Key Points
Rituximab prevents steroid-requiring chronic graft-vs-host disease when given after peripheral blood stem cell transplantation.
Overall survival is improved with rituximab after allogeneic peripheral blood stem cell transplantation when compared with a control cohort.
Abstract
B cells are implicated in the pathophysiology of chronic graft-vs-host disease (GVHD), and phase 2 trials suggest that B cell depletion can treat established chronic GVHD. We hypothesized that posttransplantation B cell depletion could prevent the occurrence of chronic GVHD. We performed a 65-patient phase 2 trial of rituximab (375 mg/m2 IV), administered at 3, 6, 9, and 12 months after transplantation. Rituximab administration was safe without severe infusional adverse events. The cumulative incidences of chronic GVHD and systemic corticosteroid-requiring chronic GVHD at 2 years from transplantation were 48% and 31%, respectively, both lower than the corresponding rates in a concurrent control cohort (60%, P = .1, and 48.5%, P = .015). There was no difference in relapse incidence, but treatment-related mortality at 4 years from transplantation was significantly lower in treated subjects when compared with controls (5% vs 19%, P = .02), and overall survival was superior at 4 years (71% vs 56%, P = .05). At 2 years from transplantation, the B-cell activating factor/B-cell ratio was significantly higher in subjects who developed chronic GVHD in comparison with those without chronic GVHD (P = .039). Rituximab can prevent systemic corticosteroid-requiring chronic GVHD after peripheral blood stem cell transplantation and should be tested in a prospective randomized trial. This trial was registered at www.clinicaltrials.gov as NCT00379587.
Introduction
Chronic graft-versus-host disease (GVHD) is the leading cause of late morbidity, impaired quality of life, and mortality after allogeneic stem cell transplantation.1-3 Efforts to pharmacologically prevent chronic GVHD by extending the period of immunosuppression after allogeneic transplantation have not been successful.4,5 The use of T-cell depletion, either with in vivo (polyclonal or monoclonal antibody therapy)6-8 or ex vivo (T-cell depletion or CD34+ selection)9 methodologies has been shown to prevent chronic GVHD; however, this has not been associated with an improvement in overall survival because of excess mortality associated with opportunistic infections and possibly malignant disease relapse. Finally, allogeneic tolerance induction with the use of posttransplantation cyclophosphamide has been shown to prevent chronic GVHD, but long-term outcomes have not been compared with traditional GVHD prevention strategies.10
Because prolongation of calcineurin inhibition after transplantation does not prevent the occurrence of chronic GVHD, alternative, non-T-cell–dependent pathways that can lead to alloreactivity can be implicated in the pathogenesis of chronic GVHD in some patients. B-cell-dependent processes have thus been implicated following several lines of evidence: antibodies against minor histocompatibility antigens have been associated with the occurrence of chronic GVHD11 ; B-cell depletion in the peritransplantation period has been correlated with a reduction in chronic GVHD incidence12 ; and most important, B-cell–depletion therapy with rituximab is effective in the therapy of established chronic GVHD.13-19 In addition, murine models of chronic GVHD and bronchiolitis obliterans have implicated donor B-cell alloantibodies in the pathogenesis of this disease.20
Much work has focused on the potential role of B cells in pathobiology of chronic GVHD. It is known that B-cell reconstitution in patients with chronic GVHD is delayed, and these patients have elevated plasma B-cell activating factor (BAFF) to B-cell ratios.21 Altered B-cell homeostasis in chronic GVHD is associated with persistence of circulating, potentially autoreactive, B cells.21,22 Further supporting a mechanistic role for B cells in human chronic GVHD are studies demonstrating altered signaling through the BAFF-associated and B-cell-receptor–associated pathways.23,24
Recently, in a small series of patients with aggressive B-cell malignancies treated with rituximab in the early posttransplantation period, a reduction in the rate of chronic GVHD was noted25 ; however, patients were treated using a preparative regimen that traditionally is associated with low rates of chronic GVHD.26 Because chronic GVHD occurs more frequently after peripheral blood stem cell transplantation,27,28 we conducted a phase 2 trial of rituximab given specifically for the prevention of chronic GVHD after allogeneic peripheral blood stem cell transplantation.
Methods
This was a prospective, open-label, phase 2 trial of prophylactic rituximab given to prevent the occurrence of chronic GVHD after allogeneic stem cell transplantation. The clinical trial was approved by the Office for Human Research Studies at the Dana-Farber Cancer Institute/Harvard Cancer Center and was registered at www.clinicaltrials.gov (NCT00379587). Genentech (San Francisco, CA) provided rituximab for all enrolled subjects. Informed consent was obtained in accordance with the Declaration of Helsinki.
The primary objectives of the trial were to determine the incidence of chronic GVHD and corticosteroid-requiring chronic GVHD at 1 and 2 years after allogeneic stem cell transplantation. Corticosteroid-requiring chronic GVHD was added as an end point after the completion of the trial, but before data analysis. Secondary objectives were to determine the incidence of hematological and nonhematological adverse events when rituximab was administered in the posttransplantation setting. Eligible patients were 18 years of age or greater and received a nonmyeloablative or myeloablative transplantation using peripheral blood stem cells from an 8/8 HLA-matched donor or single antigen/allele-mismatched donor approximately 100 days prior to enrollment. Adequate performance status and organ function was required, and subjects were free of uncontrolled infection and active acute GVHD at the time of enrollment. Prior resolved acute GVHD was permitted. Subjects were required to have evidence of sustained donor chimerism and underwent disease restaging to exclude relapse at the time of enrollment. Subjects participating in clinical trials of primary GVHD prophylaxis in which chronic GVHD was a secondary end point were not enrolled. Any subject with evidence of classic chronic GVHD or overlap chronic GVHD was ineligible to participate, as was any subject who had previously received donor lymphocyte infusions to treat relapse or falling donor chimerism.
Rituximab (375 mg/m2 IV) was administered at 100 days, and again at 6, 9, and 12 months after transplantation. Premedication with H1- and H2-blockers and acetaminophen was provided. Corticosteroids were not used as premedication. Systemic immunosuppressive medications were tapered at the treating clinician’s discretion per institutional standards. Upon the occurrence of chronic GVHD, initial and subsequent immunosuppressive treatment was administered according to institutional standards as well.
Correlative studies for B-cell reconstitution and BAFF levels
A commercially available sandwich enzyme-linked immunosorbent assay (ELISA) was used in order to measure soluble BAFF/Blys levels from patient plasma (R&D Systems, Minneapolis, MN). Whole blood or viable frozen ficolled peripheral blood mononuclear cells were processed for flow cytometry. Lymphocytes were gated by size using forward and side scatter criteria. A total of 50 000 lymphocytes were collected for all samples to ensure adequate total numbers of B cells for subset analysis. Anti-CD19 PC7 (Beckman Coulter, Brea, CA) was used per manufacturer’s instructions. CD19 expression was analyzed to exclude CD3+ cells from the analysis of CD38 or CD27 expression. Red blood cells (RBCs) were lysed, and leukocytes were fixed prior to fluorescence-activated cell sorter (FACS). A MACSQuant Analyzer (Miltenyi Biotech, Auburn, CA) was used, and data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Statistical design and analysis
Because of the differences in the incidence rates of chronic GVHD after matched, related donor transplantation and matched, unrelated donor transplantation, this study was designed to accrue these 2 cohorts separately but in parallel, with the null hypothesis of a rate of chronic GVHD of 45% after matched, related transplantation and 65% after matched, unrelated transplantation. The alternative hypothesis was a 20% reduction with the use of rituximab in both cohorts. With this hypothesis, using an exact binomial distribution, the probability of concluding rituximab promising was 0.88 if the true but unknown rate of developing chronic GVHD is 25% and 0.09 if the true rate is 45% in matched, related donor transplantation and 0.86 if the true but unknown rate of developing chronic GVHD is 45% and 0.10 if the true rate is 65% in matched, unrelated transplantation. These statistical parameters do not apply to the estimates of corticosteroid-requiring chronic GVHD.
Although not part of the original statistical design, a contemporaneous control cohort was constructed from our database using patients treated during the same time interval who chose not to participate in this trial. Control patients were selected randomly from the database using the identical selection criteria as for the clinical trial population. A similar proportion of recipients of matched, related and unrelated donors as accrued in the prospective trial was purposefully chosen for comparison.
Baseline characteristics were reported descriptively, and compared using the χ2 test, Fisher’s exact test, or Wilcoxon rank-sum test. Overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier method. OS was defined as the time from stem cell infusion to death from any cause. Patients who were alive or lost to follow-up were censored at the time last seen alive. PFS was defined as the time from stem cell infusion to disease relapse or progression or death from any cause, whichever occurred first. Patients who were alive without disease relapse or progression were censored at the time last seen alive and progression-free. The log-rank test was used for comparisons of Kaplan-Meier curves. The cumulative incidence of grade 3 or higher infections was calculated considering death unrelated to infections and relapse as competing events. Cumulative incidences of chronic GVHD and systemic corticosteroid-requiring chronic GVHD were calculated using competing risks reflecting time to relapse or death without developing chronic GVHD or corticosteroid-requiring chronic GVHD as competing events. Late acute GVHD was not considered to be chronic GVHD unless overlap features were present. Similarly, cumulative incidences of nonrelapse death and relapse with or without death were calculated using competing risks, reflecting time to relapse and time to nonrelapse death, respectively, as competing risks. The difference between cumulative incidence curves in the presence of a competing risk was tested using the Gray method.29 Potential prognostic factors for OS, PFS, and corticosteroid-requiring chronic GVHD were examined in the proportional hazards model as well as in the competing risks regression model.30 Factors included in the multivariable models were age, patient–donor sex mismatch, myeloid disease (Y/N), donor type, conditioning intensity, disease relapse index,31 and grades II-IV acute GVHD. The occurrence of grade II-IV acute GVHD was treated as a time-dependent variable in multivariable analysis. The proportional hazards assumption was tested, and interaction terms were examined. All tests are 2-sided at a significance level of 0.05. All calculations were done using SAS 9.3 (SAS Institute, Inc., Cary, NC) and R version 2.13.2 (the Comprehensive R Archive Network [CRAN] project [http://cran.us.r-project.org/]).
Results
Between December 2006 and October 2010, 65 subjects were enrolled and treated on this clinical trial. The data set was locked and analyzed in March 2012, when all subjects had at least 2 years of posttransplantation follow-up, with a median follow-up of 48 months.
The median age of enrolled subjects was 54 years, and 31 subjects received peripheral blood stem cell grafts from matched, related donors. One subject received a 7/8 unrelated donor transplant and was analyzed with the 8/8 matched, unrelated donor recipient group (n = 33). Forty of 65 subjects (61.5%) had undergone reduced-intensity conditioning, and acute leukemia was the most common diagnosis (52.3%), with advanced B-cell malignancies accounting for 32.3% of enrolled subjects. All subjects received peripheral blood stem cells, but only 13.8% had grades II-IV acute GVHD. Only 2 subjects received anti-thymocyte globulin as part of their conditioning regimen (Table 1).
Safety and adverse events
A total of 181 doses of rituximab were administered, with a median of 3 doses per subject. The reasons to not receive the complete 4 doses included the development of chronic GVHD (18), relapse (13), and patient preference (2). Thirty-two subjects completed all 4 doses of rituximab, including some individuals who had developed chronic GVHD during the first year after transplantation.
There were no grades 3-4 events associated with rituximab infusion. There were 2 reports of grades 3-4 thrombocytopenia considered possibly or probably related to therapy with rituximab. Leukopenia or neutropenia considered possibly or probably related to therapy with rituximab was reported in 9 subjects and was managed with growth factor where appropriate. Other toxicities are shown in Table 2. Hypogammaglobulinemia was not recorded as an adverse event, and subjects were permitted to receive replacement therapy outside of the transplantation center. Ten patients experienced 15 episodes of grade 3 or higher infections. The 1- and 2-year cumulative incidence of grade 3 or higher infections was 11% and 15%, respectively. There were 2 lethal infections, with 1 being considered possibly related to rituximab.
Incidence of chronic GHVD
The cumulative incidence of clinician-diagnosed chronic GVHD at 1 and 2 years after transplantation was 38% and 48%, respectively. The corresponding figures for related and unrelated donor recipients were 29% and 35% for related donors, and 47% and 59% for unrelated donors, which were significantly different (P = .046) (Figure 1A-B).
Chronic GVHD incidence. (A) Overall incidence of chronic GVHD. Dashed line, incidence of all chronic GVHD; solid line, incidence of corticosteroid-requiring chronic GVHD. (B) Incidence of chronic GVHD stratified by donor type. Dotted line, incidence of all chronic GVHD, matched related donors; solid line, incidence of corticosteroid-requiring chronic GVHD, matched related donors; dot-dash line, incidence of all chronic GVHD, matched unrelated donors; dashed line, incidence of corticosteroid-requiring chronic GVHD, matched unrelated donors.
Chronic GVHD incidence. (A) Overall incidence of chronic GVHD. Dashed line, incidence of all chronic GVHD; solid line, incidence of corticosteroid-requiring chronic GVHD. (B) Incidence of chronic GVHD stratified by donor type. Dotted line, incidence of all chronic GVHD, matched related donors; solid line, incidence of corticosteroid-requiring chronic GVHD, matched related donors; dot-dash line, incidence of all chronic GVHD, matched unrelated donors; dashed line, incidence of corticosteroid-requiring chronic GVHD, matched unrelated donors.
We measured the requirement of initiation of systemic corticosteroids as a surrogate for chronic GVHD severity. The cumulative incidence of systemic corticosteroid-requiring chronic GVHD was only 21.5% and 31% at 1 and 2 years from transplantation, respectively. The corresponding figures at 1 and 2 years were 16% and 23% for related donors, and 26% and 38% for unrelated donors (P = .17) (Figure 1A-B). At 1 year from transplantation, 19 subjects (5 related donors, 14 unrelated donors) were using systemic corticosteroids, and 47.7% of subjects were using some form of systemic immunosuppression (38.7% related donors, 55.9% unrelated donors).
Mucosal chronic GVHD, with ocular and oral involvement, was the most common manifestation of chronic GVHD (24.5% and 22.6% of subjects, respectively). Because there was much less systemic corticosteroid use in these patients, it appeared that mucosal GVHD was not considered severe enough to warrant corticosteroids. Notably, both cutaneous and myofascial disease, which have previously been reported to be responsive to rituximab therapy,13 was noted only in 13.2% and 5.7% of subjects, respectively.
Immunophenotypic correlations
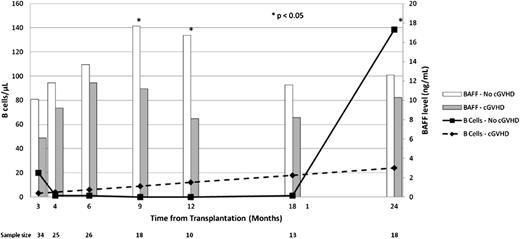
B-cell lymphopenia was profound, and B cells were unmeasurable during the year following rituximab administration in almost all treated subjects. At 18 months from transplantation (6 months following the final rituximab administration), B cells remained essentially absent, but by 24 months from transplantation, B-cell reconstitution was noted. At 24 months from transplantation, the median number of B cells in treated patients who did not develop chronic GVHD was 138.5 cells/μL in comparison with only 9.9 cells/μL in treated patients with chronic GVHD (P = .047) (Figure 2). Serum BAFF levels were not different between patients who eventually did and did not develop chronic GVHD at study entry, and at months 4 and 6 following transplantation (1 and 3 months following initial rituximab administration). BAFF levels were significantly higher in chronic GVHD-free patients at 9 and 12 months from transplantation (17.7 ng/mL vs 11.2 ng/mL, P = .033; 16.7 ng/mL vs 8.1 ng/mL, P < .01, respectively); however, there was no difference at 18 and at 24 months, at which point B cells had reconstituted (Figure 2). Consistent with prior observations,21 the BAFF/B-cell ratio was significantly higher in chronic GVHD patients than in chronic GVHD-free patients at 24 months (P = .039).
Immunophenotypic outcomes. Bar graph shows median BAFF levels. Open bar, no chronic GVHD; shaded bar, chronic GVHD. Line graph shows median B-cell numbers. Dashed line, chronic GVHD; solid line, no chronic GVHD.
Immunophenotypic outcomes. Bar graph shows median BAFF levels. Open bar, no chronic GVHD; shaded bar, chronic GVHD. Line graph shows median B-cell numbers. Dashed line, chronic GVHD; solid line, no chronic GVHD.
Relapse and survival
The median follow-up among surviving patients was 48 months (range, 26 to 77 months). The 4-year cumulative incidence of relapse was 34%. There was no difference in relapse between CD20+ malignancies and CD20− malignancies in rituximab-treated subjects (32% vs 36%). Nonrelapse mortality, an additional surrogate of chronic GVHD severity, was very low, with only 2 subjects dying of nonrelapse-related causes at 2 years from transplantation with a cumulative incidence of 5.1% at 4 years from transplantation. The 4-year estimates of PFS and OS were 61% and 71%, respectively.
Comparison with control cohort
After completion of the trial, a contemporaneous control cohort was constructed from our database using patients treated during the same time interval who chose not to participate in this trial. Baseline clinical characteristics of the control cohort can be found in Table 1. On the basis of selection criteria, there were no significant differences in age, sex, donor sex or match, malignant diagnosis, stem cell source, conditioning intensity, or the occurrence of acute GVHD.
Although there was a trend toward a reduction in the overall rate of chronic GVHD when comparing rituximab-treated cases and controls (48% vs 60%, P = .1), the 2-year cumulative incidence of systemic corticosteroid-requiring chronic GVHD was 31% among rituximab-treated subjects and 48.5% among controls (P = .015) (Figure 3A). In a multivariable competing risks regression analysis, treating acute GVHD as a time-dependent variable, the use of rituximab (hazard ratio, 0.47; 95% confidence interal [CI], 0.23-0.99; P = .046) and myeloid disease (hazard ratio, 0.37; 95% CI, 0.17-0.79; P = .01) was significantly associated with protection from corticosteroid-requiring chronic GVHD, whereas age, donor-recipient sex matching, donor type, conditioning intensity, disease relapse index, and acute GVHD had no statistically significant influence.
Comparison of rituximab-treated cases and controls. (A) Incidence of corticosteroid-requiring chronic GVHD. Solid line, incidence of corticosteroid-requiring chronic GVHD, rituximab-treated cases; dashed line, incidence of corticosteroid-requiring chronic GVHD, untreated controls. (B) Nonrelapse mortality and disease recurrence. Dotted line, incidence of relapse, rituximab-treated cases; solid line, incidence of nonrelapse mortality, rituximab-treated cases; dot-dash line, incidence of relapse, untreated controls; dashed line, of nonrelapse mortality, untreated controls. (C) Overall survival. Solid line, rituximab-treated cases; dashed, untreated controls.
Comparison of rituximab-treated cases and controls. (A) Incidence of corticosteroid-requiring chronic GVHD. Solid line, incidence of corticosteroid-requiring chronic GVHD, rituximab-treated cases; dashed line, incidence of corticosteroid-requiring chronic GVHD, untreated controls. (B) Nonrelapse mortality and disease recurrence. Dotted line, incidence of relapse, rituximab-treated cases; solid line, incidence of nonrelapse mortality, rituximab-treated cases; dot-dash line, incidence of relapse, untreated controls; dashed line, of nonrelapse mortality, untreated controls. (C) Overall survival. Solid line, rituximab-treated cases; dashed, untreated controls.
The 4-year cumulative incidence of nonrelapse mortality was 19% in the control population, in comparison with 5.1% in rituximab-treated subjects (P = .02). Of 4 subjects who died of nonrelapse causes in the rituximab cohort, the causes of death are chronic GVHD (3) and respiratory failure (1). Of 13 patients who died of nonrelapse causes in the control cohort, the causes of death are chronic GHVD (8) and cardiac, traumatic, secondary malignancy, respiratory failure, and unknown (1 each). Of these 13, 10 developed chronic GVHD prior to death. The 4-year cumulative incidence of relapse was no different between rituximab-treated patients and controls (34% vs 28%, P = .79) (Figure 3B), even when stratified by CD20 status. PFS at 4 years was no different (61% vs 53%, P = .27); however, 4-year OS was significantly higher in rituximab-treated subjects when compared with controls (71% vs 56%, P = .05) (Figure 3C). In a multivariable Cox regression analysis examining the same potential variables as for the occurrence of steroid-requiring chronic GVHD, the use of rituximab (hazard ratio for death, 0.56; 95% CI, 0.31-1.00; P = .048) and high or very high disease relapse index (hazard ratio for death, 1.90; 95% CI, 1.02-3.53; P = .04) were significantly associated with OS.
Discussion
Several lines of evidence implicate B cells in the pathophysiology of chronic GVHD.32,33 In this clinical trial, we have demonstrated that the posttransplantation administration of rituximab is safe and reduces the incidence of systemic corticosteroid-requiring chronic GVHD when compared with concurrent controls. Although the overall rate of chronic GVHD was only modestly affected and did not attain the 20% reduction hypothesized, the majority of patients treated with prophylactic rituximab who were affected had only mucocutaneous disease that did not require systemic therapy and was not associated with excess transplant-associated mortality. In fact, we noted an extremely low rate of transplant-associated mortality of 5% at 4 years from transplantation in a landmark analysis of patients who were enrolled in this clinical trial 3 months after transplantation.
This clinical trial did not use the National Institutes of Health (NIH) Staging System for the diagnosis and severity assessment of chronic GVHD because the trial was conceived before the NIH diagnostic system was initially published,34 and long before this staging system was validated.35-37 Similarly, the recommendations from the NIH Consensus Conference for the conduct of clinical trials in chronic GVHD was not published until after the initiation of this trial.38 Faced with the lack of a standardized and recognized marker of chronic GVHD severity, we chose 2 end points to measure the efficacy of our intervention: the occurrence of any-degree chronic GVHD by clinician assessment, and the requirement of systemic corticosteroids specifically to treat chronic GVHD, as a surrogate for chronic GVHD severity. We felt this latter end point was valid because chronic GVHD involving mucocutaneous tissues treated with topical therapy alone generally does not impact posttransplantation survival. In acute GVHD clinical trial designs in which corticosteroid-sparing strategies are being tested, it is recommended that corticosteroid doses be incorporated into the outcome assessment, providing further justification for this end point.39 It should be noted that this end point is not entirely objective and may be difficult to use in phase 3 clinical trials. As such, in addition to corticosteroid use, we measured nonrelapse mortality as a marker of prophylaxis efficacy. This measurement captures adverse outcomes related to the occurrence of chronic GVHD as well as adverse outcomes as a result of the therapy given to prevent or treat chronic GVHD, and is not subjective, as is the initiation of systemic corticosteroids. Another potential measure of chronic GVHD severity proposed around the time of this trial concept incorporates the composite outcomes of death, disability, recurrent infections, or prolonged hospitalizations and suicidal ideation as a marker for chronic GVHD severity; however, this outcome measurement is not commonly used.40
We compared outcomes in subjects treated in this trial with those of a contemporaneous control population chosen to match the entry criteria for this clinical trial. We specifically chose a similar proportion of related and unrelated donor recipients, and included several patients who received ATG as part of their GVHD prophylaxis regimen. Although we did not note any differences in baseline characteristics between enrolled subjects and control patients, bias cannot be completely excluded, and only a prospective randomized trial can adequately compare outcomes after rituximab administration to outcomes in the absence of rituximab administration. Survival outcomes in both rituximab and control populations were better than anticipated because they represent landmark analyses of patients who survived to day 100 and were eligible to participate in a clinical trial. It is possible that control patients were offered participation in this clinical trial but chose not to participate because of unmeasured morbidity precluding participation.
We empirically chose the timing and duration of rituximab administration in this study, with a goal of inducing long-lasting B lymphopenia prior to the robust recovery of B cells after transplantation. Repeated dosing was given on the basis of the reported half-life of rituximab to ensure B lymphopenia during the first year after transplantation. Other experiences have demonstrated the safety of even earlier administration of rituximab after transplantation. When given prophylactically as early as day +5 after transplantation to prevent Epstein-Barr virus (EBV) reactivation in T-cell–depleted transplantation, no effect on chronic GVHD incidence was apparent, because only donor B cells delivered with the graft were affected, and not donor-derived B cells that were generated upon immunologic recovery.41 Others have also demonstrated cytopenias commonly when rituximab was administered early after T-cell–depleted transplantation.19,25 In addition, we did not note excess opportunistic infections despite persistent hypogammaglobulinemia, which was treated at investigator discretion. Because rituximab administration ended at 12 months from transplantation, B-cell recovery occurred in the majority of patients between 18 months and 2 years after transplantation. We noted an ongoing incidence of chronic GVHD during year 2 from transplantation, coinciding with ongoing B-cell recovery, suggesting that prolonged rituximab administration during year 2 after transplantation or longer may be required to fully prevent B-cell-mediated chronic GVHD, or that non-B-cell–dependent processes are involved in later chronic GVHD.
One third of the subjects enrolled in our trial had high-risk B-cell malignancies. There was likely biased enrollment for these patients, because posttransplantation rituximab has been used in an attempt to prevent malignant disease relapse in B-cell malignancies. One concern was that the elimination of chronic GVHD would be associated with loss of a graft-vs-malignancy effect and an increase in malignant relapse. We did not note an excess incidence of malignant relapse after the administration of rituximab when compared with untreated controls, suggesting that the graft-vs-malignancy effect is more dependent on T-cell–mediated processes, or involves antigen-presenting cells that are not of B-cell lineage, or do not express CD20.
The mechanism through which B cells interact with and control T-cell responses is complex and has not been elucidated in chronic GVHD. B cells can participate in priming and maintaining antigen-specific CD4+ T-cell responses,42 so B-cell depletion could be associated with antigen-specific T-cell anergy, although other antigen-presenting cells are involved in productive immune responses as well. An additional mechanism potentially involved in prevention of chronic GVHD is the expansion of regulatory T cells, a phenomenon that has been noted in association with B-cell depletion in autoimmune diseases,42 although we did not note an expansion of regulatory T cells in this study (data not shown). Finally, regulatory B cells—a poorly immunophenotypically characterized, IL-10-secreting B-cell subset—may be instrumental in suppressing immune reactions.43 At least one study has shown that these B cells express CD20,44 suggesting that codepletion of conventional and regulatory B cells can prevent chronic GVHD. Further studies will be required to better understand the phenotype of B cells that recover in chronic GVHD, and in chronic GVHD-free subjects, and it is possible that regulatory B cells have different recovery kinetics than do conventional B cells. In patients with chronic GVHD, as with patients with systemic lupus erythematosis, B-cell–depleted patients in continuous remission recover with an IgD+CD27− transitional B-cell phenotype predominantly, whereas those with progressive disease have a predominant CD27+ B-cell compartment.22,43 Chronic GVHD patients have decreased recovery of naïve and transitional cells21,45 and increased proportions of CD27−CD21− B cells.46,47 Thus, the continued study of relevant pathologic and protective B-cell subsets in chronic GVHD will likely lead to the development of additional targeted therapeutic agent for affected patients.
In our study, we were able to confirm prior observations that the ratio of BAFF:B cells correlates with the occurrence of chronic GVHD.21 We also noted intermittent differences in BAFF levels at varying times after transplantation, comparing chronic GVHD and chronic GVHD-free subjects of unclear significance. Because BAFF is known in murine models to be vital for B-cell reconstitution after myeloablation,48 the significant increase in BAFF in patients without chronic GVHD (Figure 2) may relate to recovery of the B-cell compartment in this patient group. It has been suggested that BAFF levels can differentiate GVHD phenotypes,49 although this has not been examined within patients diagnosed solely with chronic GVHD. Further study of the differences in BAFF levels might explain why we noted mucosal chronic GVHD as the predominant manifestation of disease. One possibility is that mucosal tissues are sanctuary sites for B cells and are inaccessible to circulating monoclonal antibodies, or that tissue resident T cells in mucosal tissues have alternative non-B-cell–dependent pathways instrumental in chronic GVHD.
In summary, in a prospective phase 2 trial of posttransplantation rituximab administration, we noted a trend toward a reduction in the overall incidence of chronic GVHD, and that the incidence of corticosteroid-requiring chronic is reduced when compared with historical controls. This approach to prevention of chronic GVHD is safe, not associated with excess infections, and results in very low posttransplantation treatment-related mortality and improved overall survival when compared with a historical control. A prospective phase 3 trial is being designed to further test this strategy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Genentech for providing rituximab for investigation.
The study was funded in part by the Gateway for Cancer Research P-06-005 (to C.C.), P-01 CA142106 (to J.H.A.), P01 AI056299 (to B.B.), and K08-HL107756 (to S.S.); by the Jock and Bunny Adams Research and Education Endowment (to J.H.A.); and by the Ted and Eileen Pasquarello Research Fund (to J.R.). P.W. is supported by the King Chulalongkorn Memorial Hospital, Thailand. C.C. is supported by the Stem Cell Cyclists of the Pan-Mass Challenge.
Authorship
Contribution: C.C. designed the study, cared for patients, collected and analyzed data, and drafted and approved the manuscript; H.T.K. designed the study, analyzed data, and approved the manuscript; B.B. collected data and approved the manuscript; S.S., P.W., B.R.B., and J.R. conducted scientific analyses and approved the manuscript; V.T.H. cared for patients, collected data, and approved the manuscript; Y.-B.C., J.R., P.A., J.K., B.G., E.A., and R.J.S. cared for patients and approved the manuscript; S.M. conducted scientific analyses, collected data, and approved the manuscript; and J.H.A. designed the study, cared for patients, and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Corey Cutler, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA 02215; e-mail: corey_cutler@dfci.harvard.edu.