Key Points
The DNAM-1 adhesion and costimulatory pathway promotes GVHD via effects on regulatory T cells.
Effective GVL can still occur in the absence of DNAM-1, making the pathway an attractive therapeutic target.
Abstract
Donor T cells play pivotal roles in graft-versus-host disease (GVHD) and graft-versus-leukemia (GVL) effects following bone marrow transplantation (BMT). DNAX accessory molecule 1 (DNAM-1) is a costimulatory and adhesion molecule, expressed mainly by natural killer cells and CD8+ T cells at steady state to promote adhesion to ligand-expressing targets and enhance cytolysis. We have analyzed the role of this pathway in GVHD and GVL. The absence of DNAM-1 on the donor graft attenuated GVHD in major histocompatibility complex (MHC)-mismatched and MHC-matched BMT following conditioning with lethal and sublethal irradiation. In contrast, DNAM-1 was not critical for GVL effects against ligand (CD155) expressing and nonexpressing leukemia. The effects on GVHD following myeloablative conditioning were independent of CD8+ T cells and dependent on CD4+ T cells, and specifically donor FoxP3+ regulatory T cells (Treg). The absence of DNAM-1 promoted the expansion and suppressive function of Treg after BMT. These findings provide support for therapeutic DNAM-1 inhibition to promote tolerance in relevant inflammatory-based diseases characterized by T-cell activation.
Introduction
Allogeneic bone marrow transplantation (BMT) is a curative therapy for many hematologic malignancies. Unfortunately, graft-versus-host disease (GVHD) is a major complication and is mediated by donor T cells, which respond to recipient-derived alloantigens presented predominantly by recipient-derived antigen-presenting cells (APCs).1-4 The strength of this T-cell–APC interaction is modulated by multiple costimulatory pathways.
DNAX accessory molecule 1 (DNAM-1, CD226) is an adhesion/costimulatory molecule belonging to the immunoglobulin superfamily; it triggers cell-mediated cytotoxicity against cells expressing the ligands CD155 and CD112.5-8 In humans and mice, DNAM-1 is mainly expressed on natural killer (NK) cells and CD8 T cells8,9 and has been shown to enhance the cytotoxic effector function of NK cells and T cells against malignancies10,11 and virus-infected cells.12,13 DNAM-1 has also been associated with susceptibility to type 1 diabetes and multiple sclerosis by genome-wide association studies.14,15 CD155 and CD112 (the known ligands for DNAM-1) also bind to an alternative T-cell immunoglobulin and immunoreceptor tyrosine inhibitory motif (ITIM) domain (TIGIT) molecule which counterregulates this pathway,16-18 and indeed DNAM-1 and TIGIT cross-compete for binding to CD155 in vitro.19 TIGIT is expressed preferentially on regulatory T cells (Treg), activated and memory T cells, and NK cells and has been shown to promote interleukin-10 (IL-10) secretion and immune regulation via effects on both dendritic cells16 and effector T cells.18,19 However, the effects of DNAM-1 or TIGIT on Treg have not been described.
We thus examined whether DNAM-1 influences GVHD and graft-versus-leukemia (GVL) and the mechanisms therein within well-established BMT models that use conditioning with lethal doses of irradiation. In this scenario, we demonstrate that donor DNAM-1 expression promotes GVHD in a CD4+ T-cell–dependent manner via the inhibition of donor Foxp3+ Treg whereas costimulatory effects on effector CD8+ T cells are redundant. In addition, we show that donor Treg and effector T cells increase their expression of DNAM-1 and TIGIT after transplantation.
Materials and methods
Mice
Female C57BL/6 (B6.WT, H-2b, CD45.2+), B6.Ptprca (B6.Ptp, H-2b, CD45.1+), B6D2F1 (H-2b/d), BALB/B (H-2b), BALB/c (H-2d), C3H/Hej (C3H, H-2k), and B6C3F1 (H-2b/k) mice were purchased from the Animal Resources Centre (Perth, WA, Australia). H-2Kbm1 (bm1) mice were supplied by the Walter and Elisa Hall Institute. C57BL/6-background DNAM-1–deficient (B6.DNAM-1−/−) mice were provided by M. Colonna (Washington University School of Medicine, St. Louis, MO).20 B6-background FoxP3.EGFP mice were provided by A.Y. Rudensky (University of Washington, Seattle, WA).21
BMT
Mice were transplanted as described previously.22 On day −1, lethal doses of total body irradiation (137Cs source at 108 cGy/min) were administered to B6C3F1, B6D2F1, bm1 (1100 cGy), C3H (950 cGy), BALB/B, and BALB/c (900 cGy) mice. In some experiments, B6D2F1 recipients received only sublethal irradiation (500 cGy). On day 0, lethally irradiated mice were transplanted with 5 × 106 whole or T-cell–depleted bone marrow (BM) cells with or without purified splenic T cells. Sublethally irradiated mice were transplanted with 50 × 106 splenocytes or T-cell–depleted grafts as non-GVHD controls. In some experiments, splenic CD4+ cells (>85% CD4+CD3+, <0.8% CD8+CD3+) or CD8+ cells (>88% CD8+CD3+, <3.9% CD4+CD3+) were positively selected using the magnetically activated cell sorter (MACS) system (Miltenyi Biotec, Bergisch Gladbach, Germany). CD25+CD4+ cells (>95% purity) were purified from spleen by cell sorting (MoFlo, Beckman Coulter) following CD4+ cell selection using the MACS system. Treg depletion was performed by injecting 500 μg CD25 antibody (PC61, generated in house) intraperitoneally on days −3 and −1. Animal procedures were undertaken by using protocols approved by the institutional Queensland Institute of Medical Research (QIMR) animal ethics committee.
Assessment of GVHD
The degree of systemic GVHD was assessed by using a cumulative scoring system that measured changes in five clinical parameters: weight loss, posture (hunching), activity, fur texture, and skin integrity (maximum index, 10).23 Mice were monitored daily, those with GVHD clinical scores ≥6 were culled, and the date of death was recorded as the next day, in accordance with institutional animal ethics guidelines.
Histology
Slides of formalin-fixed, hematoxylin and eosin–stained tissue were coded and scored in a blinded fashion by A.D.C., as previously described.24 Images were acquired by using an Olympus BX51 microscope (Olympus), an Evolution MP 5.0 camera, and Qcapture software (Qimaging).
Leukemia challenge
Assays were undertaken as previously described.22 In brief, the luciferase-transfected mastocytoma cell line P815 (H-2Dd, DBA/2) or the chronic myeloid leukemia cell line P210 (H-2Dk, C3H) were injected intravenously into B6D2F1 or B6C3F1 recipients on the day of transplantation. Survival and clinical scores were monitored. Mice were imaged weekly by using a Xenogen imaging system (Xenogen IVIS 100; Caliper Life Sciences) to determine the level of tumor burden. Leukemic death was defined as a significant tumor burden assessed by imaging (>107 photons/sec) and/or the development of hind-limb paralysis (because of P815 chloromas within vertebrae). GVHD death was defined as low tumor burden (<107 photons/sec) and significant GVHD scores (≥5).
Fluorescence activated cell sorting analysis
The following antibodies were purchased from BioLegend (San Diego, CA): phycoerythrin (PE)-conjugated anti-H-2Kk (36-7-5), CD3 (2C11), anti-NK1.1 (PK136), CD155 (4.24.1), anti–interferon gamma (IFN-γ; XMG1.2), anti–tumor necrosis factor (TNF; MP6-XT22), rat immunoglobulin G2b (IgG2b) isotype control (RTK4530), allophycocyanin-conjugated anti-NK1.1 (PK136), anti–IFN-γ (XMG1.2) and rat IgG2b isotype control (RTK4530), Alexa Fluor 647-conjugated CD226 (TX42.1), and rat IgG2b isotype control (RTK4530). The following antibodies were purchased from BD Bioscience (San Jose, CA): fluorescein isothiocyanate–conjugated anti-H-2Db (KH95), PE-conjugated CD25 (7D4), PE-Cy7–conjugated CD4 (RM4-5), APC-Cy7–conjugated CD3 (17A2), and Pacific Blue-conjugated CD8 (53-6.7). Alexa Fluor 647–conjugated anti-TIGIT (GIGD7) was purchased from eBioscience (San Diego, CA). Fluorescein isothiocyanate–conjugated CD3 (KT31.1), rat IgG2a isotype control (Mac4), and IgG2b isotype control (Mac5) were generated in-house. Anti-FoxP3 was stained with Alexa Fluor 647–conjugated Foxp3 (150D) and the FoxP3 Staining Buffer set (eBioscience).
Cytokine analysis
Serum cytokine levels were determined by using the BD FACSArray Bioanalyzer System (BD Biosciences), according to the manufacturer’s protocol. Ex vivo splenocytes were stimulated with soluble anti-CD3 (2 μg/mL; 2C11; produced in-house) and brefeldin A (1:1000 dilution; BioLegend) for 4 hours. Cells were processed for intracellular cytokine staining, according to the manufacturer’s protocol (BD Cytofix/Cytoperm Kit, BD Bioscience).
51Cr release assay
Assays were undertaken as previously described.24 In brief, 51Cr-labeled target cells (donor type EL4, H-2b; host-type P210, H-2k) were then cultured with donor CD8+ effectors, purified from recipient spleens 12 days after BMT by using CD8 microbeads and the MACS system (Miltenyi Biotec) for 5 hours at 37°C and 5% CO2. 51Cr release into supernatants was determined via gamma counter (TopCount microplate scintillation counter; Packard Instrument). Spontaneous release was defined from wells receiving targets only, and total release was defined from wells receiving targets plus 1% Triton X-100. Percentage of cytotoxicity was calculated as follows: percentage of cytotoxicity = (experimental release – spontaneous release)/(total release – spontaneous release) × 100.
Statistical analysis
Survival curves for GVHD were plotted by using Kaplan-Meier estimates and compared by log-rank analysis. The curves for leukemia death were plotted and compared by using cumulative incidence analysis of competing risks by R_2.10.1 software. The Mann-Whitney U test was used for the statistical analysis of remaining data.
Results
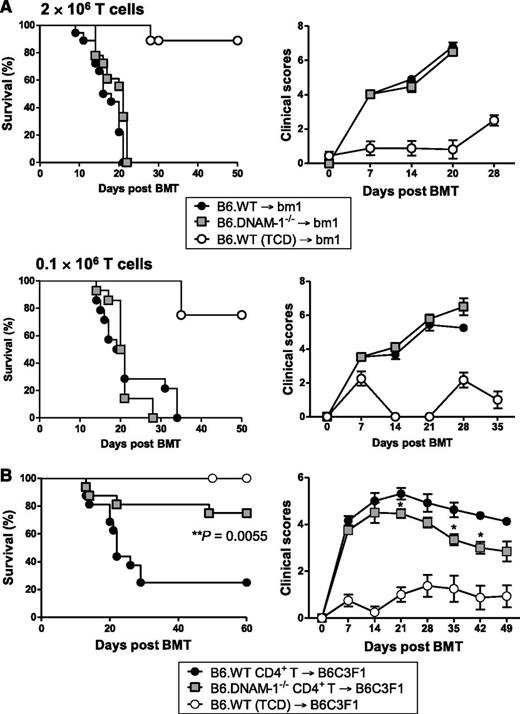
To determine the effect of donor DNAM-1 expression on acute GVHD, lethally irradiated C3H mice were transplanted with BM and T cells from major histocompatibility complex (MHC)-mismatched B6.WT or B6.DNAM-1−/− mice. The absence of DNAM-1 on donor cells significantly improved overall survival such that 67% of recipients survived beyond day 60, whereas the expression of DNAM-1 on donor cells resulted in a median survival of only 26 days with more than 90% of recipients succumbing to GVHD by day 40 (Figure 1A). Furthermore, GVHD severity in surviving mice (as determined by clinical scores) was reduced beyond day 7 in recipients of DNAM-1 deficient grafts (Figure 1B). This was primarily the result of attenuated GVHD within the gastrointestinal tract (Figure 1C-D). We next confirmed the role of DNAM-1 in GVHD in an MHC-matched BMT model (B6 → BALB/B) where GVHD is directed to multiple minor histocompatibility antigens (Figure 1E-F). GVHD lethality was reduced following transplantation with 5 × 106 DNAM-1−/− T cells, and GVHD severity was also attenuated following transplantation with 1 × 106 DNAM-1−/− T cells. The lower T-cell dose induced minimal GVHD mortality (data not shown). Together, these data establish that following myeloablative conditioning, the expression of DNAM-1 on donor cells is critical for the induction of lethal GVHD directed to either MHC antigens or multiple minor histocompatibility antigens in isolation.
The absence of DNAM-1 on donor cells attenuates acute GVHD. (A-D) 5 × 106 BM and 0.5 × 106 CD3+ T cells from B6.WT or B6.DNAM-1−/− donor mice were transplanted into lethally irradiated C3H recipients. Survival (A) and clinical score (B) are shown. P < .0001, B6.DNAM-1−/− vs B6.WT. *P < .05, B6.DNAM-1−/− vs B6.WT. Data shown are combined from 2 replicate experiments (n = 15 per T-cell–replete group and n = 7 in T-cell–depleted [TCD] control). (C,D) Tissues from skin, liver, and small intestine of C3-recipient mice were taken 20 days after transplantation. (C) Semiquantitative histopathology and (D) representative images (magnification ×250) from small intestine. Scale bars represent 100 μm. (n = 8 to 9 in T-cell–replete groups and n = 3 in TCD control). (E,F) 5 × 106 BM and (E) 5 × 106 or (F) 1 × 106 CD3+ T cells from B6.WT or B6.DNAM-1−/− donor mice was transplanted into lethally irradiated BALB/B recipients. (E) P = .0026, B6.DNAM-1−/− vs B6.WT. Survival data shown are combined from 2 replicate experiments (n = 15 per T-cell–replete group and n = 3 in TCD control). (F) ***P = .0002; **P < .008; *P < .02; B6.DNAM-1−/− vs B6.WT. Survival data shown are combined from 2 replicate experiments (n = 14 per T-cell–replete group and n = 5 in TCD control).
The absence of DNAM-1 on donor cells attenuates acute GVHD. (A-D) 5 × 106 BM and 0.5 × 106 CD3+ T cells from B6.WT or B6.DNAM-1−/− donor mice were transplanted into lethally irradiated C3H recipients. Survival (A) and clinical score (B) are shown. P < .0001, B6.DNAM-1−/− vs B6.WT. *P < .05, B6.DNAM-1−/− vs B6.WT. Data shown are combined from 2 replicate experiments (n = 15 per T-cell–replete group and n = 7 in T-cell–depleted [TCD] control). (C,D) Tissues from skin, liver, and small intestine of C3-recipient mice were taken 20 days after transplantation. (C) Semiquantitative histopathology and (D) representative images (magnification ×250) from small intestine. Scale bars represent 100 μm. (n = 8 to 9 in T-cell–replete groups and n = 3 in TCD control). (E,F) 5 × 106 BM and (E) 5 × 106 or (F) 1 × 106 CD3+ T cells from B6.WT or B6.DNAM-1−/− donor mice was transplanted into lethally irradiated BALB/B recipients. (E) P = .0026, B6.DNAM-1−/− vs B6.WT. Survival data shown are combined from 2 replicate experiments (n = 15 per T-cell–replete group and n = 3 in TCD control). (F) ***P = .0002; **P < .008; *P < .02; B6.DNAM-1−/− vs B6.WT. Survival data shown are combined from 2 replicate experiments (n = 14 per T-cell–replete group and n = 5 in TCD control).
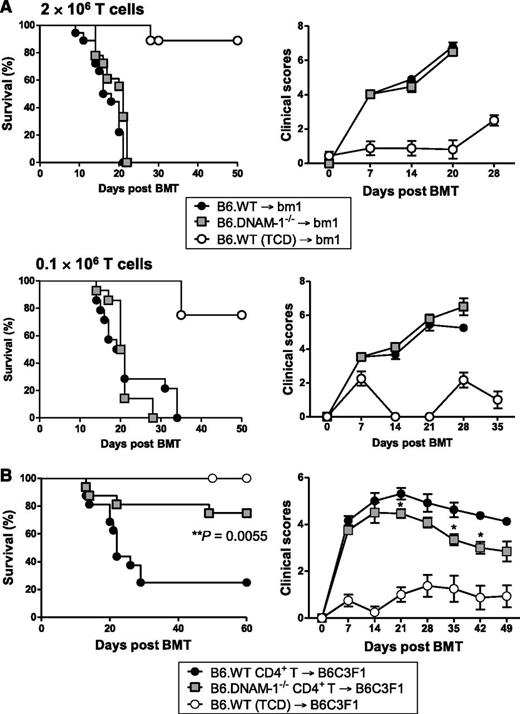
Next we examined the donor populations expressing DNAM-1, which mediates this effect. Since donor T cells are critical for the initiation of GVHD, we first focused on donor T-cell subsets. The B6 → C3H GVHD system described in Figure 1 is CD4+ and CD8+ T-cell dependent since the addition of CD8 T cells to CD4 T cells within the donor graft accelerates GVHD mortality (median survival, 19.5 vs 37 days; P = .013; data not shown). We investigated the effect of DNAM-1 using the B6 → bm1 BMT model. In this system, there is a single mutation in the H-2Kb allele in bm1 recipients (H-2Kbm1), while all other major and minor histocompatibility antigens are intact. Thus, there is no capability of CD4 T cells to be primed and invoke GVHD here, because there are no additional minor histocompatibility antigen disparities that can be presented in class II to drive such a response. We thus used this system to analyze the requirement for the DNAM-1 pathway in inducing GVHD mediated by donor CD8 T cells in isolation, recognizing that the system does not examine GVHD that is induced by both CD4 and CD8 T cells, as occurs clinically. Surprisingly, DNAM-1 had negligible impact on acute GVHD severity in this setting, regardless of the donor CD8 T-cell dose (2 × 106 or 0.1 × 106 T cells; Figure 2A). This is in contrast to the previous description of DNAM-1 expression on CD8+ T cells exacerbating GVHD when very high numbers of donor T cells are transplanted (50 × 106 splenocytes or 12 × 106 T cells) after sublethal conditioning,25 a system that may reflect GVHD after nonmyeloablative conditioning. We therefore analyzed the effect of DNAM-1 expression on donor CD4+ T cells on acute GVHD by transplanting only purified donor CD4 T cells from B6.WT or B6.DNAM-1−/− donors into lethally irradiated B6C3F1 recipients. We used B6C3F1 recipients here to remove graft rejection in the absence of donor CD8 T cells as a confounding variable. The absence of DNAM-1 on donor CD4 T cells alone significantly attenuated acute GVHD such that 75% of recipients of DNAM-1–deficient CD4+ T cells survived beyond day 60 whereas only 25% of recipients of WT CD4+ T cells survived at this time, and median survival was reduced to only day 22. The severity of GVHD was also reduced in surviving mice of B6.DNAM-1−/− grafts (Figure 2B). Thus, following lethal irradiation, the protection from GVHD seen in the absence of DNAM-1 is CD4+ T-cell dependent.
The DNAM-1 effects on GVHD are dependent on CD4+ T cells. (A) 5 × 106 BM and 2 × 106 or 0.1 × 106 CD3+ T cells from B6.WT or B6.DNAM-1−/− donor mice were transplanted into lethally irradiated bm1 recipients. Survival data (left) and clinical scores (right) with 2 × 106 or 0.1 × 106 T cells are combined from 2 replicate experiments, respectively. 2 × 106 T cells (n = 18 per T-cell–replete group and n = 8 in TCD control). 0.1 × 106 T cells (n = 14 per T-cell–replete group and n = 4 in TCD control). (B) 5 × 106 BM from B6.WT and 1.8 × 106 CD4+ T cells from B6.WT or B6.DNAM-1−/− donor mice were transplanted into lethally irradiated B6C3F1 recipients. P = .0055, B6.DNAM-1−/− vs B6.WT. Survival data shown are combined from 2 replicate experiments (n = 16 per T-cell–replete group and n = 8 in TCD control).
The DNAM-1 effects on GVHD are dependent on CD4+ T cells. (A) 5 × 106 BM and 2 × 106 or 0.1 × 106 CD3+ T cells from B6.WT or B6.DNAM-1−/− donor mice were transplanted into lethally irradiated bm1 recipients. Survival data (left) and clinical scores (right) with 2 × 106 or 0.1 × 106 T cells are combined from 2 replicate experiments, respectively. 2 × 106 T cells (n = 18 per T-cell–replete group and n = 8 in TCD control). 0.1 × 106 T cells (n = 14 per T-cell–replete group and n = 4 in TCD control). (B) 5 × 106 BM from B6.WT and 1.8 × 106 CD4+ T cells from B6.WT or B6.DNAM-1−/− donor mice were transplanted into lethally irradiated B6C3F1 recipients. P = .0055, B6.DNAM-1−/− vs B6.WT. Survival data shown are combined from 2 replicate experiments (n = 16 per T-cell–replete group and n = 8 in TCD control).
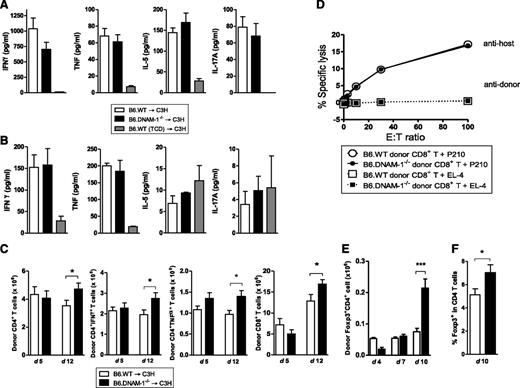
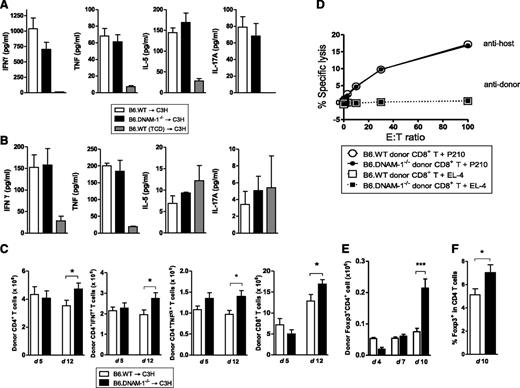
To investigate the mechanisms by which DNAM-1 expression controls GVHD, inflammatory cytokines and donor effector T-cell function were analyzed. In the B6 → C3H model, there were no significant differences in systemic inflammatory cytokine levels (IFN-γ, TNF, IL-5, and IL-17A) in recipients of B6.WT and B6.DNAM-1−/− grafts on either day 5 or day 12 posttransplantation (Figure 3A-B). Similarly, the expansion of donor CD4+ and CD8+ T cells and their effector function, including cytokine production (IFN-γ, TNF) from CD4+ T cells and cytolytic function of CD8+ T cells were not attenuated in recipients of B6.DNAM-1−/− grafts at these time points (Figure 3C-D). In contrast, donor Treg cells were expanded in the recipients of B6.DNAM-1−/− grafts late after BMT (day 10 but not earlier at days 4 or 7; Figure 3E-F). Indeed, the absence of DNAM-1 appears to result in an increased expansion of all donor T-cell subsets 12 days after BMT (Figure 3C-E); however, this occurs preferentially in the Treg population (Figure 3E-F). We confirmed that the percentages of splenic Foxp3+ cells were not different between naive B6.WT and B6.DNAM-1−/− mice. The proportion of CD4+ cells within the CD3+ population of splenic T cells from naive B6.WT and B6.DNAM-1−/− mice was 52.6% ± 1.2% and 52.2% ± 5.3% (median ± standard deviation [SD]; n = 4 per group), respectively. The proportion of Foxp3+ cells in the CD4+ population from the same mice was 14.6% ± 1.9% and 15.6% ± 2.5% (median ± SD; n = 6 per group), respectively. In addition, proliferation and IFN-γ production were not different in T cells from naive B6.WT and B6.DNAM-1−/− mice in mixed lymphocyte reaction when stimulated by purified allogeneic dendritic cells (data not shown).
The absence of DNAM-1 on donor cells promotes Treg expansion. 5 × 106 BM and 0.5 × 106 CD3+ T cells from B6.WT or B6.DNAM-1−/− donor mice were transplanted into lethally irradiated C3H recipients. TCD bone marrow from B6.WT donors was transplanted into irradiated C3H recipients as non-GVHD controls. (A) Serum cytokines at day 5 (n = 14 to 17 per T-cell–replete group and n = 6 in TCD control). (B) Serum cytokines at day 12 (n = 4 to 5 per T-cell–replete group and n = 3 in TCD control). (C) Total, IFN-γ+, and TNF+ donor CD4+ T-cell numbers (H-2Db+CD3+CD4+) and the total donor CD8+ T cell numbers (H-2Db+CD3+CD8+) at day 5 and 12 (n = 10 per group, combined from 2 replicate experiments). (D) 51Cr release assay with allogeneic (P210) and syngeneic (EL4) tumor targets at day 12. Representative data of mean with standard error of the mean (SEM) from triplicate wells are shown from 2 replicate experiments. (E) Donor FoxP3+ CD4+ T-cell numbers (Foxp3+H-2Db+CD3+CD4+) at days 4, 7, and 10 after BMT (n = 3 per group at days 4 and 7; n = 9 to 10, combined from 2 replicate experiments at day 10). (F) The FoxP3+ cell percentage within donor CD4+ T cells (H-2Db+CD3+CD4+) at day 10 after BMT (n = 13 to 14, combined from 3 replicate experiments at day 10). ***P = .0007; *P < .05 ; B6.DNAM-1−/− vs B6.WT.
The absence of DNAM-1 on donor cells promotes Treg expansion. 5 × 106 BM and 0.5 × 106 CD3+ T cells from B6.WT or B6.DNAM-1−/− donor mice were transplanted into lethally irradiated C3H recipients. TCD bone marrow from B6.WT donors was transplanted into irradiated C3H recipients as non-GVHD controls. (A) Serum cytokines at day 5 (n = 14 to 17 per T-cell–replete group and n = 6 in TCD control). (B) Serum cytokines at day 12 (n = 4 to 5 per T-cell–replete group and n = 3 in TCD control). (C) Total, IFN-γ+, and TNF+ donor CD4+ T-cell numbers (H-2Db+CD3+CD4+) and the total donor CD8+ T cell numbers (H-2Db+CD3+CD8+) at day 5 and 12 (n = 10 per group, combined from 2 replicate experiments). (D) 51Cr release assay with allogeneic (P210) and syngeneic (EL4) tumor targets at day 12. Representative data of mean with standard error of the mean (SEM) from triplicate wells are shown from 2 replicate experiments. (E) Donor FoxP3+ CD4+ T-cell numbers (Foxp3+H-2Db+CD3+CD4+) at days 4, 7, and 10 after BMT (n = 3 per group at days 4 and 7; n = 9 to 10, combined from 2 replicate experiments at day 10). (F) The FoxP3+ cell percentage within donor CD4+ T cells (H-2Db+CD3+CD4+) at day 10 after BMT (n = 13 to 14, combined from 3 replicate experiments at day 10). ***P = .0007; *P < .05 ; B6.DNAM-1−/− vs B6.WT.
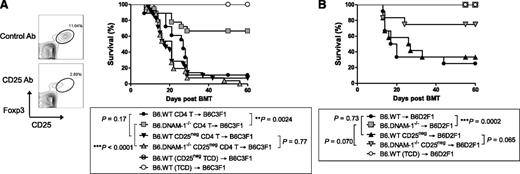
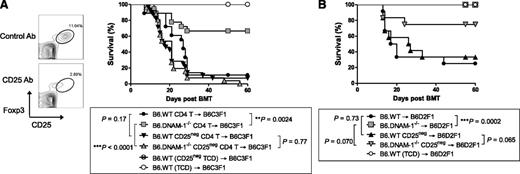
To investigate whether the effects of DNAM-1 on GVHD were dependent on regulatory T cells, we depleted natural Treg from donor B6.WT or B6.DNAM-1−/− grafts via the administration of CD25 antibody (Figure 4A). This treatment depleted the majority of Foxp3+CD25+CD4+ T cells (Figure 4A) within the donor graft, and when lethally irradiated B6C3F1 mice were transplanted with these T cells, the protective effect of the absence of DNAM-1 seen in Figure 2B was lost (Figure 4A). Thus the protective effect of DNAM-1 deficiency was dependent on Treg after myeloablative conditioning. Next we examined whether the protective effect of DNAM-1 deficiency after sublethal conditioning was also dependent on Treg. Sublethally irradiated B6D2F1 mice were transplanted with 50 × 106 splenocytes from Treg-depleted or replete B6.WT or B6.DNAM-1−/− mice. All recipients of Treg-replete B6.DNAM-1−/− graft survived beyond day 60, whereas only 25% of recipients of Treg-replete B6.WT graft survived at the same point (P = .0002). Again, in the setting of sublethal conditioning, the protective effect of DNAM-1 deficiency was reduced following Treg depletion (Figure 4B); however, this effect was relatively minor compared with that after lethal (myeloablative) conditioning (Figure 4A). In the sublethal conditioning system, we noted a trend to protection from GVHD in the absence of DNAM-1 that was also independent of Treg (Figure 4B), in contrast to that seen after myeloablative conditioning (Figure 4A). This is consistent with effects of DNAM-1 on other donor T-cell populations, particularly CD8 T cells, after sublethal conditioning, as described by Nabekura et al.25
The absence of DNAM-1 on donor cells attenuates GVHD in a Treg-dependent fashion after lethal and sublethal conditioning. (A,B) All donor mice were treated with 500 μg CD25 antibody (Ab; PC61) for Treg depletion or rat IgG1 (isotype control; MAC49, Treg replete) intraperitoneally on days −3 and −1. (A) Lethally irradiated B6C3F1 mice were transplanted with 5 × 106 BM from CD25 Ab– or IgG–treated B6.WT mice and 2 × 106 MACS-purified CD4+ T cells from CD25 Ab– or IgG–treated B6.WT or B6.DNAM-1−/− mice on day 0. TCD BM from B6.WT donors was transplanted into irradiated B6C3F1 recipients as non-GVHD controls. Representative fluorescence activated cell sorting (FACS) dots of Treg depletion with CD25 Ab or isotype control (left) and survival data (right) are shown. P = .77, CD25 Ab–treated B6.WT vs B6.DNAM-1−/−. **P = .0024, IgG-treated B6.WT vs B6.DNAM-1−/−. Data combined from 4 experiments (n = 26 to 28 per T-cell–deplete group, n = 18 per T-cell–replete group, and n = 6 to 8 in TCD control). (B) Sublethally irradiated (500 cGy) B6D2F1 mice were transplanted with 50 × 106 splenocytes from CD25 Ab– or IgG–treated B6.WT or B6.DNAM-1−/− mice on day 0. T-cell depleted (TCD) grafts from IgG-treated B6.WT donors were transplanted as non-GVHD controls. P = .0002, IgG-treated B6.WT vs B6.DNAM-1−/−; P = .065, CD25 Ab–treated B6.WT vs B6.DNAM-1−/−. Data combined from 2 replicate experiments (n = 12 per T-cell–replete group and n = 8 in TCD control).
The absence of DNAM-1 on donor cells attenuates GVHD in a Treg-dependent fashion after lethal and sublethal conditioning. (A,B) All donor mice were treated with 500 μg CD25 antibody (Ab; PC61) for Treg depletion or rat IgG1 (isotype control; MAC49, Treg replete) intraperitoneally on days −3 and −1. (A) Lethally irradiated B6C3F1 mice were transplanted with 5 × 106 BM from CD25 Ab– or IgG–treated B6.WT mice and 2 × 106 MACS-purified CD4+ T cells from CD25 Ab– or IgG–treated B6.WT or B6.DNAM-1−/− mice on day 0. TCD BM from B6.WT donors was transplanted into irradiated B6C3F1 recipients as non-GVHD controls. Representative fluorescence activated cell sorting (FACS) dots of Treg depletion with CD25 Ab or isotype control (left) and survival data (right) are shown. P = .77, CD25 Ab–treated B6.WT vs B6.DNAM-1−/−. **P = .0024, IgG-treated B6.WT vs B6.DNAM-1−/−. Data combined from 4 experiments (n = 26 to 28 per T-cell–deplete group, n = 18 per T-cell–replete group, and n = 6 to 8 in TCD control). (B) Sublethally irradiated (500 cGy) B6D2F1 mice were transplanted with 50 × 106 splenocytes from CD25 Ab– or IgG–treated B6.WT or B6.DNAM-1−/− mice on day 0. T-cell depleted (TCD) grafts from IgG-treated B6.WT donors were transplanted as non-GVHD controls. P = .0002, IgG-treated B6.WT vs B6.DNAM-1−/−; P = .065, CD25 Ab–treated B6.WT vs B6.DNAM-1−/−. Data combined from 2 replicate experiments (n = 12 per T-cell–replete group and n = 8 in TCD control).
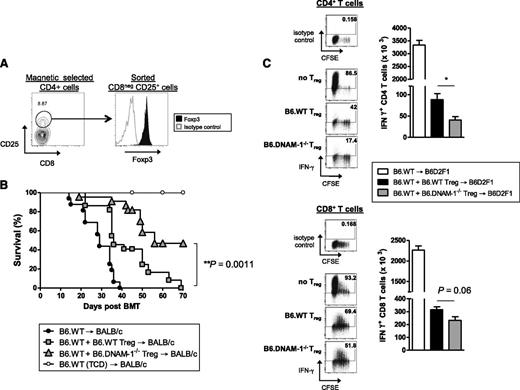
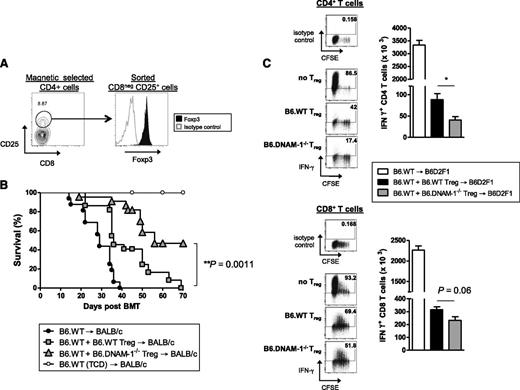
To study whether functional differences may also exist in Treg in addition to the numerical effects seen in the absence of DNAM-1, adoptive transfer experiments were undertaken, as previously described,26 comparing the relative ability of B6.WT and B6.DNAM-1−/− Treg to suppress GVHD. Lethally irradiated BALB/c mice were transplanted with BM from B6.WT donors with or without 2 × 105 FAC-sorted CD25+CD4+ T cells (>90% Foxp3+; Figure 5A) from B6.WT or B6.DNAM-1−/− mice. Two days later, 5 × 105 Treg-depleted WT T cells were transplanted to induce acute GVHD. The majority of recipients of DNAM-1−/− Treg survived beyond day 50 (median survival, day 56), demonstrating a superior ability to suppress acute GVHD relative to WT Treg in which the median survival was day 36 (Figure 5B). This confirms that the expression of DNAM-1 on Treg inhibits their suppressive function in vivo. Since B6.DNAM-1−/− Treg expanded differentially beyond day 7 after BMT (Figure 3E-F), we analyzed the effect of WT versus DNAM-1−/− Treg on effector T cell function at this time. In this assay, BM and Treg were transplanted on day 0, and Treg-depleted CD45.1+ T cells were transplanted 7 days later. Effector function in CD45.1+ T cells was examined 4 days later. In support of the notion that DNAM-1 expression dampens Treg function, DNAM-1−/− Treg were more potent than WT Treg in suppressing IFN-γ generation in effector T cells, and this was seen in both CD4 and CD8 T-cell compartments (Figure 5C).
The absence of DNAM-1 on donor Treg promotes regulatory function in vivo. (A) Representative FACS plots of the sort strategy on CD25+CD8− T cells from naive B6.WT or B6.DNAM-1−/− mice and Foxp3 expression on sorted cells. (B) 5 × 106 BM from B6.WT mice with or without 0.2 × 106 FACS sort-purified CD25+CD4+ T cells from B6.WT or B6.DNAM-1−/− mice were transplanted into lethally irradiated BALB/c mice. Two days later, those recipients were given 0.5 × 106 CD3+ T cells from CD25 Ab–treated B6.WT mice. TCD bone marrow from B6.WT donors was transplanted into irradiated BALB/c recipients as non-GVHD controls. Survival data are shown. P < .0001, B6.DNAM-1−/− Treg vs no Treg; P = .0001, B6.WT Treg vs no Treg; P = .0011, B6.WT Treg vs B6.DNAM-1−/− Treg. Data shown are combined from 3 replicate experiments (n = 16 T-cell–replete group without Treg, n = 22 per T-cell–replete group with Treg, and n = 9 in TCD control). (C) 5 × 106 BM from B6.WT mice with or without 0.4 × 106 FACS sort-purified CD25+CD4+ T cells from B6.WT or B6.DNAM-1−/− mice were transplanted into lethally irradiated B6D2F1 mice. Seven days later, those recipients were given 2 × 106 CD3+ carboxyfluorescein succinimidyl ester (CFSE)–labeled T cells from CD25 Ab–treated B6 (CD45.1+) mice. Four days later, spleens from recipients were analyzed. CD45.1+IFN-γ+CD4+ T cells (CD45.1+CD3+CD4+) (top right) and CD45.1+IFN-γ+CD8+ T cells (CD45.1+CD3+CD8+) (bottom right). Representative FACS plots for CFSE dilution and IFN-γ secretion in CD4+ and CD8+ T cells from each group are shown. IFN-γ+CD4+ T cells: P = .016, B6.DNAM-1−/− Treg vs no Treg; P = .029, B6.WT Treg vs no Treg; P = .016, B6.WT Treg vs B6.DNAM-1−/− Treg. IFN-γ+CD8+ T cells; P = .016, B6.DNAM-1−/− Treg vs no Treg; P = .029, B6.WT Treg vs no Treg; P = .064, B6.WT Treg vs B6.DNAM-1−/− Treg. n = 4 to 5 per group. Representative data from 1 of 2 experiments is shown.
The absence of DNAM-1 on donor Treg promotes regulatory function in vivo. (A) Representative FACS plots of the sort strategy on CD25+CD8− T cells from naive B6.WT or B6.DNAM-1−/− mice and Foxp3 expression on sorted cells. (B) 5 × 106 BM from B6.WT mice with or without 0.2 × 106 FACS sort-purified CD25+CD4+ T cells from B6.WT or B6.DNAM-1−/− mice were transplanted into lethally irradiated BALB/c mice. Two days later, those recipients were given 0.5 × 106 CD3+ T cells from CD25 Ab–treated B6.WT mice. TCD bone marrow from B6.WT donors was transplanted into irradiated BALB/c recipients as non-GVHD controls. Survival data are shown. P < .0001, B6.DNAM-1−/− Treg vs no Treg; P = .0001, B6.WT Treg vs no Treg; P = .0011, B6.WT Treg vs B6.DNAM-1−/− Treg. Data shown are combined from 3 replicate experiments (n = 16 T-cell–replete group without Treg, n = 22 per T-cell–replete group with Treg, and n = 9 in TCD control). (C) 5 × 106 BM from B6.WT mice with or without 0.4 × 106 FACS sort-purified CD25+CD4+ T cells from B6.WT or B6.DNAM-1−/− mice were transplanted into lethally irradiated B6D2F1 mice. Seven days later, those recipients were given 2 × 106 CD3+ carboxyfluorescein succinimidyl ester (CFSE)–labeled T cells from CD25 Ab–treated B6 (CD45.1+) mice. Four days later, spleens from recipients were analyzed. CD45.1+IFN-γ+CD4+ T cells (CD45.1+CD3+CD4+) (top right) and CD45.1+IFN-γ+CD8+ T cells (CD45.1+CD3+CD8+) (bottom right). Representative FACS plots for CFSE dilution and IFN-γ secretion in CD4+ and CD8+ T cells from each group are shown. IFN-γ+CD4+ T cells: P = .016, B6.DNAM-1−/− Treg vs no Treg; P = .029, B6.WT Treg vs no Treg; P = .016, B6.WT Treg vs B6.DNAM-1−/− Treg. IFN-γ+CD8+ T cells; P = .016, B6.DNAM-1−/− Treg vs no Treg; P = .029, B6.WT Treg vs no Treg; P = .064, B6.WT Treg vs B6.DNAM-1−/− Treg. n = 4 to 5 per group. Representative data from 1 of 2 experiments is shown.
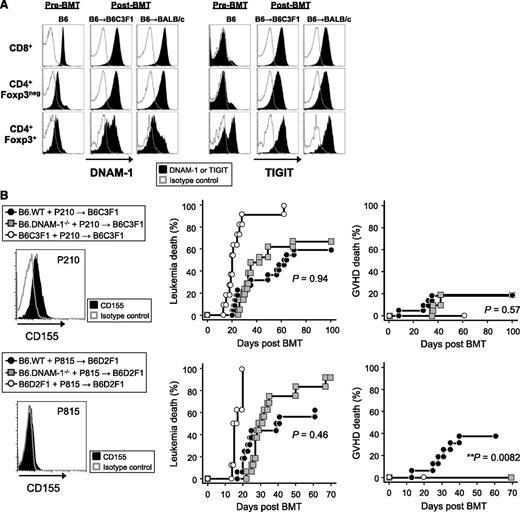
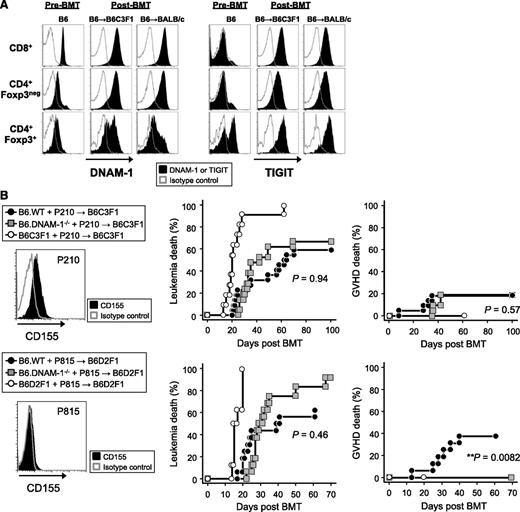
We next quantified DNAM-1 expression on donor Treg and effector T-cell populations before and after BMT. Interestingly, DNAM-1 was more highly expressed on donor CD8+ T cells relative to CD4+ T cells at steady state, but after BMT, it was highly expressed on both CD8+ and CD4+ T cells, including the Foxp3+ CD4+ Treg fraction (Figure 6A). In order to understand how the absence of DNAM-1 on Treg may so profoundly alter their function, we quantified the expression of TIGIT, the alternative ligand to DNAM-1 for CD155 and CD112. TIGIT was expressed preferentially on Treg in naive mice but was upregulated on all T-cell fractions after BMT (Figure 6A). The expression of both DNAM-1 and TIGIT following T-cell activation has also been reported in human samples.27 The ligation of TIGIT inhibits antigen-reactive T-cell responses directly and indirectly via effects on APCs,16,18 and TIGIT-deficient T cells exacerbate GVHD,19 demonstrating an important regulatory function. Thus, in the absence of DNAM-1, TIGIT may be able to interact with CD155/CD112 unopposed to enhance Treg expansion and function.
The absence of DNAM-1 on donor cells maintains GVL effects. (A) Lethally irradiated B6C3F1 or BALB/c mice were transplanted with 5 × 106 BM and 2 × 106 or 0.5 × 106 T cells, respectively, from B6.Foxp3.GFP mice. The expression of DNAM-1 or TIGIT was analyzed on each described T-cell subset from mesenteric lymph nodes at day 10 and from naive mice. Data are representative of 2 to 3 replicate experiments. The same pattern of staining was seen in T cells from spleen and peripheral lymph nodes (not shown). (B) 5 × 106 BM and 0.5 × 106 T cells from B6.WT, B6.DNAM-1−/−, B6C3F1, or B6D2F1 mice with 5 × 106 P210luc+ or 5 × 103 P815luc+ tumor cells were transplanted into lethally irradiated B6C3F1 recipients (upper panel) or B6D2F1 recipients respectively (lower panel). Representative FACS dots of CD155 expression on each tumor cell line (left), leukemia death (middle), and GVHD death (right) are shown. Data shown with P210luc+ are combined from 3 replicate experiments (n = 21 to 22 per T-cell–replete group and n = 11 in syngeneic control). P < .0001, B6C3F1 vs B6.WT or B6.DNAM-1−/−; P = .94, leukemia death of B6.WT vs B6.DNAM-1−/−; P = .57, GVHD death of B6.WT vs B6.DNAM-1−/−. Data shown with P815luc+ are combined from 2 replicate experiments (n = 16 per T-cell–replete group and n = 8 in syngeneic control). P < .0001, B6D2F1 vs B6.WT or B6.DNAM-1−/−; P = .46, leukemia death of B6.WT vs B6.DNAM-1−/−; P = .0082, GVHD death of B6.WT vs B6.DNAM-1−/−. Fisher’s exact test confirmed that there was a significant relationship between GVHD death and group (P = .007).
The absence of DNAM-1 on donor cells maintains GVL effects. (A) Lethally irradiated B6C3F1 or BALB/c mice were transplanted with 5 × 106 BM and 2 × 106 or 0.5 × 106 T cells, respectively, from B6.Foxp3.GFP mice. The expression of DNAM-1 or TIGIT was analyzed on each described T-cell subset from mesenteric lymph nodes at day 10 and from naive mice. Data are representative of 2 to 3 replicate experiments. The same pattern of staining was seen in T cells from spleen and peripheral lymph nodes (not shown). (B) 5 × 106 BM and 0.5 × 106 T cells from B6.WT, B6.DNAM-1−/−, B6C3F1, or B6D2F1 mice with 5 × 106 P210luc+ or 5 × 103 P815luc+ tumor cells were transplanted into lethally irradiated B6C3F1 recipients (upper panel) or B6D2F1 recipients respectively (lower panel). Representative FACS dots of CD155 expression on each tumor cell line (left), leukemia death (middle), and GVHD death (right) are shown. Data shown with P210luc+ are combined from 3 replicate experiments (n = 21 to 22 per T-cell–replete group and n = 11 in syngeneic control). P < .0001, B6C3F1 vs B6.WT or B6.DNAM-1−/−; P = .94, leukemia death of B6.WT vs B6.DNAM-1−/−; P = .57, GVHD death of B6.WT vs B6.DNAM-1−/−. Data shown with P815luc+ are combined from 2 replicate experiments (n = 16 per T-cell–replete group and n = 8 in syngeneic control). P < .0001, B6D2F1 vs B6.WT or B6.DNAM-1−/−; P = .46, leukemia death of B6.WT vs B6.DNAM-1−/−; P = .0082, GVHD death of B6.WT vs B6.DNAM-1−/−. Fisher’s exact test confirmed that there was a significant relationship between GVHD death and group (P = .007).
Finally, we examined whether DNAM-1 is required for GVL effects. Lethally irradiated B6C3F1 mice were transplanted with BM and 0.5 × 106 T cells from B6.WT, B6.DNAM-1−/− or B6C3F1 mice, along with luciferase-expressing p210 leukemia cells, which are C3H derived and express CD155 but not NKG2D ligands. DNAM-1 has been suggested previously to be dispensable if tumor cells express an NKG2D ligand.28 Lower doses of T cells were used in these experiments to minimize GVHD mortality, such that GVL could be more fully examined. We focused on CD155 since both TIGIT and DNAM-1 have a much higher affinity for this molecule than CD112.16,17 Surprisingly, the absence of donor DNAM-1 had little effect on the ability of the donor T cell to eradicate recipient-type leukemia and did not influence leukemia-specific mortality (Figure 6B). Identical results were obtained when luciferase-expressing P815 leukemia cells (DBA/2-derived mastocytoma cell line–expressing NKG2D ligands but not CD155) were cotransplanted in the B6 → B6D2F1 system (Figure 6B). These data suggest that the inhibition of DNAM-1 will maintain GVL effects after BMT, regardless of whether the tumor expresses CD155 or not. Despite the low numbers of T cells transplanted, the protective effect from GVHD was still observed in the B6 → B6D2F1 system (Figure 6B). These data are consistent with the fact that DNAM-1 has little effect on cytotoxic T lymphocyte generation and cytotoxicity after BMT, which represents the major effector pathway of GVL.
Discussion
In these studies, we predominantly used lethal doses of total body irradiation as conditioning to achieve rapid, complete, and equivalent donor engraftment status, which is a prerequisite for the comparative analysis of GVHD or GVL. In this setting, we have demonstrated that DNAM-1 controls the induction of GVHD after BMT and that although DNAM-1–dependent costimulatory effects on CD8 T cells are redundant, DNAM-1 controls Foxp3+CD4+ Treg expansion and function.
Previous studies have suggested that DNAM-1 on donor CD8+ T cells promotes GVHD, putatively behaving as a classical costimulatory molecule since donor CD8 T-cell expansion was reduced in the absence of DNAM-1.25 However, these studies were undertaken in BMT systems that used sublethal conditioning and very high numbers of T cells that may or may not reflect GVHD after nonmyeloablative conditioning. Furthermore, cause-and-effect relationships between CD8 T-cell expansion and GVHD were not clearly established. In addition, this previous study did not examine the effects of DNAM-1 on Treg nor did it examine effects on GVL.25 In our studies using multiple models of GVHD that were either CD4- or CD8-dependent, we could not demonstrate any effect of DNAM-1 when GVHD was mediated by CD8 T cells in isolation. Protective effects after myeloablative conditioning were instead all dependent on donor CD4 Treg. We also demonstrated that donor Treg were still relevant for the protection from GVHD in the absence of DNAM-1 after sublethal conditioning. However, there was clearly protection from GVHD in the absence of DNAM-1 that was also independent of Treg in this system. This is consistent with effects on other donor T-cell populations, particularly CD8 T cells, after sublethal conditioning, as described by Nabekura et al.25 These differences likely reflect the fact that following myeloablative conditioning and the inflammatory environment invoked thereafter, a level of redundancy exists for individual costimulatory pathways.
Although these protective effects from the absence of donor DNAM-1 after myeloablative conditioning were dependent on Treg and independent of CD8+ T cells in isolation, this does not necessarily imply that the reduction of GVHD is restricted to settings in which CD4+ T cells alone induce GVHD. Although clinical GVHD after HLA-matched BMT is predominantly mediated by donor CD8 T cells, CD4 T cells are important,29-31 and the absence of DNAM-1 clearly attenuates GVHD that is mediated by both CD4 and CD8 T cells when both cell subsets are primed (Figures 1 and 5). Furthermore, after nonmyeloablative conditioning, DNAM-1 can also influence GVHD independently of Treg (Figure 4C) via effects on CD8 T cells.25
Recent studies have shown that GVHD is enhanced in the absence of CD155 on recipient tissues,32 consistent with the concept that this molecule is critical for invoking inhibitory signals within the donor T-cell compartment. Since this effect is not mediated through DNAM-1, it supports the notion that CD155 binds to an alternative ligand to inhibit donor T-cell function and GVHD. Given the expression of TIGIT on regulatory T cells and the fact that GVHD is enhanced in the absence of TIGIT,19 it is likely that this pathway dominates in the absence of DNAM-1, and in so doing, may enhance donor Treg expansion and function. However, alternative explanations are possible for the inhibitory effects of DNAM-1 on Treg during GVHD. The CD155–DNAM-1 pathway promotes cell adhesion, and it is possible that Treg trafficking and adherence differs in the presence or absence of DNAM-1, such that Treg may preferentially remain within lymphoid organs in the absence of DNAM-1. In addition, the costimulatory nature of the pathway may preferentially induce cell death in Treg following ligation, such that Treg survival is enhanced when DNAM-1 is absent.
Given that DNAM-1–blocking antibodies attenuate GVHD,25 the pharmacologic inhibition of this pathway would appear to be an attractive strategy to promote Treg-mediated tolerance. With this in mind, the potential effects of DNAM-1 inhibition on GVL are a critical issue. DNAM-1 could be potentially important in GVL effects at a number of levels. First, it may be important in promoting cytotoxicity directly against ligand (CD155) expressing tumors.8,28 However, the intact GVL response against P815 that does not express CD155 suggests that this is not critical and that other pathways predominate. Second, DNAM-1 may be important at the donor T-cell level by promoting donor T-cell expansion and function following interaction with APCs expressing CD155. Again, the fact that this pathway is not required for donor T-cell expansion and cytotoxicity suggest that this is also not the case. Thus, although the notion that DNAM-1 is important for GVHD but not GVL appears paradoxical at first glance, the explanation is readily apparent, given the effect of DNAM-1 on Treg and studies of the effect of Treg on GVL. These studies have clearly demonstrated that the inhibition of GVHD by Treg have limited or no effects on GVL.33-35 This reflects the ability of Treg to inhibit T-cell proliferation and effector function in relation to cytokine function while having limited effects on T-cell–dependent cytoxicity,35 the critical pathway required for mediating GVL effects. Thus, by enhancing Treg expansion and function, DNAM-1 inhibition can effectively promote the separation of GVHD and GVL. We used tumor cell lines P210 and P815 to determine GVL effects since their phenotype in regard to CD155 expression is likely to be maintained more consistently than leukemia generated by retroviral oncogene insertion.36 However, the limitations of using cell lines is that they may not as faithfully recapitulate the phenotype and etiology of clinical human leukemia. Importantly, the nature of the GVL response required to eliminate these cell lines is well characterized and involves perforin and TNF-mediated CD8-dependent cytotoxicity in the presence of CD4 T-cell help.37-40 In this regard, CD8 T cells are well established as mediators of GVL responses clinically.41,42 Nevertheless, we acknowledge that we have not excluded impairment of GVL mediated directly by CD4 T cells against MHC class II–bearing leukemia in the absence of DNAM-1.
The modulation of Treg, by promoting either expansion, function, or both, is an attractive strategy for preventing and treating conditions characterized by aberrant T-cell function. In particular, this approach appears highly feasible for preventing or treating GVHD. To date, this involves either the expansion of Treg in vitro prior to adoptive transfer or the expansion of Treg directly in vivo.33,43-46 The in vitro expansion typically involves polyclonal expansion of natural Treg with CD3 and CD28 antibodies in the presence of IL-2 and transforming growth factor beta, with or without rapamycin.43,45,46 The expansion in vivo revolves around the administration of IL-2, usually in recipients receiving immune suppression with rapamycin.47 At least in the latter setting, the concurrent inhibition of DNAM-1 may be an attractive strategy for further enhancing this effect.
In summary, this is the first study demonstrating the ability of DNAM-1 to promote pathology via effects on Treg, and it provides further support for therapeutic DNAM-1 inhibition by antibody-mediated blockade25 in relevant inflammatory-based diseases in which the induction of tolerance is desirable.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Professor Marco Colona for DNAM-1−/− mice, P. Hall and G. Chojnowski (QIMR) for expert cell sorting, and L. Wockner (QIMR) and K. Matsuo (Aichi Cancer Center Research Institute) for statistical analysis.
This work was supported by grants from the Queensland Cancer Council and the National Health and Medical Research Council (NHMRC). G.R.H. and M.J.S. are NHMRC Australia Fellows. K.P.A.M. is a Cancer Council Queensland Senior Research Fellow. K.A.M. is an NHMRC Clinical Training Fellow.
Authorship
Contribution: M.K. designed and performed research, analyzed data, and wrote the paper. R.D.K., S.D.O., K.E.L., M.L., B.E.T., N.C.R., L.L., C.J.C., R.J.R., K.A.M., K.A.A., and A.V. performed research. A.D.C. performed blinded histological assessment. M.J.S. supplied mice and aided in data interpretation, K.P.A.M. aided in data interpretation and experimental design, and G.R.H. aided in experimental design and helped write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Motoko Koyama, Bone Marrow Transplantation Laboratory, Queensland Institute of Medical Research, 300 Herston Rd, Brisbane, QLD 4006, Australia; e-mail: Motoko.Koyama@qimr.edu.au.

![Figure 1. The absence of DNAM-1 on donor cells attenuates acute GVHD. (A-D) 5 × 106 BM and 0.5 × 106 CD3+ T cells from B6.WT or B6.DNAM-1−/− donor mice were transplanted into lethally irradiated C3H recipients. Survival (A) and clinical score (B) are shown. P < .0001, B6.DNAM-1−/− vs B6.WT. *P < .05, B6.DNAM-1−/− vs B6.WT. Data shown are combined from 2 replicate experiments (n = 15 per T-cell–replete group and n = 7 in T-cell–depleted [TCD] control). (C,D) Tissues from skin, liver, and small intestine of C3-recipient mice were taken 20 days after transplantation. (C) Semiquantitative histopathology and (D) representative images (magnification ×250) from small intestine. Scale bars represent 100 μm. (n = 8 to 9 in T-cell–replete groups and n = 3 in TCD control). (E,F) 5 × 106 BM and (E) 5 × 106 or (F) 1 × 106 CD3+ T cells from B6.WT or B6.DNAM-1−/− donor mice was transplanted into lethally irradiated BALB/B recipients. (E) P = .0026, B6.DNAM-1−/− vs B6.WT. Survival data shown are combined from 2 replicate experiments (n = 15 per T-cell–replete group and n = 3 in TCD control). (F) ***P = .0002; **P < .008; *P < .02; B6.DNAM-1−/− vs B6.WT. Survival data shown are combined from 2 replicate experiments (n = 14 per T-cell–replete group and n = 5 in TCD control).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/17/10.1182_blood-2012-07-444026/4/m_3511f1.jpeg?Expires=1768753281&Signature=drzlDjj9kOtpWUKxq9HxW5b5-Mv3rGVq68MIDTdK3vhAz06GNdJUrZggDjd~PnraNyVIjqjMkGIxDoIzZp6UzXKQMkCiP7GElAjOM1Q5Qp4kKZY0IDb0bRDSVQvxzntZK1nebxzReD0M1l8jFF-ABNEq6S4Dz1Y-tabMa6MHVMzaOskLaTVjrlTecBi14UojRyuemWjrqaQSxgdfKI7qLhE6WgQvdNv3IBasyl3VwgVOtrxV-39yY-2hKuRsLGl64D4atMqSa9~6~~E--K3exekt47ww4lbNc~ncvVAZJvRzpNEuCxv~9Kp2mmaTpcGvtzCVtl7I-NNJSb86JhPnmQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. The absence of DNAM-1 on donor cells attenuates acute GVHD. (A-D) 5 × 106 BM and 0.5 × 106 CD3+ T cells from B6.WT or B6.DNAM-1−/− donor mice were transplanted into lethally irradiated C3H recipients. Survival (A) and clinical score (B) are shown. P < .0001, B6.DNAM-1−/− vs B6.WT. *P < .05, B6.DNAM-1−/− vs B6.WT. Data shown are combined from 2 replicate experiments (n = 15 per T-cell–replete group and n = 7 in T-cell–depleted [TCD] control). (C,D) Tissues from skin, liver, and small intestine of C3-recipient mice were taken 20 days after transplantation. (C) Semiquantitative histopathology and (D) representative images (magnification ×250) from small intestine. Scale bars represent 100 μm. (n = 8 to 9 in T-cell–replete groups and n = 3 in TCD control). (E,F) 5 × 106 BM and (E) 5 × 106 or (F) 1 × 106 CD3+ T cells from B6.WT or B6.DNAM-1−/− donor mice was transplanted into lethally irradiated BALB/B recipients. (E) P = .0026, B6.DNAM-1−/− vs B6.WT. Survival data shown are combined from 2 replicate experiments (n = 15 per T-cell–replete group and n = 3 in TCD control). (F) ***P = .0002; **P < .008; *P < .02; B6.DNAM-1−/− vs B6.WT. Survival data shown are combined from 2 replicate experiments (n = 14 per T-cell–replete group and n = 5 in TCD control).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/17/10.1182_blood-2012-07-444026/4/m_3511f1.jpeg?Expires=1768771707&Signature=FwSBzGAZNh9fsUI96W3x606ixxo-kVKUCKT~monm7vnwvLuKHwkaBEzDIV4J5eXQIlhamfxdj4Rbyq9UjrT~rpVevnsbNFeC7Ci~bYqiF6Lrn0qgq6hUUuoRVF28LDNk8e~-WbQivfDWhFpw3ADioRukquY6OTaZ2HIM66rQDiURNx9A1emW-NYYlmShf~zymIyo7G4qgMxqa~vBBzeGuvPiGqgmVLMtEI5CNEVZ58d0zJGwUmTvlz-~IjQIQ4KsB8ZiMBLJQ1WCVJ09j6VJZ0K1Vn-iBBlnBPlph2EISztDC-PnT0MfTdkJ03vc-R8hVD~q89s772rR1mYd~NK2Mw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)