Abstract
The complex microRNA (miRNA) network plays an important role in the regulation of cellular processes such as development, differentiation, and apoptosis. Recently, the presence of cell-free miRNAs that circulate in body fluids was discovered. The ability of these circulating miRNAs to mirror physiological and pathophysiological conditions as well as their high stability in stored patient samples underlines the potential of these molecules to serve as biomarkers for various diseases. In this review, we describe recent findings in miRNA-mediated cell-to-cell communication and the functions of circulating miRNAs in the field of hematology. Furthermore, we discuss current approaches to design biomarker studies with circulating miRNAs. This article critically reviews the novel field of circulating miRNAs and highlights their suitability for clinical and basic research in addition to their potential as a novel class of biomarkers.
Introduction
Tissue-bound miRNAs classify hematological diseases
MicroRNAs (miRNAs) are 19 to 25–nucleotide-long, noncoding RNA molecules that were recently identified to play key roles in regulating gene expression by inhibiting translation and/or triggering degradation of target mRNAs.1 Already at their time of discovery, miRNAs such as lin-4 were found to coordinate the temporal transition of tissues between developmental stages.2 ,3 The results of extensive profiling approaches further supported the idea that miRNA expression patterns encode the developmental history of both human tissues and cancers.4 Despite the relatively small number of miRNA species, they seem to contain a large amount of diagnostic information. In a pivotal study, Lu et al4 demonstrated that miRNAs are able to discriminate leukemias of different origin. The potential of miRNAs to distinguish between acute leukemias was further emphasized by Mi et al,5 showing that 4 miRNAs (miR-223, miR-128a, miR-128b, and let-7b) were able to discriminate between acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). In fact, a combination of any 2 of these miRNAs could differentiate ALL from AML cases with an overall diagnostic accuracy of 97% to 99%. Subsequently, researchers further stratified lymphoid and myeloid disease entities according to cytogenetics, molecular genetics, and prognostic information based on their tissue-bound miRNA expression patterns6 -10 (see supplemental Table 1 on the Blood website).
Circulating miRNAs, from intracellular to extracellular and back
Recently, circulating miRNAs were discovered in a variety of body fluids11 -13 that can be obtained in a noninvasive or minimally invasive manner. Especially in blood, the levels and composition of circulating miRNAs were found to reflect the presence of malignant and nonmalignant diseases, even of localized tumors such as glioblastomas and lung cancer.14 ,15 Based on their origin, cell-free miRNAs are referred to in the current literature as, for example, blood-, serum- or plasma-borne. Here, we will refer to cell-free miRNAs as “circulating miRNAs” regardless of their origin or the body fluid they are derived from.
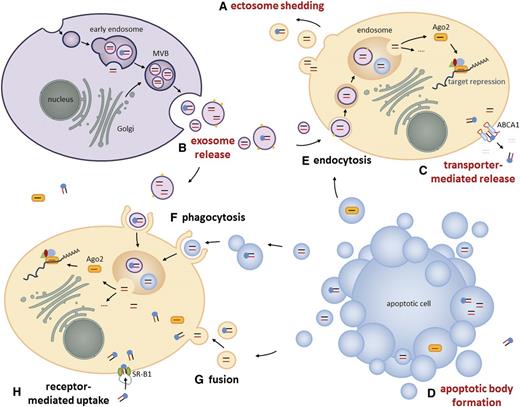
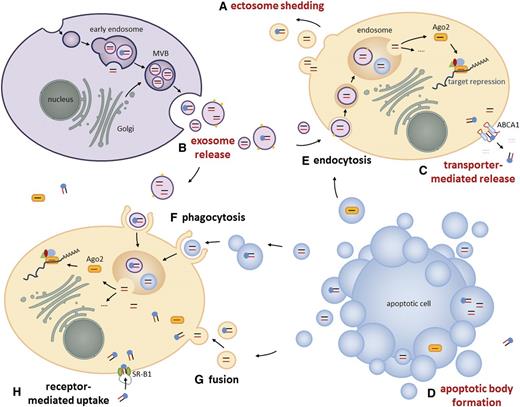
Due to their accessibility, the most common miRNA sources reported in literature are whole blood, serum, and plasma (Table 1). Despite the omnipresence of ribonucleases, miRNAs were found to be highly stable in blood and other body fluids. In fact, serum-borne miRNAs were documented to be stable for more than 10 days at room temperature and more than 10 years if stored at −20°C.16 Two distinct mechanisms have been shown to protect circulating miRNAs from degradation. First, miRNAs form complexes with proteins such as AGO-2,17 a component of the RNA-induced silencing complex, or NPM-1,18 which is involved in the biogenesis of ribosomes as well as with high-density lipoproteins (HDLs).19 Second, precursor miRNAs20 and mature miRNAs are packaged into small vesicles (Figure 1)21 ,22 that are either derived from the endosomal membrane compartment or shed directly from the plasma membrane17 ,23 (Figure 1A-B). Although it has been reported that the majority of miRNAs is present in both packaging types, there is growing evidence that extracellular miRNAs are sorted selectively either into vesicles24 -26 or into ribonucleoprotein complexes,17 ,27 and that the association of circulating miRNAs with the exosome or protein compartment is disease dependent.28 In 2012, Palma et al29 discovered that breast cancer cells selectively release miRNAs into anomalous vesicles, which are larger than conventional exosomes and exhibit metastasis-relevant surface markers such as CD44. The identification of such cancer-associated exosomes based on their expression of specific marker proteins like EpCAM, CD2430 or CD44 could possibly serve as a new diagnostic tool for minimal residual disease in solid tumors as well as hematological neoplasias.
Long-distance intercellular miRNA transfer. In addition to short-distance miRNA exchange, for example, via gap junctions or during immunological synapse formation,21 ,22 miRNAs can also be released by cells either into extracellular vesicles or complexed to proteins for long-distance communication. These packaged, mature miRNAs and pre-miRNAs are protected from extracellular ribonuclease activity and can be taken up by recipient cells, where they influence cellular processes. (A) During “plasma membrane budding,” ectosomes containing cytoplasmic components like miRNAs are released into the extracellular surrounding. (B) The fusion of exocytic multivesicular bodies (MVBs) with the plasma membrane releases miRNA-containing intraluminal vesicles called exosomes. When derived from healthy cells, exosomes reach a diameter of 60 to 90 nm. Exosomes released by cancer cells were reported to differ in size or to display metastasis-associated surface markers.29 ,30 (C) The ABCA1 transporter mediates the release of HDL-complexed miRNAs.19 (D) Apoptotic bodies are released from a cell that undergoes apoptosis. These large vesicles contain fragmented DNA and cytoplasmic components including miRNAs. General mechanisms of vesicle uptake in recipient cells involve (E) endocytosis, (F) phagocytosis, and (G) fusion with the plasma membrane. The uptake of HDL-complexed miRNAs in recipient cells is mediated by (H) SR-B1 receptors.19 Release and uptake mechanisms of extravesicular AGO- or NPM-1–complexed miRNAs are not described yet.
Long-distance intercellular miRNA transfer. In addition to short-distance miRNA exchange, for example, via gap junctions or during immunological synapse formation,21 ,22 miRNAs can also be released by cells either into extracellular vesicles or complexed to proteins for long-distance communication. These packaged, mature miRNAs and pre-miRNAs are protected from extracellular ribonuclease activity and can be taken up by recipient cells, where they influence cellular processes. (A) During “plasma membrane budding,” ectosomes containing cytoplasmic components like miRNAs are released into the extracellular surrounding. (B) The fusion of exocytic multivesicular bodies (MVBs) with the plasma membrane releases miRNA-containing intraluminal vesicles called exosomes. When derived from healthy cells, exosomes reach a diameter of 60 to 90 nm. Exosomes released by cancer cells were reported to differ in size or to display metastasis-associated surface markers.29 ,30 (C) The ABCA1 transporter mediates the release of HDL-complexed miRNAs.19 (D) Apoptotic bodies are released from a cell that undergoes apoptosis. These large vesicles contain fragmented DNA and cytoplasmic components including miRNAs. General mechanisms of vesicle uptake in recipient cells involve (E) endocytosis, (F) phagocytosis, and (G) fusion with the plasma membrane. The uptake of HDL-complexed miRNAs in recipient cells is mediated by (H) SR-B1 receptors.19 Release and uptake mechanisms of extravesicular AGO- or NPM-1–complexed miRNAs are not described yet.
Circulating miRNAs require specific considerations for their detection and quantification.
Whole blood vs plasma vs serum vs exosomes: what is the best source? Challenges in the analysis of circulating miRNAs include preanalytical decisions, such as sample storage, sample processing, the profiling method, and postanalytical decisions concerning, for example, data normalization. So far, a comparison of circulating miRNA levels among plasma, serum, and whole blood is still missing. However, a detailed analysis of miRNA spectra in serum and plasma was performed by Wang et al,31 who investigated potential biases with regard to the preparation of blood samples on miRNA quantification. In their analysis, they detected higher RNA concentrations in serum compared with the corresponding plasma samples.31 The authors31 suggest that this difference in concentration is attributed to the release of RNA during the coagulation process and is not caused by cell lysis, as the amount of blood-cell associated miRNAs such as miR-150, miR-16, and miR-126 did not change in their experiments. Based on these findings, the authors recommend plasma over serum to reduce sample-to-sample variations induced by serum coagulation. In contrast, van Schooneveld et al32 highlighted the advantages of serum for miRNA expression analyses, due to its more consistent and higher yield of small RNAs. Hence, the choice between plasma and serum for miRNA analysis may depend on more practical reasons, such as availability and handling. More recent approaches include the analysis of purified exosome-associated miRNAs (exosomal miRNAs). Considering the protein-complexed nature of circulating miRNAs,17 ,19 ,27 it does not seem generally advisable to focus on exosomal miRNAs for biomarker studies. However, exosomal miRNA profiling could turn out to be superior for detecting abnormalities in secretory cell types and for targeted analyses of cancer-related vesicles. Other issues in the choice of sample material concern the influence of red blood cell counts and hemolysis as well as the frequent presence of platelets in blood plasma and partly in serum samples upon miRNA quantification.31 ,33 -36 These problems can only be addressed through standardized protocols.
Current approaches and postanalytical processing to quantify circulating miRNAs.
The lack of standardized protocols for small RNA extraction combined with a growing variety of miRNA profiling approaches particularly complicates the comparison of independent data sets. In addition, postanalysis decisions such as data normalization have the potential to bias results and conclusions. An overview of currently employed methods reported in recent literature (2011-2012), which were used for miRNA extraction and quantification in serum, plasma, whole blood, and cerebrospinal fluid (CSF) and for data normalization, is shown in supplemental Table 2.
Despite reasonable enrichment of small RNAs by RNA extraction protocols, little is known about the composition of the extracted RNA. Do RNA subgroups behave differently in isolation protocols based on their size or sequence? This issue has been addressed recently by Kim et al,37 who demonstrated that small RNAs with low guanine-cytosine content are recovered inefficiently from small cell numbers in RNA extractions using TRIzol. In addition, theses authors found that some pre-miRNAs, small interfering RNAs, and transfer RNAs are also affected by this phenomenon.37 A possible explanation for this finding is that small RNAs need to base pair with carrier molecules such as longer RNAs for effective precipitation, thus resulting in a biased RNA extraction. It remains to be shown whether this is true for other situations, for example, affinity-based RNA extraction protocols using columns.
Because of its high sensitivity, fast readout, and universal application, quantitative realtime polymerase chain reaction (qRT-PCR) is widely used for miRNA quantification. Improvements of this technology include the incorporation of locked nucleic acids into the PCR primers, which further increased specificity and sensitivity and circumvented the need for input RNA preamplification due to enhanced base stacking, RNA backbone stabilization, and the balancing of differences in melting temperatures caused by variances in RNA guanine-cytosine content.38 Other approaches to quantify circulating miRNAs include next-generation sequencing (NGS) and hybridization-based methods such as miRNA microarray technologies, NanoString,39 or Scano-miR.40 Older methods like northern blot analysis are not suitable due to the necessity of high amounts of RNA input and the difficulty of accurately quantifying miRNAs. A shared limitation is the lack of absolute miRNA quantification41 ,42 and their insufficient comparability due to platform-dependent detection biases.41 ,43
In contrast to qRT-PCR and microarray technology, NGS allows the identification of novel miRNAs, miRNA sequence changes, and isomiRs.44 Notably, not all novel miRNAs discovered by NGS are indeed true miRNAs. For example, Jones et al45 proposed miR-720 and miR-1308 as tools to differentiate myeloma patients from healthy controls. However, in 2010, miR-720 was deleted from miRBase, a reference database for miRNAs, as miR-720 was validated to be a transfer RNA fragment.45 The major advantages and disadvantages of the currently used miRNA quantification methods are presented in supplemental Table 3 and were extensively reviewed in 2012 by Pritchard et al.46
A further challenge remains the bioinformatical processing of large miRNA expression data sets, including data normalization. The lack of endogenous normalization controls for circulating miRNAs, in particular, is still discussed controversially. Frequently used genes for normalizing qRT-PCR data include small nuclear RNAs like RNU6B or RNU43. However, these molecules are considered inadequate due to their low or varying abundance in body fluids47 -49 and a nonexcludable disease-specificity, as shown by Baraniskin et al for RNU2 in colorectal cancer.50 The ideal case for normalization and quantification of circulating miRNAs would be a universal housekeeping gene. Until such a gene (or RNA) is identified, fluid-specific housekeeping genes will have to be identified individually for each analysis. In general, all ubiquitously expressed miRNAs are candidates for normalization controls, although a systematic screen for endogenous controls in various body fluids has not yet been performed. Because of this drawback, many studies report the use of the mean expression value of all expressed miRNAs (global mean normalization) to normalize their data sets. This method has been reported to exhibit superior performance over the normalization with reference genes in large and unbiased profiling approaches.51 ,52
Current design of circulating miRNA biomarker studies
Numerous published biomarker studies (Table 253 -56 and supplemental Table 4) use a set of preselected miRNAs, often with known tissue-based expression, and correlate their circulating levels to healthy control vs disease or different disease subgroups. Regarding diffuse large B-cell lymphoma (DLBCL), Lawrie et al57 were the first to investigate whether circulating miRNAs can be detected in serum and if their expression levels differ between DLBCL patients and healthy individuals. In their retrospective study, they found deregulated levels of all 3 examined miRNAs (miR-155, miR-210, and miR-21), but only increased miR-21 levels were associated with an increased relapse-free survival. A similar study was published in 2012 by Fang et al,58 who confirmed elevated levels of miR-21 and miR-155 in DLBCL patients but could not demonstrate a significant association with any clinical parameters. It is not clear if the closed study design, the sample numbers, or an inhomogeneity of the profiled DLBCL samples led to different results.
Zuo et al59 measured 2 myelodysplastic syndrome (MDS)–related miRNAs, miR-16 and let-7a, in the plasma of 50 patients with MDS and showed that levels of these miRNAs had significant prognostic relevance. In a multivariate analysis, the authors demonstrated that reduced plasma levels of let-7a were an independent predictor for overall survival.59 Other studies also proposed the prognostic validity of serum and plasma miRNAs, for example, in multiple myeloma (MM),60 ,61 AML,62 and T-cell leukemia63 (see Table 2).
So far, the most comprehensive study of circulating miRNAs was published by Keller et al,14 who investigated the miRNA transcriptome of whole blood samples in 14 selected diseases, including multiple cancers, using miRNA microarrays.64 The resulting miRNA profiles allowed the identification of 2 miRNAs that discriminated between healthy controls and each profiled disease with an average accuracy of 88.5% or 72.5%, respectively. Despite the high discriminative potential, the predictive value of the total miRNome (the full complement of miRNAs in a genome) amounted on average to only 67.45%. Confounding variables for such an experimental approach in whole blood include existing known and unknown comorbidities and varying blood counts, which might distort disease-specific miRNA profiles.
But how do circulating miRNAs compare with established biomarkers? Can they complement existing markers to improve their sensitivity and specificity? These questions were partially addressed in a study by Moussay et al,65 who investigated the potential of circulating miRNAs as biomarkers in the plasma of patients with chronic lymphocytic leukemia (CLL) and compared their presence to the ZAP-70 status, which is an established clinical risk stratifier.66 A small set of 3 miRNAs, miR-195, miR-29a, and miR-222, allowed the separation of patients with CLL from healthy controls. Furthermore, a signature of 6 miRNAs (miR-29a, miR-483-5p, miR-195, miR-185, miR-135a*, and miR-15a) could segregate ZAP-70+ and ZAP-70− samples. In contrast, IgVH mutation status did not correlate with circulating miRNA levels, whereas plasma levels of miR-20a positively correlated with a longer time to treatment interval. These initial studies strengthen the concept of circulating miRNAs as potential biomarkers. However, to understand the connection of circulating miRNAs with the pathology of hematological diseases, comprehensive signatures that compare circulating and tissue-bound miRNA levels within the same individuals are necessary. Such prospective profiling approaches would not only identify disease-specific miRNA signatures, but they would create new opportunities to monitor treatment responses and identify early relapses. Nevertheless, to establish circulating miRNAs as biomarkers, well-designed clinical trials are required. So far, there is only 1 trial registered that is relevant to hematology and investigates circulating miR-155 as a predictive marker in acute graft-versus-host disease (clinicaltrials.gov identifier NCT01521039).
Functional aspects of circulating miRNAs in hematopoietic cells
The discovery of circulating miRNAs raised several questions about their function. Are these molecules mere degradation products without any functional relevance, or are they actively secreted to serve as messengers in cell-to-cell communication? Based on multiple studies, endothelial cells in particular seem to have the ability to communicate through miRNA trafficking in physiological and pathophysiological conditions. For instance, Zhang et al24 reported the active secretion of miR-150 by THP-1, a cell line derived from human acute myelomonocytic leukemia, as well as by plasma cells. Secreted miR-150 was taken up by cocultured human microvascular endothelial cells in which miR-150 regulated the protein level of c-MYB, a bona fide miR-150-target.24 A similar setup was used by Umezu et al,67 who visualized the transport of fluorescently labeled miR-92a via exosomes from K562, a human chronic myeloid leukemia cell line, to human umbilical vein endothelial cells (HUVECs). This led to the repression of miR-92a targets like integrin α5 in the recipient cells.67 A very different mechanism of cellular miRNA communication was recently identified by Fabbri et al,68 who showed that 2 tumor-secreted exosomal miRNAs, miR-21 and miR-29b, can bind and thereby activate Toll-like receptors on immune cells. This activation triggered an inflammatory response in the recipient cells and ultimately resulted in a benefit for tumor growth and metastasis in vivo.68 These and similar studies69 -78 (Table 3) indicate that exosome-shuttled miRNAs can serve as in vivo messengers that are able to regulate signaling pathways in recipient cells and might represent a novel platform for tumor cells to modulate their environment.
Regarding cell-to-cell transfer of protein-complexed miRNAs, Vickers et al19 demonstrated that cellular release of HDL-complexed miRNAs is mediated by the adenosine triphosphate–binding cassette transporter A1 (ABCA1) (Figure 1C) and that their uptake is guided by the scavenger receptor class B type I (SR-BI) (Figure 1H). Furthermore, they could show that HDL can carry distinct miRNA populations depending on the health status of the tested individual and that HDL-associated miRNA profiles significantly differ from exosome-associated miRNAs.19 Another class of microvesicles that is involved in miRNA trafficking represent apoptotic bodies, which derive from the cellular membrane of apoptotic cells (Figure 1D) and contain cytoplasmic compounds such as miRNAs. Zernecke et al79 were the first to show that miR-126–enriched apoptotic bodies shed by endothelial cells are taken up by neighboring vascular cells, where they promote proliferation as well as production of CXCL12, a chemokine known to counteract apoptosis. Similarly, the relevance of miRNA trafficking in the bone marrow compartment was highlighted by Salvucci et al,80 who demonstrated that granulocyte colony-stimulating factor promotes the accumulation of miR-126–containing microvesicles in the bone marrow and thereby targets the expression of surface vascular cell adhesion molecule-1 on early hematopoietic cells.80 This novel regulatory layer underlines the growing impact of circulating miRNAs on stem cell physiology.
Naturally, the concept of circulating miRNAs inspired new therapeutic approaches such as the use of nanotechnology-based carrier strategies to enrich or antagonize miRNAs in diseased cells.81 Both strategies proved to be effective in different murine lymphoma models.82 ,83 However, both the clinical administration of exosomes84 and the drug-induced inhibition of exosomal release68 ,85 from, for example, leukemia cells remain highly speculative.
It is a fascinating idea that cells can trigger or repress pathways in recipient cells via miRNAs during or even after cell death. Whether similar transport mechanisms account for the exchange of circulating miRNAs between the blood-brain and the blood-CSF barriers, as recently shown for central nervous system lymphomas,86 still needs to be investigated. Despite all the novel insights in this field, it is not yet clear whether cell-mediated miRNA transfer is a highly specialized fine-tuning mechanism or a substantial pathway and whether it can be targeted therapeutically to counter, for example, leukemogenesis.
Conclusion
Despite the recognized potential of circulating miRNAs as biomarkers, only little is known about their origin in physiological and pathological conditions or about the factors that influence their levels and composition. Circulating miRNAs are profiled in an increasing number of diseases and body fluids, but to introduce them as biomarkers into clinical routine, carefully conducted clinical studies are needed. Importantly, preanalytic factors such as the collection, processing, and storage of samples have to be monitored and standardized (supplemental Tables 2 and 3). Although the idea of functional circulating miRNAs is intriguing, their relevance is still a matter of debate. It remains to be seen whether circulating miRNAs are mere fine-tuners or central players in hematological diseases, a question that functional studies only recently started to focus on. However, judged by their advantageous properties and the continuously increasing amount of studies, circulating miRNAs have the potential to become reasonable diagnostic tools once their infancy has passed.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by grants from Deutsche Krebshilfe (109420), fellowship 2010/04 from the European Hematology Association, and grants from Deutsche Forschungsgemeinschaft (D.3955 (SFB 1074)).
Authorship
Contribution: S.G., A.S., C.L., C.B., H.D., D.M., and F.K. wrote the paper.
Conflict-of-interest disclosure: A.S. is employed by Exiqon. The remaining authors declare no competing financial interests.
Correspondence: Florian Kuchenbauer, University of Ulm, Albert-Einstein-Allee 23, 89081 Ulm, Germany; e-mail: florian.kuchenbauer@uni-ulm.de.