Abstract
Comorbidites play a crucial role in the prognosis, treatment and outcomes of patients with hematological malignancies. We have previously reported the impact of comorbidities independent of age and IPSS score (1). The aim of this study was to determine whether comorbidities continued to have a similar impact when patients were reclassified according to the Revised-International Prognostic Scoring System (RIPSS).
We reviewed the medical records of 600 consecutive MDS patients who presented to MD Anderson Cancer Center from January 2002 to June 2004. The Adult Comorbidity Evaluation-27 (ACE-27), a validated 27-item comorbidity index for cancer patients (2), was used to assess the severity of comorbid conditions. For each patient, we obtained demographic data and specific staging information based on the R-IPSS. We also collected information on stem cell transplantation (SCT), mortality and survival. Kaplan-Meier methods and log-rank tests were used to assess survival.
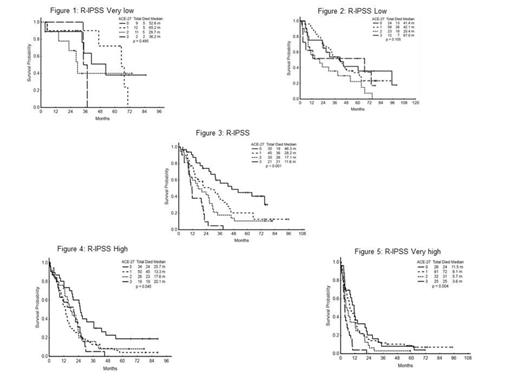
Of the 600 patients included in this study, 402 (67%) were male, and 517 (86%) were white; median age at presentation was 66 years (17 – 94); mean duration of follow-up was 54 months (1 - 100). A total of 34, 117, 131, 129, 165 patients were R-IPSS very low, low, intermediate, high and very high respectively. The ACE-27 comorbidity scores were as follows: none, 137 patients (23%); mild, 254 (42%); moderate, 127 (21%); and severe, 82 (14%). 476 (79%) patients died, and 54 (9%) patients underwent SCT. Overall median survival using the Kaplan-Meier method was 16 months (1 – 100). Median survival according to ACE-27 scores was: 28 months for no comorbidity, 16 months for mild comorbidity, 14 months for moderate comorbidity, and 9 months for severe comorbidity with a P-value of P<0.001. The median survival by R-IPSS was 47 , 34, 21, 16, 6 months for patients in very low, low, intermediate, high and very high risk groups respectively (P<0.001). Of the 54 patients who had SCT, 27 (50 %) died. The median survival of patients who did not undergo stem cell transplantation was 23, 16, 4, 8 months for patients with no, mild, moderate and severe comorbidity scores, respectively (P<0.001). The ACE-27 comorbidity score significantly impacted the median OS of patients in the intermediate (P<0.001) , high (P=0.045) , and very high (P=0.004) R-IPSS groups; but did not significantly impact the median OS in the low (P=0.11) and very low (P=0.49) groups. See figures 1 – 5 below. The ACE-27 comorbidity score significantly impacted the median OS of patients </=65 years (P<0.001) but did not significantly impact those >65 years (P=0.18).
Comorbidities had a significant impact on the survival of patients with myelodysplastic syndrome, especially among those with more advanced disease and younger age (</= 65). Among the intermediate, high and very high R-IPSS groups the patients with higher ACE-27 comorbidity scores had a significantly shorter survival than those with no comorbidities. The comorbidity scores did not significantly impact survival in the R-IPSS very low and low groups, which may reflect the improved survival and limited number of events that occur in these favorable subsets. Perhaps with longer follow-up a difference in survival may emerge among the favorable R-IPSS subsets. A comprehensive assessment of comorbidity is therefore needed to determine the prognosis in patients with MDS.
References
1. Naqvi K, Garcia-Manero G, Sardesai S. Association of comorbidities with overall survival in myelodysplastic syndrome: Development of a prognostic mode. J Clin Oncol. 2011;29(16):2240-6.
2. Piccirillo JF, Tierney RM, Costas I, et al. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441-47.
Ravandi:Sunesis: Consultancy, Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Teva: Consultancy, Honoraria; Pfizer: Honoraria; Merck: Research Funding; Bayer/Onyx: Consultancy, Honoraria; EMD Serono: Research Funding; Medimmune: Research Funding; Amgen: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria. Garcia-Manero:Novartis Pharmaceutical: Research Funding. Kantarjian:Sanofi-Aventis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.