI inhibits the FAD-reducing activity of the gp91 (or gp91-like, ie, NOX) catalytic subunit of NADPH oxidase and inhibits the activity of all known NADPH oxidase isoforms.31 Pretreatment of SS RBC with DPI significantly reduced their level of ROS production, whereas comparable pretreatment with oxypurinol or rotenone had no significant effect (Figure 2B). We also tested the effect of the NADPH oxidase inhibitors gp91ds-Tat, a gp91-homologous peptide, and apocynin, a phytochemical, both of which inhibit NADPH activity by blocking the translocation of cytosolic components of the enzyme complex to the membrane surface.31 As with DPI, both gp91ds-Tat and apocynin significantly decreased the level of ROS production in SS RBC (Figure 2C). The inhibitory effects of NADPH oxidase inhibitors on RBC ROS production and the lack of effect of other superoxide-generating enzyme inhibitors support the conclusion that a significant proportion of ROS generation in SS RBC is due to NADPH oxidase activity.
Production of ROS in SS RBC is mediated in part by NADPH oxidase activity. (A) A schematic depiction of ROS metabolism along with inhibitors of enzymatic sources of ROS. (B) The ROS signal in SS RBC is reduced by preincubation with the NADPH oxidase inhibitor DPI (10 μM) prior to staining with CM-H2-DCFDA, but not by the xanthine oxidase inhibitor oxypurinol (500 µM) or the mitochondrial electron transport inhibitor rotenone (50 µM) (n = 6). (C) The ROS signal in SS RBC is reduced by preincubation with any of the known inhibitors of NADPH oxidase (DPI at 50 µM, gp91-dsTat at 50 µM, and apocynin at 100 µM), supporting the involvement of NADPH oxidase in ROS generation in these cells (n = 3 and P < .05 where designated by asterisks). (D) Whole-blood samples from AA and SS subjects were colabeled with PE-Cy7 anti-CD45, PE–anti-GPA, and CM-H2-DCFDA to permit the identification of WBC (CD45+;GPA−) and RBC (CD45−;GPA+) populations and the quantitation of the total ROS signal from each population (shown in the histograms as red for the RBC population, blue [thin line along the x-axis] for WBC and purple for the total RBC + WBC populations, in representative samples). (E) RBC ROS production, identified by gating out the CD45+ WBC population, constituted almost 80% of the combined RBC and WBC ROS signal. In AA samples (n = 10), the signal from RBC alone was 79% ± 1.5% of the total signal, whereas in SS samples (n = 7) this proportion was 77% ± 4.7%. FSC, forward light scatter; SSC, side light scatter.
Production of ROS in SS RBC is mediated in part by NADPH oxidase activity. (A) A schematic depiction of ROS metabolism along with inhibitors of enzymatic sources of ROS. (B) The ROS signal in SS RBC is reduced by preincubation with the NADPH oxidase inhibitor DPI (10 μM) prior to staining with CM-H2-DCFDA, but not by the xanthine oxidase inhibitor oxypurinol (500 µM) or the mitochondrial electron transport inhibitor rotenone (50 µM) (n = 6). (C) The ROS signal in SS RBC is reduced by preincubation with any of the known inhibitors of NADPH oxidase (DPI at 50 µM, gp91-dsTat at 50 µM, and apocynin at 100 µM), supporting the involvement of NADPH oxidase in ROS generation in these cells (n = 3 and P < .05 where designated by asterisks). (D) Whole-blood samples from AA and SS subjects were colabeled with PE-Cy7 anti-CD45, PE–anti-GPA, and CM-H2-DCFDA to permit the identification of WBC (CD45+;GPA−) and RBC (CD45−;GPA+) populations and the quantitation of the total ROS signal from each population (shown in the histograms as red for the RBC population, blue [thin line along the x-axis] for WBC and purple for the total RBC + WBC populations, in representative samples). (E) RBC ROS production, identified by gating out the CD45+ WBC population, constituted almost 80% of the combined RBC and WBC ROS signal. In AA samples (n = 10), the signal from RBC alone was 79% ± 1.5% of the total signal, whereas in SS samples (n = 7) this proportion was 77% ± 4.7%. FSC, forward light scatter; SSC, side light scatter.
We next compared the intensity of total RBC and WBC ROS signals in whole-blood samples to determine the relative contribution of the two populations to ROS produced by the blood cells. Our analysis of ROS signal intensity in both AA and SS blood samples within 24 hours of collection revealed that the total RBC ROS signal was approximately 4-fold that of the total WBC ROS signal, in both AA and SS samples (Figure 2D-E), indicating that RBC are a significant source of oxidative stress in the bloodstream in comparison with WBCs.
Both normal and sickle RBC contain several NADPH oxidase isoforms
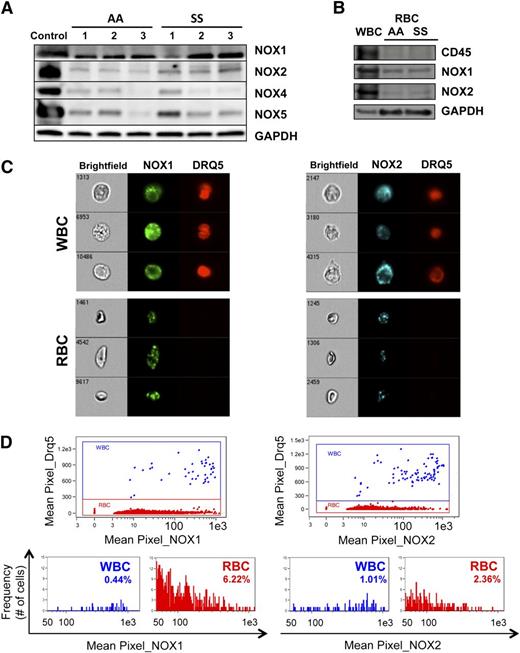
To confirm the presence of NADPH oxidase in RBC and to determine the relative abundance of the 5 known isoforms of the gp91 catalytic subunit, we performed western blotting analysis of cell lysates from total AA and SS RBC samples with isoform-specific antibodies. Our analysis revealed that NOX1, NOX2, NOX4, and NOX5 were detectable in both AA and SS RBC whereas NOX3 was not (Figure 3A and supplemental Figure 3). NOX2 appeared more abundant in SS than AA RBC, whereas the levels of NOX1, NOX4, and NOX5 demonstrated significant phenotypic variability between the different patient (SS) as well as control (AA) samples.
AA and SS RBC contain several NOX isoforms. (A) NOX isoforms are detectable in AA and SS RBC. Western blotting of total RBC lysates reveals the presence of the NOX isoforms NOX1, NOX2 (gp91), NOX4, and NOX5 in both AA and SS RBC. NOX3 could not be detected. Control samples for the different NOX isoforms were human WBC lysate (NOX1 and NOX2), human kidney lysate (NOX4), and human testis lysate (NOX5). (B) NOX1 and NOX2, but not CD45, are detected in AA and SS RBC, indicating that the presence of NOX isoforms in the RBC lysate is not due to WBC contamination. (C) NOX1 and NOX2 are detected by immunofluorescence in intact WBC, as expected, as well as in intact RBC of whole SS blood samples, by multiparameter high-speed cell imaging in flow. (D) Up to 80% of sickle RBC have positive stain for NOX1, with 6% of the erythrocytes having a mean pixel value (expressing the MFI) of NOX1 >50 (arbitrary units), at the level of positivity for NOX1 in WBC within the same sample. NOX2 positivity appear less intense for the sickle erythrocytes in comparison with WBC, with only 2% of the RBC staining as strongly positive as the WBC.
AA and SS RBC contain several NOX isoforms. (A) NOX isoforms are detectable in AA and SS RBC. Western blotting of total RBC lysates reveals the presence of the NOX isoforms NOX1, NOX2 (gp91), NOX4, and NOX5 in both AA and SS RBC. NOX3 could not be detected. Control samples for the different NOX isoforms were human WBC lysate (NOX1 and NOX2), human kidney lysate (NOX4), and human testis lysate (NOX5). (B) NOX1 and NOX2, but not CD45, are detected in AA and SS RBC, indicating that the presence of NOX isoforms in the RBC lysate is not due to WBC contamination. (C) NOX1 and NOX2 are detected by immunofluorescence in intact WBC, as expected, as well as in intact RBC of whole SS blood samples, by multiparameter high-speed cell imaging in flow. (D) Up to 80% of sickle RBC have positive stain for NOX1, with 6% of the erythrocytes having a mean pixel value (expressing the MFI) of NOX1 >50 (arbitrary units), at the level of positivity for NOX1 in WBC within the same sample. NOX2 positivity appear less intense for the sickle erythrocytes in comparison with WBC, with only 2% of the RBC staining as strongly positive as the WBC.
To confirm that the Rac-dependent NADPH-oxidase subunit isoforms NOX1 and NOX2 detected in RBC lysates were indeed endogenous in erythrocytes, we performed immunoblots for NOX1, NOX2, and CD45 (pan-leukocyte marker) in AA and SS RBC, using WBC lysate as control (Figure 3B). CD45 was not detectable in either SS or AA RBC lysates, whereas NOX1 and NOX2 were clearly present, indicating that WBC contamination of our RBC preps was minimal. Of note, mRNA for a variety of the subunits of the different NADPH oxidase subtypes have been detected in human adult and cord blood reticulocytes (supplemental Figure 1; data retrieved from www.ncbi.nlm.nih.gov/gds/, records 2655 and 2656),32 supporting our findings that the NOX isoforms present in RBC are endogenous.
In addition, we evaluated the presence of NOX1 and NOX2 by immunofluorescence in intact RBC and WBC of whole-blood samples by multiparameter high-speed cell-imaging analysis in flow using ImagestreamX (Figure 3C). Up to 80% of sickle RBC had positive stain for NOX1 with 6% of the erythrocytes having mean NOX1 fluorescent intensity comparable to that of the WBC within the same sample (Figure 3D). NOX2 signal appeared less intense in RBC compared with WBC, indicating that these NOX isoforms seen in RBC are not within granulocyte membrane particles attached to RBC membranes (in which case we would expect NOX2 to be more abundant than NOX1).
RBC ROS production is regulated by intracellular signaling pathways involving Rac GTPases, PKC, and intracellular calcium ions
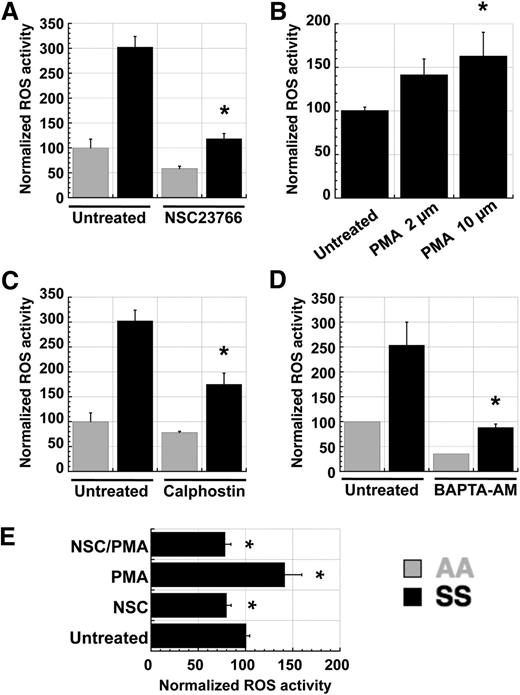
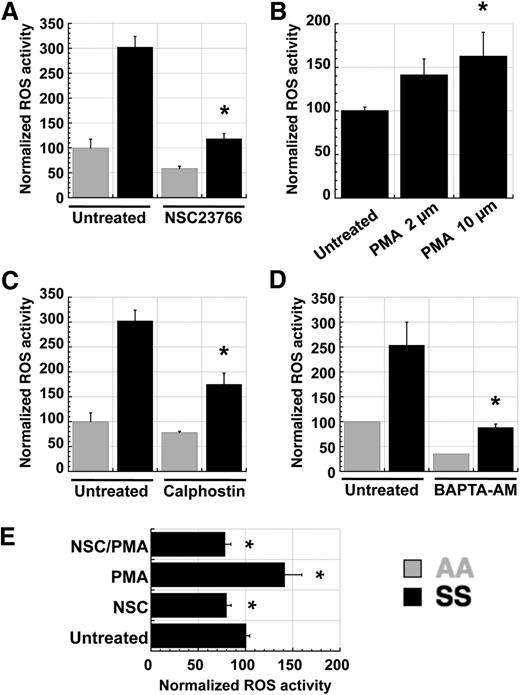
In many cell types, NADPH oxidase activity is mediated by PKC-mediated activation of the small Ras-like GTPase Rac, which is a component of the NOX1, NOX2, and NOX3 enzyme complexes.33 Because PKC activity is known to be elevated in SS RBC relative to AA RBC,34 we tested whether PKC and Rac might function in a signaling axis to induce NADPH oxidase activity in RBC. Pretreatment of fraction 1 AA or SS RBC with the small-molecule Rac inhibitor NSC23766 resulted in decreased ROS production (Figure 4A). Additionally, pretreatment with the phorbol ester PMA increased ROS production in SS RBC in a dose-dependent fashion (Figure 4B), whereas the PKC inhibitor calphostin decreased it (Figure 4C). Because intracellular calcium is a major activator of classic PKC isoforms and SS RBC have increased calcium permeability relative to AA RBC,35 we also tested the effect of calcium chelation on RBC ROS production. Pretreatment of AA and SS RBC with the cell-permeable calcium chelator BAPTA-AM decreased ROS production significantly (Figure 4D). Finally, to confirm that Rac acts downstream of PKC in inducing ROS production in sickle RBC, we pretreated RBC with NSC23766 alone, PMA alone, or the two simultaneously. NSC23766 cotreatment abolished the PMA-mediated increase in ROS production (Figure 4E), indicating that PKC stimulation of ROS production requires active Rac GTPases.
ROS production in SS RBC is mediated by a PKC–RacGTP signaling axis. Fraction 1 SS or AA RBC were preincubated with inhibitors or inducers for 1 hour prior to staining with CM-H2-DCFDA. (A) ROS levels in SS RBC are decreased by preincubation with the Rac-specific small-molecule inhibitor NSC23766 (NSC), indicating that Rac-GTP mediates ROS production in erythrocytes. (B,C) ROS levels in SS RBC are (B) increased by preincubation with the PKC activator PMA and (C) decreased by the PKC inhibitor calphostin (500 nM), indicating that PKC activity increases ROS production in erythrocytes. (D) ROS levels in SS RBC are decreased by preincubation with the cell-permeable calcium chelator BAPTA-AM (50 µM), indicating that free calcium, a key activator of classic PKC isoforms, is necessary for ROS generation in erythrocytes. (E) Fraction 1 SS RBC were incubated with 500 µM NSC23766 (NSC), 2 µM PMA (PMA), or both (NSC/PMA) for 1 hour before staining with CM-H2-DCFDA to detect ROS levels. Inhibition of Rac-GTP activity with NSC23766 blocks the PKC-mediated induction of ROS production in SS RBC, indicating that Rac-GTP acts downstream of PKC in the activation of NADPH oxidase in erythrocytes. Data presented are representative of repeated experiments with batched samples. n = 4 each for AA and SS samples in all graphs above, and P < .05 where designated by asterisks.
ROS production in SS RBC is mediated by a PKC–RacGTP signaling axis. Fraction 1 SS or AA RBC were preincubated with inhibitors or inducers for 1 hour prior to staining with CM-H2-DCFDA. (A) ROS levels in SS RBC are decreased by preincubation with the Rac-specific small-molecule inhibitor NSC23766 (NSC), indicating that Rac-GTP mediates ROS production in erythrocytes. (B,C) ROS levels in SS RBC are (B) increased by preincubation with the PKC activator PMA and (C) decreased by the PKC inhibitor calphostin (500 nM), indicating that PKC activity increases ROS production in erythrocytes. (D) ROS levels in SS RBC are decreased by preincubation with the cell-permeable calcium chelator BAPTA-AM (50 µM), indicating that free calcium, a key activator of classic PKC isoforms, is necessary for ROS generation in erythrocytes. (E) Fraction 1 SS RBC were incubated with 500 µM NSC23766 (NSC), 2 µM PMA (PMA), or both (NSC/PMA) for 1 hour before staining with CM-H2-DCFDA to detect ROS levels. Inhibition of Rac-GTP activity with NSC23766 blocks the PKC-mediated induction of ROS production in SS RBC, indicating that Rac-GTP acts downstream of PKC in the activation of NADPH oxidase in erythrocytes. Data presented are representative of repeated experiments with batched samples. n = 4 each for AA and SS samples in all graphs above, and P < .05 where designated by asterisks.
RBC ROS production is modulated by plasma factors
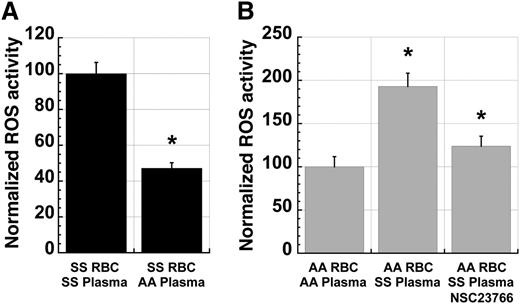
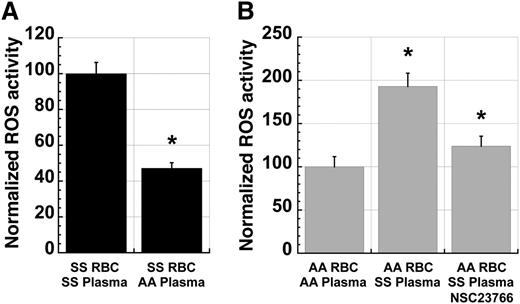
The presence of a PKC-Rac-NADPH oxidase signaling pathway in SS RBC raised the possibility that there might be extracellular signals that modulate NADPH oxidase activity in RBC. To test this possibility, we incubated fraction 1 SS RBC in plasma from ABO-matched SS patients or AA control subjects prior to analysis of ROS production. Preincubation of SS cells in AA plasma for 24 hours resulted in a significant decrease in levels of ROS production compared with preincubation in SS plasma (Figure 5A). Conversely, incubation of AA cells in ABO group-matched SS plasma for 4 hours resulted in a significant increase in ROS production relative to that seen in cells incubated in AA plasma (Figure 5B). Addition of the Rac inhibitor NSC23766 to the preincubation decreased this SS plasma-mediated activation of ROS production in AA cells (Figure 5B), demonstrating that this effect is mediated via Rac activity.
Sickle plasma activates ROS production in erythrocytes in a manner dependent on Rac-GTPase activity. (A) ROS production in fraction 1 SS RBC decreases significantly with incubation in ABO-matched AA plasma when compared with RBC incubated in endogenous or exogenous ABO-matched SS plasma. Cells were incubated for 24 hours in a standard tissue-culture incubator at 37°C before incubating with CM-H2-DCFDA for ROS quantitation. n = 20 and P < .05. (B) ROS production in fraction 1 AA RBC increases significantly with incubation in ABO-matched SS plasma compared with RBC incubated in endogenous or exogenous ABO-matched AA plasma. This induction is partially blocked by coincubation with NSC23766 (500 µM). Cells were incubated for 4 hours in a standard tissue-culture incubator before staining with CM-H2-DCFDA. n = 17 and P < .05.
Sickle plasma activates ROS production in erythrocytes in a manner dependent on Rac-GTPase activity. (A) ROS production in fraction 1 SS RBC decreases significantly with incubation in ABO-matched AA plasma when compared with RBC incubated in endogenous or exogenous ABO-matched SS plasma. Cells were incubated for 24 hours in a standard tissue-culture incubator at 37°C before incubating with CM-H2-DCFDA for ROS quantitation. n = 20 and P < .05. (B) ROS production in fraction 1 AA RBC increases significantly with incubation in ABO-matched SS plasma compared with RBC incubated in endogenous or exogenous ABO-matched AA plasma. This induction is partially blocked by coincubation with NSC23766 (500 µM). Cells were incubated for 4 hours in a standard tissue-culture incubator before staining with CM-H2-DCFDA. n = 17 and P < .05.
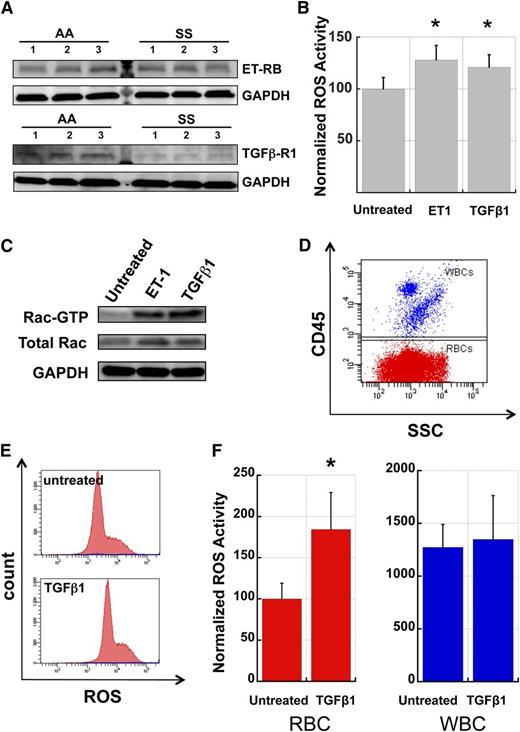
RBC ROS production is stimulated by TGFβ1 and ET-1
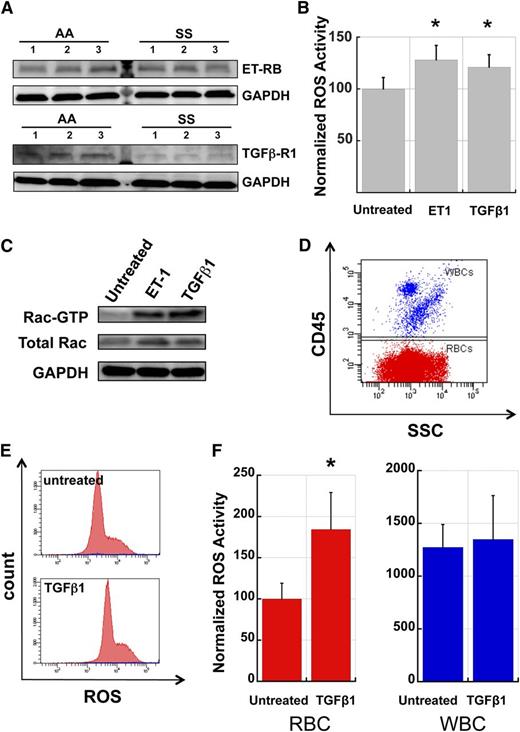
A number of plasma factors have been shown to stimulate NADPH oxidase activity in a range of cell types, including neutrophils, monocytes, and endothelial cells, and several of these factors are known to be present at elevated levels in the plasma of SCD patients, presumably due to the inflammatory state associated with SCD.36,37 To test the involvement of TGFβ1 and ET-1, two plasma cytokines previously implicated in SCD pathophysiology, we first assayed AA and SS RBC for the presence of receptors to these molecules on the membrane. Western blots of RBC membrane preparations revealed that both AA and SS RBC membranes carry the TGFβ receptor type 1 and ET-1 receptor B subtypes (Figure 6A). The apparently lower levels of TGFβ-R1 in SS RBC relative to AA RBC may be due to receptor tachyphylaxis induced by higher levels of circulating TGFβ1, a phenomenon described before for TGFβ receptors in transformed and nontransformed fibroblasts in response to exposure to TGFβ.38,39 We then conducted incubation experiments of AA fraction 1 RBC with ET-1 and TGFβ1 to determine if these factors could stimulate ROS production and activate Rac GTPases in RBC. Preincubation of fraction 1 AA RBC in endogenous plasma with added TGFβ1 or ET-1 for 4 hours resulted in a significant increase in ROS production in these cells compared with plasma-alone controls (Figure 6B). Additionally, pull-down assays of GTP-bound Rac indicated that incubation of AA RBC with ET-1 or TGFβ1 resulted in increased levels of active Rac (Figure 6C). Finally, to compare the relative MFI of ROS production for WBC and RBC populations and the effect of cytokine stimulation on RBC and WBC ROS production, we used a mix of fraction 1 AA RBC with WBC from the same donor to create a cell suspension containing 3% to 5% WBC. After incubation with TGFβ1 or ET-1 for 4 hours, cells were stained with anti-CD45 to label the WBC (Figure 6D) and loaded with CM-H2-DCFDA to quantitate ROS production. ET-1 stimulation caused an increase in RBC ROS production by 12% and an increase in WBC ROS by 9%, both not statistically significant for the number of samples (n = 5) tested, likely due to phenotypic variability between different human samples. Stimulation with TGFβ1 increased RBC ROS production almost 2-fold whereas it had no discernible effect on WBC ROS production (Figure 6E-F). WBC ROS production was approximately 10 to 15 times higher than in RBC before stimulation and only 5 to 7 times higher after stimulation with TGFβ1. Given these ratios and the relative numbers of RBC and WBC in circulation (typically on the order of 500:1), we conclude that enzymatically produced RBC-derived oxidative species contribute significantly to overall SCD-related oxidative stress.
TGFβ1 and Endothelin-1 activate Rac-GTP and stimulate ROS production in AA RBC. (A) AA and SS RBC carry surface receptors to TGFβ1 and ET-1. RBC membrane preparations (pink ghosts) were probed with antibodies against ET-1 Receptor B or TGFβ1 Receptor type 1 as indicated. GAPDH at the same specimen is shown as loading control. (B) Fraction 1 RBC from healthy (AA) donors were incubated for 4 hours in endogenous plasma with or without the addition of exogenous ET-1 (2.5 µM) or TGFβ1 (0.5 µg/ml) and then stained for ROS with CM-H2-DCFDA. n = 15 and P < .05. (C) Fraction 1 AA RBC were incubated with ET-1 or TGFβ-1 as in (B) and then lysed and processed in pull-down assays to determine active Rac (Rac-GTP). Control aliquots of total lysate were immunoblotted for total Rac and GAPDH proteins as quantitative controls for pull-down and loading. The experiment was repeated three times with similar results. (D) Representative flow cytogram demonstrating the CD45 positive WBC mixed with fraction 1 AA RBC (CD45-negative), in order to evaluate relative erythrocyte and leukocyte ROS production. (E) Representative histograms of AA RBC (red) and WBC (blue population along the x-axis) samples without and with TGFβ1 stimulation. (F) Comparative ROS signals and induction of ROS production in AA RBC and WBC by TGFβ-1 stimulation. All ROS signals are normalized to the unstimulated RBC signal. TGFβ1 caused an increase of 84% in RBC ROS production (P < .05) while it only caused a 6% increase in WBC ROS production (n = 5). SSC, side light scatter.
TGFβ1 and Endothelin-1 activate Rac-GTP and stimulate ROS production in AA RBC. (A) AA and SS RBC carry surface receptors to TGFβ1 and ET-1. RBC membrane preparations (pink ghosts) were probed with antibodies against ET-1 Receptor B or TGFβ1 Receptor type 1 as indicated. GAPDH at the same specimen is shown as loading control. (B) Fraction 1 RBC from healthy (AA) donors were incubated for 4 hours in endogenous plasma with or without the addition of exogenous ET-1 (2.5 µM) or TGFβ1 (0.5 µg/ml) and then stained for ROS with CM-H2-DCFDA. n = 15 and P < .05. (C) Fraction 1 AA RBC were incubated with ET-1 or TGFβ-1 as in (B) and then lysed and processed in pull-down assays to determine active Rac (Rac-GTP). Control aliquots of total lysate were immunoblotted for total Rac and GAPDH proteins as quantitative controls for pull-down and loading. The experiment was repeated three times with similar results. (D) Representative flow cytogram demonstrating the CD45 positive WBC mixed with fraction 1 AA RBC (CD45-negative), in order to evaluate relative erythrocyte and leukocyte ROS production. (E) Representative histograms of AA RBC (red) and WBC (blue population along the x-axis) samples without and with TGFβ1 stimulation. (F) Comparative ROS signals and induction of ROS production in AA RBC and WBC by TGFβ-1 stimulation. All ROS signals are normalized to the unstimulated RBC signal. TGFβ1 caused an increase of 84% in RBC ROS production (P < .05) while it only caused a 6% increase in WBC ROS production (n = 5). SSC, side light scatter.
Discussion
In this study, we present evidence for a novel mechanism of oxidative stress production in human SCD erythrocytes. We show that NADPH oxidase catalytic subunits are present in erythrocytes and that RBC NADPH oxidase activity is regulated intracellularly by PKC and Rac GTPases and extracellularly by signaling factors present in plasma from sickle cell patients. Finally, we identify TGFβ1 and ET-1, both known to be present at elevated levels in SCD plasma,40,41 as two of the signaling molecules mediating this plasma-induced production of ROS in erythrocytes. Therefore, in addition to the known nonenzymatic production of ROS in sickle RBC by HbS auto-oxidation and iron-mediated Fenton chemistry reactions, we propose here an enzymatic mechanism of ROS production within erythrocytes mediated by NADPH oxidase and stimulated by plasma signaling factors. The preponderance of reticulocytes and young RBC in the sickle red cell mass further increases the cumulative oxidative load in sickle cell patients relative to AA subjects because younger cells produce more ROS than older ones due to their higher enzymatic activity.
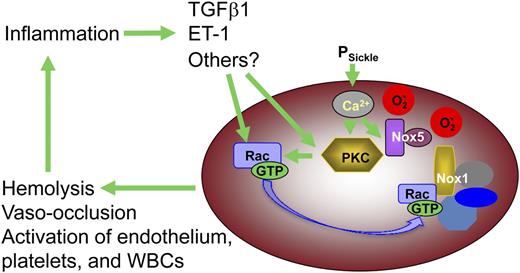
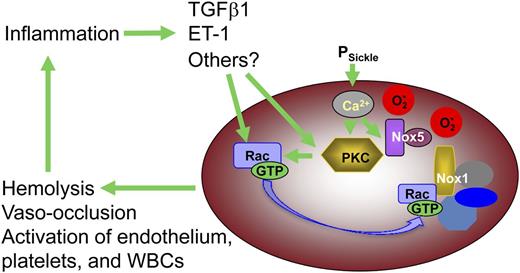
The catalytic subunits of 4 of the 5 known NADPH oxidase complexes can be detected in erythrocytes by western blotting (Figure 3A), and transcripts for several of the NADPH oxidase subunit peptides are present in the human reticulocyte transcriptome.32 The relative abundance of NOX1 and NOX5 suggests that these may be the primarily active isoforms in both AA and SS cells, whereas NOX2 appears to be present at higher levels in sickle RBC. The presence of several isoforms is of interest because it suggests that there may be diverse mechanisms regulating NADPH oxidase–mediated ROS generation in erythrocytes. NOX1, 2, and 4 require the association of membrane-bound activating subunits; Rac-GTP is required for NOX1 and NOX2 activity, whereas NOX5 is independent of other subunits and can be activated directly by the binding of intracellular Ca2+.33 PKC-mediated activation of Rac is an established mechanism of NADPH oxidase activation in a variety of cell types,42 and our results indicate that this signaling pathway is present and active in sickle RBC as well. PKC may also directly activate NADPH oxidase by phosphorylation of the p47- and p22-like subunits of this enzyme complex.43 Ca2+, a key activator of classical PKC isoforms and a direct activator of NOX5, may also activate ROS production in sickle erythrocytes as it enters the cell via the deoxygenation-induced nonselective cation influx pathway (PSickle).30 The role of plasma signaling factors such as TGFβ1 and ET-1 in stimulating NADPH oxidase activity in erythrocytes is especially intriguing because it suggests that oxidative stress within the sickle RBC can drive a system-wide vicious cycle of inflammation in conjunction with activated endothelium, platelets, and WBC (Figure 7).
Proposed model for the contribution by erythrocyte-derived ROS to SCD pathophysiology. Erythrocyte NADPH oxidase-derived ROS, along with that derived from HbS auto-oxidation, induces structural damage in the RBC that renders the cell more vulnerable to lysis or sickling deformation and vaso-occlusion. Additionally, ROS escaping from the RBC effect changes in plasma proteins, WBC, endothelial cells, and platelets, which in conjunction with RBC lysis and vaso-occlusion induce a state of chronic systemic inflammation. Extracellular signaling molecules associated with this inflammatory state act back on the RBC, via cell surface receptors, to induce higher levels of NADPH oxidase activity, closing a positive-feedback loop that drives hemolysis, irreversible sickling, and vaso-occlusion.
Proposed model for the contribution by erythrocyte-derived ROS to SCD pathophysiology. Erythrocyte NADPH oxidase-derived ROS, along with that derived from HbS auto-oxidation, induces structural damage in the RBC that renders the cell more vulnerable to lysis or sickling deformation and vaso-occlusion. Additionally, ROS escaping from the RBC effect changes in plasma proteins, WBC, endothelial cells, and platelets, which in conjunction with RBC lysis and vaso-occlusion induce a state of chronic systemic inflammation. Extracellular signaling molecules associated with this inflammatory state act back on the RBC, via cell surface receptors, to induce higher levels of NADPH oxidase activity, closing a positive-feedback loop that drives hemolysis, irreversible sickling, and vaso-occlusion.
NADPH oxidase-derived ROS in SS RBC may cause direct oxidative damage to a variety of subcellular structures, reducing erythrocyte deformability and resulting in increased RBC fragility and hemolysis. Moreover, NADPH oxidase activity may deplete the cellular pool of NADPH, thus impairing the ability of the RBC to maintain its antioxidant defenses.2,13 In addition to inducing endogenous damage, erythrocyte-derived ROS, which can exit the cell via the membrane anion channel,25 may also contribute to systemic oxidative stress by modifying the activity of plasma proteins, WBC, platelets, and endothelial cells.1
The potential utility of antioxidant therapy in SCD is intriguing but with limited evidence to date that it can ameliorate the acute or chronic pathophysiology of the disease. The antioxidant N-acetylcysteine, a source of sulfhydryl groups in cells and scavenger of free radicals, has been shown to reduce the formation of irreversibly sickled RBC under hypoxic conditions in vitro.44 In a phase 2 clinical trial of N-acetylcysteine in SCD patients, administration of the drug decreased the percentage of dense cells, normalized RBC glutathione levels, and decreased the frequency of vaso-occlusive crises.45 One of the multiple beneficial mechanisms of hydroxyurea in SCD may be reduction of systemic oxidative stress in humans,46,47 and there is an inverse correlation between hemoglobin F levels and markers of systemic oxidative stress in an SCD mouse model.48 Trials of antioxidant agents in mouse models of SCD also appear to reduce markers of acute and chronic inflammation.49,50 Of note, all of these studies attempt scavenging of free radicals rather than inhibition of their production. On the basis of the data we present in this study, NADPH oxidase activity is implicated not only as a source of oxidative stress in SCD coming from WBC and endothelial cells but also as an endogenous, erythrocyte-specific mechanism that contributes to RBC rigidity and fragility. As such, NADPH oxidase inhibitors could contribute to prevention of sickle RBC-induced vaso-occlusion and hemolysis, the primary pathophysiologic mechanisms of sickle cell anemia, and may merit inclusion, along with hydroxyurea and agents targeting other pathophysiological mechanisms, in a multimodal approach to SCD therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the CCHMC Hematology Repository support team for assistance with obtaining patient and control blood samples and Jose Cancelas, Robert Franco, and members of the Zheng laboratory at CCHMC for valuable critiques of this work. The authors acknowledge with gratitude the patients with SCD followed at the CCHMC Hematology Clinic and their families for their continued participation in and support of hematology research at our institution.
This work was supported by grants from the National Institutes of Health (K08HL088126 and R01HL116352) (T.A.K.) (P30DK090971) (Y.Z.) (U54HL070871) (C.H.J.) (DK26263) (NM).
National Institutes of Health
Authorship
Contribution: A.G. participated in the planning of the project, designed, performed, and analyzed experiments, and wrote the manuscript. S.P., D.G.K., and S.K. designed, performed, and analyzed experiments. N.M., P.M., and C.H.J. participated in the planning of the project and interpreted and critiqued experimental results. Y.Z. participated in the planning of the project, provided key reagents, and interpreted and critiqued experimental results. T.A.K. participated in the planning of the project, designed and analyzed experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alex George, Texas Children's Hematology Center, Texas Children's Hospital, 6701 Fannin St, Suite 1580, Houston, TX 77030; e-mail: axgeorge@txch.org.

![Figure 2. Production of ROS in SS RBC is mediated in part by NADPH oxidase activity. (A) A schematic depiction of ROS metabolism along with inhibitors of enzymatic sources of ROS. (B) The ROS signal in SS RBC is reduced by preincubation with the NADPH oxidase inhibitor DPI (10 μM) prior to staining with CM-H2-DCFDA, but not by the xanthine oxidase inhibitor oxypurinol (500 µM) or the mitochondrial electron transport inhibitor rotenone (50 µM) (n = 6). (C) The ROS signal in SS RBC is reduced by preincubation with any of the known inhibitors of NADPH oxidase (DPI at 50 µM, gp91-dsTat at 50 µM, and apocynin at 100 µM), supporting the involvement of NADPH oxidase in ROS generation in these cells (n = 3 and P < .05 where designated by asterisks). (D) Whole-blood samples from AA and SS subjects were colabeled with PE-Cy7 anti-CD45, PE–anti-GPA, and CM-H2-DCFDA to permit the identification of WBC (CD45+;GPA−) and RBC (CD45−;GPA+) populations and the quantitation of the total ROS signal from each population (shown in the histograms as red for the RBC population, blue [thin line along the x-axis] for WBC and purple for the total RBC + WBC populations, in representative samples). (E) RBC ROS production, identified by gating out the CD45+ WBC population, constituted almost 80% of the combined RBC and WBC ROS signal. In AA samples (n = 10), the signal from RBC alone was 79% ± 1.5% of the total signal, whereas in SS samples (n = 7) this proportion was 77% ± 4.7%. FSC, forward light scatter; SSC, side light scatter.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/11/10.1182_blood-2012-07-441188/4/m_2099f2.jpeg?Expires=1769253947&Signature=ooE~xC2Gq7HGfeed6vsXXh42ucdrwTvolnDpBkDtlRzAfeXj3~6y4sL336hZul2ziw4g9RcllUupgVYKFywnkjO2g0qltOh81JQlNpZbU8srLao-NMzyUixBtADkj1LkCAKVNY5XUQQd3-Vtt7kWFDTZr8z4xmYJSv3sRP3q3VqpNBe9GHhi2gt81JrZdRE4llkdzjfrT5~XiMRkiHzIX9UzVXJJoIU4KJXbMy34gr4Xh7wF~QDOPQHk3Yvyeh7Joa7CP8wWnexQgQzWv0H6bynLnIyk6JU9s6iwViVPv~NZbx~8gjTi~kN9flpy4XiyuPaFQid4iwelj1GNp4rpgQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Production of ROS in SS RBC is mediated in part by NADPH oxidase activity. (A) A schematic depiction of ROS metabolism along with inhibitors of enzymatic sources of ROS. (B) The ROS signal in SS RBC is reduced by preincubation with the NADPH oxidase inhibitor DPI (10 μM) prior to staining with CM-H2-DCFDA, but not by the xanthine oxidase inhibitor oxypurinol (500 µM) or the mitochondrial electron transport inhibitor rotenone (50 µM) (n = 6). (C) The ROS signal in SS RBC is reduced by preincubation with any of the known inhibitors of NADPH oxidase (DPI at 50 µM, gp91-dsTat at 50 µM, and apocynin at 100 µM), supporting the involvement of NADPH oxidase in ROS generation in these cells (n = 3 and P < .05 where designated by asterisks). (D) Whole-blood samples from AA and SS subjects were colabeled with PE-Cy7 anti-CD45, PE–anti-GPA, and CM-H2-DCFDA to permit the identification of WBC (CD45+;GPA−) and RBC (CD45−;GPA+) populations and the quantitation of the total ROS signal from each population (shown in the histograms as red for the RBC population, blue [thin line along the x-axis] for WBC and purple for the total RBC + WBC populations, in representative samples). (E) RBC ROS production, identified by gating out the CD45+ WBC population, constituted almost 80% of the combined RBC and WBC ROS signal. In AA samples (n = 10), the signal from RBC alone was 79% ± 1.5% of the total signal, whereas in SS samples (n = 7) this proportion was 77% ± 4.7%. FSC, forward light scatter; SSC, side light scatter.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/11/10.1182_blood-2012-07-441188/4/m_2099f2.jpeg?Expires=1769253948&Signature=I7fYWM6dfqWZR59eTslELZ5JrfP61Yp1vqMWn8D9KOgGYnA93rcTSrEX-DtAPh7nsqkT7zGO81loCiQ~F13oLtNIIulg4vSZfIGDftIEpNdJtOZNJ5QJVgcjcVpeKNHNm1yml4xvqTlgxRa4jmxugJGORIg6hmpP~9gYewv1r41yNiFkH4BL1ZYkexJVYOjJquqDZWtvj4VmrsA6BUwEFhnb2~-jSr6R9lia-9ki7mHpY3ElwNbJhzbpYqlaKvvTlpLR1mdW-XyrMqvzdKPF-8Ls1JbTwbHFPg74-x6tEnF-H21B~~AJ6bchmhxLK2nJIzLY-GKJX-~EF6Bd6hB71g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)