Key Points
New germ-line mutations of JAK2 in the kinase domain were identified.
Specificity for MPL and resistance to JAK2 and HSP90 inhibitors was determined.
Abstract
The main molecular basis of essential thrombocythemia and hereditary thrombocytosis is acquired, and germ-line–activating mutations affect the thrombopoietin signaling axis. We have identified 2 families with hereditary thrombocytosis presenting novel heterozygous germ-line mutations of JAK2. One family carries the JAK2 R867Q mutation located in the kinase domain, whereas the other presents 2 JAK2 mutations, S755R/R938Q, located in cis in both the pseudokinase and kinase domains. Expression of Janus kinase 2 (JAK2) R867Q and S755R/R938Q induced spontaneous growth of Ba/F3-thrombopoietin receptor (MPL) but not of Ba/F3–human receptor of erythropoietin cells. Interestingly, both Ba/F3-MPL cells expressing the mutants and platelets from patients displayed thrombopoietin-independent phosphorylation of signal transducer and activator of transcription 1. The JAK2 R867Q and S755R/R938Q proteins had significantly longer half-lives compared with JAK2 V617F. The longer half-lives correlated with increased binding to the heat shock protein 90 (HSP90) chaperone and with higher MPL cell-surface expression. Moreover, these mutants were less sensitive to JAK2 and HSP90 inhibitors than JAK2 V617F. Our results suggest that the mutations in the kinase domain of JAK2 may confer a weak activation of signaling specifically dependent on MPL while inducing a decreased sensitivity to clinically available JAK2 inhibitors.
Introduction
Thrombocytosis is a rare entity characterized by an abnormal proliferation of the megakaryocytic lineage resulting in overproduction of platelets (>450 × 109/L in adults). The overproduction of platelets is related to the activation of the thrombopoietin (TPO)/TPO receptor (MPL) axis that signals through several pathways, which include Janus kinase 2 (JAK2)/signal transducer and activator of transcription (STAT) 1-3-5, phosphatidylinositol 3-kinase (PI3K)/AKT, and mitogen-activated protein kinase.1,2
Familial thrombocytosis involves heterogeneous disorders that can be subdivided into true myeloproliferative neoplasms (MPNs) transmitted by autosomal inheritance with incomplete penetrance, and hereditary thrombocytosis with an autosomal dominant pattern and an early onset (complete penetrance) of the hematologic abnormalities.3,4 The latter are considered as nonmalignant diseases because of the polyclonal nature of hematopoiesis.
To date, germ-line mutations that have been identified in familial thrombocytosis all affect genes involved in TPO/MPL signaling. The genes targeted by germ-line mutations in familial thrombocytosis are often the same as sporadic mutations in MPNs, but the mutations disrupt different residues in the proteins. They include MPL S505N and MPL P106L, leading to the constitutive activation of the receptor and to a presumptive defect of TPO binding, respectively.5-7 An MPL W515R mutation, which is rare in sporadic MPNs compared with MPL W515L or W515K, has also recently been identified in familial thrombocytosis.8 Likewise, 2 germ-line mutations, JAK2 V617I (different from the V617F substitution classically found in 60% of sporadic essential thrombocythemias [ETs]) and JAK2 R564Q (never observed in sporadic MPNs), have been reported.9-11 Several mutations in the TPO gene that lead to increased TPO translation have also been described in families with a thrombocytosis.12-15
Here, we report 2 families with hereditary thrombocytosis carrying so far unidentified heterozygous germ-line mutations in JAK2.
Methods
Materials
Human recombinant erythropoietin (EPO) and interleukin 3 (IL-3) were generous gifts from Amgen (Neuilly, France). Stem cell factor (SCF) was from Biovitrum AB (Stockholm, Sweden), and recombinant TPO was a generous gift from Kirin (Tokyo, Japan). Restriction enzymes were purchased from Fermentas (St. Leon-Rot, Germany).
Patients and cell purification
Pedigrees of hereditary thrombocytosis registered in the French national collection of MPNs (DC 2009-957) were analyzed. All participants gave their written informed consent. Clinical and biological annotations were recorded in an Access database approved by the Commission Nationale de l'informatique et des Libertés (#815419). This study was conducted in accordance with the Declaration of Helsinki. Thrombocytosis was defined by a platelet count >450 × 109/L in patients who had no evidence for reactive thrombocytosis and no World Health Organization criteria for ET.16 Peripheral blood from patients was collected in sterile citrated tubes. Platelet-rich plasma was obtained by centrifugation at 180g for 15 minutes at 20°C. Platelet-rich plasma was mixed with acid citrate dextrose at a 9:1 ratio and centrifuged at 1500g for 15 minutes at 20°C. Platelet pellet was carefully resuspended in phosphate-buffered saline with 0.1% ethylenediaminetetraacetic acid. Blood mononuclear cells (BMCs) were plated in 6-well plates for 1 hour to remove monocytes and then seeded in semisolid cultures, or they were used to sort CD34+ and CD3+ cells by flow cytometry.
Quantification of clonogenic progenitors in semisolid cultures
BMCs were plated in methylcellulose, and burst-forming unit–erythroid (BFU-E) and colony-forming unit (CFU)–granulo-monocytic progenitors were counted 14 days later. BMCs were evaluated in serum-free fibrin clot assays to quantify CFU-megakaryocyte (MK) progenitor–derived colonies at day 10 by an indirect immuno–alkaline phosphatase staining technique using an anti-CD41a monoclonal antibody (clone HIP8; Becton Dickinson) as previously described.17,18
JAK2 molecular analysis
Amplification of the 25 exons and intron-exon boundaries of JAK2 (NM_004972.3) was performed in the probands on genomic DNA extracted from BMCs. Purified polymerase chain reaction products were sequenced in both directions using the BigDye Terminator chemistry (Life Technologies, Saint-Aubin, France) on an ABI3730 Genetic Analyzer.
DNA manipulations, production of retroviruses
The 2265T>A (S755R), 2600G>A (R867Q), and 2813G>A (R938Q) point mutations were introduced into the MIGR1-human JAK2 WT-IRES-GFP plasmid by the QuikChange site-directed mutagenesis method using the PfuUltra high-fidelity DNA polymerase (Stratagene, Amsterdam, The Netherlands). Full-length JAK2 mutant complementary DNAs were verified by sequencing. Vesicular stomatitis virus glycoprotein–pseudotyped viral particles were produced into 293EBNA cells.
Cell lines
The murine pro-B Ba/F3 cells were grown in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum (Stem Cell Technologies, Grenoble, France) and 5% conditioned medium from WEHI-3B cells as a source of murine IL-3. Ba/F3 cells were retrovirally transduced with MIGR1-IRES-CD4 to stably express the human receptor of EPO (EPOR) or TPO (MPL) harboring an N-terminal FLAG tag and maintained in the presence of 1 U/mL EPO and 5% WEHI, respectively. Ba/F3-EPOR and Ba/F3-MPL cell lines expressing the various JAK2 mutants were generated after transduction and subsequent green fluorescent protein (GFP)–positive cell sorting by flow cytometry (MoFlo; Beckman-Coulter, Villepinte, France).
Proliferation assays
The premixed WST-1 (5-[2,4-disulfophenyl]-2H-tetrazolium, monosodium salt) cell proliferation assay system was carried out according to the manufacturer’s instructions (Takara Bio Europe, Clontech, Saint-Germain-en-Laye, France). Experiments were done in triplicate. For inhibitor treatments, values were transformed to percent inhibition relative to vehicle-treated (dimethylsulfoxide) cells, and sigmoidal curves were fitted according to nonlinear regression analysis of the data using GraphPad PRISM software to calculate 50% inhibitory concentration. Dose-response curves to EPO and TPO were expressed as percent of viability of the maximal response.
Western blot analysis and coimmunoprecipitation
Signaling studies were performed on platelets and Ba/F3 cell lines by western blot analysis using polyclonal antibodies against the phosphorylated forms of JAK2 (Tyr 1007/1008), STAT1 (Tyr 701), STAT3 (Tyr 705), STAT5 (Tyr 694), extracellular signal-regulated kinase (ERK) 1/2 (Thr 202/Tyr 204), and AKT (Thr 308) and against the pan proteins (Cell Signaling Technology [Ozyme]). Heat shock cognate 70 (HSC70) (Stressgen, Victoria, Canada) or β-actin (Sigma, Saint Quentin Fallavier, France) were used as loading controls. For coimmunoprecipitation experiments, cells lysates were incubated with anti-JAK2 antibody (Ozyme) and A/G agarose beads. HSP90 and MPL were revealed with antibodies from Ozyme and Upstate (Millipore, Molsheim, France), respectively.
Cell-surface expression of FLAG-tagged MPL
Expression of MPL at the surface of Ba/F3 cells was examined by flow cytometry with 10 μg/mL monoclonal anti-FLAG M2 antibody (Sigma-Aldrich) followed with 1:400 diluted phycoerythrin (PE)–conjugated anti-mouse IgG(H+L) from Jackson ImmunoResearch Laboratories (Interchim, Montluçon, France).
Dual luciferase transcriptional assays
Transcriptional activation of STAT5, STAT3, and STAT1 was analyzed in γ2A JAK2-deficient cells using the dual pGRR5 firefly luciferase reporter (responding to STATs) and pRL-TK-driven renilla luciferase system. γ2A cells were transiently transfected using Lipofectamine reagent with complementary DNAs coding for JAK2 (or mutants thereof), MPL, and either STAT5, STAT3, or STAT1 as described.19 A Perkin-Elmer Victor X Light analyzer was used for detecting luminescence in cell lysates.
Modeling of mutation effects in JAK2 JH2 and JH1
The inactive and active crystal structures of JH1 (kinase domain)20 and the crystal structure of the JH2 (pseudokinase domain)21 were superimposed by Dr Andrew Shaw (Ludwig Institute for Cancer Research, San Diego, CA). Mutagenesis and effects on known salt bridges and intramolecular interactions were examined by the PyMol program.
Results
Identification of new germ-line JAK2 mutations
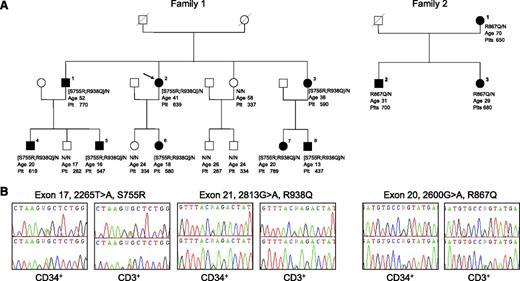
We performed a genome-wide single-nucleotide polymorphism (SNP) analysis of a large pedigree (family 1) with 7 affected members (individuals 1-7) showing only JAK2 V617F–negative thrombocytosis (Figure 1A). We genotyped the affected individuals using the Affymetrix 6.0 SNP array (906 622 SNPs) and determined 9 chromosomal regions with SNP alleles shared by all of them. One was located on chromosome 9 and encompassed the JAK2 locus (supplemental Table 1, available on the Blood Web site). Sequencing of the entire JAK2 gene identified 2 heterozygous JAK2 mutations, that is, c.2265T>A (S755R) and c.2813G>A (R938Q). Segregation analysis showed that both mutations were located on the same allele (Figure 1A). Subsequently, we analyzed 5 other families characterized by isolated elevated platelet counts ranging from 470 × 109/L to 1900 × 109/L, the absence of JAK2 V617F, an age at onset <40 years in at least 1 affected case, and a family history compatible with an autosomal dominant inheritance. They were sequenced for JAK2, and a new single heterozygous JAK2 mutation, c.2600G>A (R867Q), was found in family 2 (Figure 1A-B). Sequence analysis for 7 affected individuals from the 2 families confirmed the presence of the JAK2 mutations at heterozygous state in CD34+ and CD3+ cells. In both families, all members carrying these mutations suffer from thrombocytosis except 1 young relative of family 1 with a platelet count (437 × 109/L) close to the normal upper limit (subject 8). Their detailed hematologic and clinical characteristics are shown in supplemental Table 2.
Clinical and molecular features of 2 pedigrees with hereditary thrombocytosis. (A) Filled symbols represent individuals with JAK2 germ-line mutations. Below each symbol is reported the JAK2 genotype with “N” indicating a normal allele, the age (years) at diagnosis for affected individuals or the age at last examination for asymptomatic relatives, and the platelet level (×109/L). (B) Sequence electropherograms of the germ-line JAK2 mutations.
Clinical and molecular features of 2 pedigrees with hereditary thrombocytosis. (A) Filled symbols represent individuals with JAK2 germ-line mutations. Below each symbol is reported the JAK2 genotype with “N” indicating a normal allele, the age (years) at diagnosis for affected individuals or the age at last examination for asymptomatic relatives, and the platelet level (×109/L). (B) Sequence electropherograms of the germ-line JAK2 mutations.
The identified mutations are novel JAK2 missense mutations that have not been reported in genomic databases. The S755R mutation is located in the pseudokinase domain (JH2), whereas R867Q and R938Q mutations are situated in the kinase domain (JH1) of the protein (Figure 2A). These mutations affect amino acids that are highly conserved across species, and in silico analysis of the potential impact of these substitutions predicted a pathogenic effect on JAK2 function (supplemental Table 3). More specifically, the R867Q mutation in JH1 was predicted to abolish a salt bridge (between R867 and D869), which is present in the inactive JH1 and is lost upon activation.20 Thus, the R867Q mutation would result in an active state in the region around β sheets 2 and 3 (Figure 2B). The S755R mutation was predicted to interfere with another important salt bridge (between D635 from α helix D and K752 in the region linking α helix D to α helix G) with the bulky positive R755 triggering a steric clash (Figure 2B).21 The loss of this salt bridge may destabilize the network of α helices at the C terminus of JH2 by pushing away helix G. This network of helices has previously been shown to exert inhibitory effects on JH1.22 The R938Q is located in a sensitive region of the JH1, not far from the adenosine triphosphate (ATP) loop and substrate access site (Figure 2B).
Scheme and in silico modeling of the JAK2 mutants. (A) Scheme and location of germ-line JAK2 mutations. (B) Superimposition of the crystal structures of the active JH1 kinase and JH2 pseudokinase domains of JAK2 wild type (WT) based on 2 radiograph crystal structure studies.20,21 On the left in blue: coordinate of active JH1 (protein data bank [PDB] code: 2B7A). On the right in green: coordinate of active JH2 (PDB code: 4FVQ). Two of the mutations, R938Q and R867Q, are located in the JH1, whereas the S755R mutation is located in the JH2 (pink residues). The germ-line JAK2 S755R/R938Q mutant therefore contains mutations in both JH2 and JH1. The activation loop is represented in red in both JH1 and JH2. The glycine-rich loop is in yellow, and the catalytic loop is in orange. The salt bridge between D869 and R867 is only formed in the inactive JH1, which is the coordinate represented on the small square on the left. The coordinate of inactive JH1 is PDB code 3UGC. The salt bridge between K752 and D635 is formed in active JH2 (WT and V617F). The coordinate shown here in the small square on the right is the active JH2 WT (PDB code: 4FVQ).
Scheme and in silico modeling of the JAK2 mutants. (A) Scheme and location of germ-line JAK2 mutations. (B) Superimposition of the crystal structures of the active JH1 kinase and JH2 pseudokinase domains of JAK2 wild type (WT) based on 2 radiograph crystal structure studies.20,21 On the left in blue: coordinate of active JH1 (protein data bank [PDB] code: 2B7A). On the right in green: coordinate of active JH2 (PDB code: 4FVQ). Two of the mutations, R938Q and R867Q, are located in the JH1, whereas the S755R mutation is located in the JH2 (pink residues). The germ-line JAK2 S755R/R938Q mutant therefore contains mutations in both JH2 and JH1. The activation loop is represented in red in both JH1 and JH2. The glycine-rich loop is in yellow, and the catalytic loop is in orange. The salt bridge between D869 and R867 is only formed in the inactive JH1, which is the coordinate represented on the small square on the left. The coordinate of inactive JH1 is PDB code 3UGC. The salt bridge between K752 and D635 is formed in active JH2 (WT and V617F). The coordinate shown here in the small square on the right is the active JH2 WT (PDB code: 4FVQ).
To determine the clonality of hematopoieisis of these JAK2-mutated patients, we carried out methylation-sensitive polymerase chain reaction assays using the HUMARA and PCSK1N loci on granulocytes and CD3+ cells of 3 affected women (subjects 3 and 7 of family 1 and subject 3 of family 2).23 Results were in favor of a polyclonal hematopoiesis as opposed to a JAK2 V617F–positive patient with polycythemia vera who displayed a complete X inactivation in agreement with a clonal hematopoiesis (supplemental Table 4).
Finally, we verified that the TPO/MPL axis controlling platelet production was not affected besides the mutations of JAK2, by sequencing MPL and assessing TPO levels in plasma for these 2 families. No mutation was identified in MPL for patient 2 of family 1 and patient 3 of family 2, and TPO levels were inside the normal range (<30 pg/mL) (supplemental Table 2).
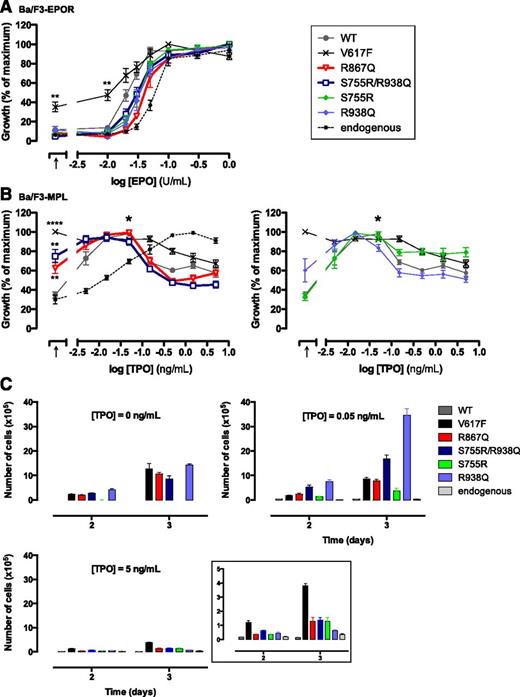
JAK2 R867Q and JAK2 S755R/R938Q are gain-of-function mutations that induce spontaneous growth and constitutive signaling of MPL-expressing cells
We modeled the JAK2 mutations in Ba/F3 cells lines expressing either MPL (Ba/F3-MPL) or EPOR (Ba/F3-EPOR). Ba/F3-MPL and Ba/F3-EPOR expressing JAK2 V617F or JAK2 WT were used as controls. We measured cellular proliferation in response to cytokines using WST-1 assays. None of the JAK2 mutants, except JAK2 V617F, conferred hypersensitivity to EPO to the Ba/F3-EPOR cell line (Figure 3A). In contrast, the Ba/F3-MPL cells expressing either of the JAK2 mutants, except for JAK2 S755R, could be maintained in a cytokine-deprived condition (>3 weeks). Independency to TPO was confirmed using a WST-1 assay (Figure 3B). TPO induced a sigmoidal dose-response curve in the Ba/F3-MPL cell line (Figure 3B, “endogenous”). As previously described for JAK2 WT and JAK2 V617F,24,25 overexpression of the JAK2 mutants resulted in TPO-induced bell curves with a peak at 0.05 ng/mL and reduced cell viability at higher doses (Figure 3B-C). Of note, JAK2 S755R did not confer TPO independency to the cells. However, at high doses of TPO, the decreased viability of Ba/F3-MPL cells in the presence of JAK2 S755R was attenuated compared with JAK2 WT and JAK2 R938Q (Figure 3B-C). These data suggest that the R938Q mutation drives the TPO-independent proliferation of the double mutant JAK2 S755R/R938Q. The S755R mutation might be involved in maintaining basal growth in the presence of high doses of TPO as seen with JAK2 V617F.
Sensitivities to EPO and TPO of Ba/F3-EPOR and Ba/F3-MPL cells. (A) Ba/F3-EPOR cells expressing each of the JAK2 forms were cultured for 48 hours either in the absence of cytokine (black arrow, x-axis) or in the presence of increasing doses of EPO (0.01, 0.02, 0.03, 0.05, 0.1, 0.3, and 1 U/mL). Viable cells were quantified by WST-1 proliferation assay. Dose-response curves are means expressed in percentages of maximum growth value ± standard error of the mean (SEM; n = 4 in triplicate). (B) Proliferation was assayed 48 hours after culturing Ba/F3-MPL cells expressing each of the JAK2 forms in the absence of cytokine (black arrow, x-axis) or in the presence of increasing doses of TPO (0.005, 0.015, 0.05, 0.15, 0.5, 1.5, and 5 ng/mL). The asterisk indicates 0.05 ng/mL TPO. Data are means ± SEM (n = 4 independent experiments performed in triplicate). Two-tailed Student t test: **P < .01; ****P < .0001. (C) Ba/F3-MPL cell lines were plated at 30 000 cells and cultured over a period of 72 hours in the absence or presence of either 0.05 ng/mL or 5 ng/mL TPO and numbered. Curves show cumulative cell number ± standard deviation of a typical experiment out of 3 performed in triplicate. Insert represents data at 5 ng/mL TPO with an adapted y-axis scale.
Sensitivities to EPO and TPO of Ba/F3-EPOR and Ba/F3-MPL cells. (A) Ba/F3-EPOR cells expressing each of the JAK2 forms were cultured for 48 hours either in the absence of cytokine (black arrow, x-axis) or in the presence of increasing doses of EPO (0.01, 0.02, 0.03, 0.05, 0.1, 0.3, and 1 U/mL). Viable cells were quantified by WST-1 proliferation assay. Dose-response curves are means expressed in percentages of maximum growth value ± standard error of the mean (SEM; n = 4 in triplicate). (B) Proliferation was assayed 48 hours after culturing Ba/F3-MPL cells expressing each of the JAK2 forms in the absence of cytokine (black arrow, x-axis) or in the presence of increasing doses of TPO (0.005, 0.015, 0.05, 0.15, 0.5, 1.5, and 5 ng/mL). The asterisk indicates 0.05 ng/mL TPO. Data are means ± SEM (n = 4 independent experiments performed in triplicate). Two-tailed Student t test: **P < .01; ****P < .0001. (C) Ba/F3-MPL cell lines were plated at 30 000 cells and cultured over a period of 72 hours in the absence or presence of either 0.05 ng/mL or 5 ng/mL TPO and numbered. Curves show cumulative cell number ± standard deviation of a typical experiment out of 3 performed in triplicate. Insert represents data at 5 ng/mL TPO with an adapted y-axis scale.
As expected, overexpression of JAK2 V617F in Ba/F3-EPOR cells induced a constitutive phosphorylation of all signaling pathways tested (STAT5, AKT, and ERK) (Figure 4A). Although the levels of expression of the JAK2 mutants in Ba/F3-EPOR cells were similar to JAK2 WT or JAK2 V617F, we observed a weak phosphorylation of ERK in the presence of all the mutants except JAK2 R938Q, and a detectable phosphorylation of AKT in presence of JAK2 S755R and JAK2 S755R/R938Q (Figure 4A). However, there was no distinct phosphorylation of STAT5 in absence of EPO, as shown by the P-STAT5/HSC70 ratios that remain below the level induced by JAK2 V617F.
Signaling studies in Ba/F3-EPOR and Ba/F3-MPL cells and STAT activities in γ2A cells. (A) Ba/F3-EPOR or (B) Ba/F3-MPL cells expressing the different JAK2 constructs were serum- and cytokine-starved for 6 hours prior to a 15-minute stimulation with (A) 1 U/mL EPO and (B) 0.05 and 5 ng/mL TPO at 37°C, as indicated. Cells were lysed, and the phosphorylation status of STAT1, STAT3, STAT5, AKT, and ERK1/2 was examined by western blotting with the respective anti-phospho specific antibodies, as indicated. Expression of HSC70 in the samples was used as loading control and was consistent with expression of total AKT, ERK1/2, and the individual STAT isoforms. Blots shown were reproduced in 2 independent experiments. The quantification of the phospho-STAT5 (P-STAT5) blots for unstimulated Ba/F3-EPOR and Ba/F3-MPL were shown as P-STAT5/HSC70 ratios (arbitrary units [AU]) and displayed below the figures. (C) JAK2-deficient γ2A cells were transfected to express the various JAK2 mutants in the presence of equal amounts of JAK2 WT and MPL. STAT1-, STAT3-, or STAT5-dependent transcriptional activity was measured 24 hours after transfection by the dual firefly (pGRR5 reporter responding to STATs) and renilla (pRL-TK reporter with constitutive expression) luciferase system. Shown are averages ± SEM of 9 experiments in triplicate. Two-tailed Student t test: *P < .05; **P < .01; ***P < .001.
Signaling studies in Ba/F3-EPOR and Ba/F3-MPL cells and STAT activities in γ2A cells. (A) Ba/F3-EPOR or (B) Ba/F3-MPL cells expressing the different JAK2 constructs were serum- and cytokine-starved for 6 hours prior to a 15-minute stimulation with (A) 1 U/mL EPO and (B) 0.05 and 5 ng/mL TPO at 37°C, as indicated. Cells were lysed, and the phosphorylation status of STAT1, STAT3, STAT5, AKT, and ERK1/2 was examined by western blotting with the respective anti-phospho specific antibodies, as indicated. Expression of HSC70 in the samples was used as loading control and was consistent with expression of total AKT, ERK1/2, and the individual STAT isoforms. Blots shown were reproduced in 2 independent experiments. The quantification of the phospho-STAT5 (P-STAT5) blots for unstimulated Ba/F3-EPOR and Ba/F3-MPL were shown as P-STAT5/HSC70 ratios (arbitrary units [AU]) and displayed below the figures. (C) JAK2-deficient γ2A cells were transfected to express the various JAK2 mutants in the presence of equal amounts of JAK2 WT and MPL. STAT1-, STAT3-, or STAT5-dependent transcriptional activity was measured 24 hours after transfection by the dual firefly (pGRR5 reporter responding to STATs) and renilla (pRL-TK reporter with constitutive expression) luciferase system. Shown are averages ± SEM of 9 experiments in triplicate. Two-tailed Student t test: *P < .05; **P < .01; ***P < .001.
In Ba/F3-MPL cells, overexpression of JAK2 V617F induced constitutive phosphorylation of STAT3/5, AKT, and ERK as expected (Figure 4B). R867Q, R938Q alone, and S755R/R938Q JAK2 mutants induced constitutive signaling of the same pathways. Noticeably, in the presence of R867Q and S755R/R938Q JAK2 mutants, we detected a spontaneous activation of STAT1. JAK2 S755R alone did not induce any constitutive phosphorylation besides a weak activation of STAT5 (Figure 4B). This last result implies that the S755R mutation compared with the R938Q mutation might play a lesser role in the constitutive signaling of JAK2 S755R/R938Q.
We then cotransfected JAK2 V617F, R867Q, or S755R/R938Q mutant in JAK2-deficient γ2A cells in the presence of a similar amount of JAK2 WT to mimic the heterozygous state of the mutations found in patients. The effect of these mutants combined with MPL on STAT1, STAT3, or STAT5 activation was measured by luciferase assays. Figure 4C shows that mutants of JAK2 induced constitutive signaling primarily via STAT1 and, to a lesser extent, STAT3 in MPL-expressing cells and under conditions of heterozygosity. Of note, JAK2 S755R had no effect on basal STAT activation (supplemental Figure 1). Activation of the STATs was dependent on MPL (supplemental Figure 2A). In addition, we used a battery of 6 coiled-coil MPL dimeric fusion proteins, with different orientations.26 The 2 new JAK2 mutants were consistently weaker than JAK2 V617F and mostly induced STAT-dependent signaling only in the presence of active or partially active MPL dimeric orientations, and they were less active in the presence of cc-MPL-II (supplemental Figure 2B).
Taken together, these results show that the constitutive signaling of JAK2 R867Q, R938Q, and S755R/R938Q is dependent on the presence of MPL and leads to growth-factor independency and hypersensitivity to TPO.
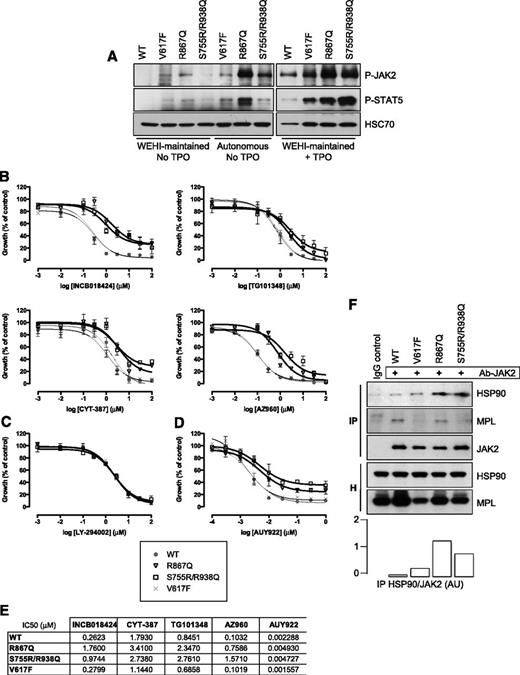
JAK2 mutants are less sensitive than JAK2 V617F to classical JAK2 inhibitors and an HSP90 inhibitor in Ba/F3 cell lines
Randomly generated point mutations in the kinase domain of JAK2 V617F have been shown to be resistant to JAK2 inhibitors while still sensitive to inhibition of HSP90, a chaperone for JAK2.27,28 Therefore, we tested the impact of the R867Q and S755R/R938Q mutations on the sensitivity of these mutated kinases to JAK2 inhibitors (INCB018424, TG101348, CYT-387, and AZ960) and to an HSP90 inhibitor (AUY922) by WST-1 assays. Autonomous Ba/F3-MPL cell lines (growing without cytokines for at least 3 weeks) expressing either JAK2 R867Q, JAK2 S755R/R938Q, or JAK2 V617F displayed constitutive phosphorylation of JAK2 and STAT5 (Figure 5A). JAK2 R867Q was reproducibly found to be more phosphorylated than JAK2 S755R/R938Q and JAK2 V617F. Cells were treated with increasing doses of inhibitors. Interestingly, the JAK2 R867Q–expressing and JAK2 S755R/R938Q–expressing cells were 5- to 15-fold less sensitive than their JAK2 WT and V617F counterparts to classical JAK2 inhibitors currently used in clinics and clinical trials such as TG101348 (SAR302503) and INCB018424 (ruxolitinib), AZ960, and CYT-387. Whereas high doses of TG101348 and AZ960 (>1 µM) prevented growth of all 3 mutated cell lines, equivalent doses of INCB018424 and CYT-387 failed to completely inhibit the proliferation of the cells expressing JAK2 R867Q and JAK2 S755R/R938Q (Figures 5B,E).
Sensitivity of JAK2 R867Q and JAK2 S755R/R938Q mutants to JAK2 and HSP90 inhibitors. (A) Ba/F3-MPL cells expressing either JAK2 R867Q, JAK2 S755R/R938Q, or JAK2 V617F could be maintained in WEHI-supplemented medium but also exhibited cytokine-independent growth (Autonomous). Autonomous or WEHI-maintained cells were serum-starved for 6 hours and stimulated, or not (no TPO), with 5 ng/mL TPO for 15 minutes (+TPO). The constitutive phosphorylation level of each of the JAK2 constructs was analyzed by western blotting. The level of P-STAT5 was also detected, and expression of HSC70 served as a loading control. Blots are representative of a typical experiment. (B) Growth of autonomous Ba/F3-MPL cells expressing JAK2 V617F (×), R867Q (▽), and S755R/R938Q mutants (□), as well as WEHI-dependent Ba/F3-MPL expressing JAK2 WT (gray circle), was determined in response to treatment with various concentrations of INCB018424, TG101348, CYT-387, AZ960, (C) the PI3K inhibitor LY-294002, or (D) AUY922. A WST-1 proliferation assay was performed after 72 hours of exposure to the inhibitors, in the presence of 5 ng/mL TPO. Data (means ± SEM) were calculated as percentages of vehicle-treated cells and were conducted in duplicate in 4 independent experiments. Significant differences for R867Q and S755R/R938Q mutants compared with JAK2 WT were found for all inhibitors except for LY-294002 (P < .05 using the 2-tailed Student t test). (E) The 50% inhibitory concentration (IC50) values of cytokine-independent Ba/F3-MPL cells exposed to inhibitors for 72 hours were calculated using GraphPad PRISM software. (F) Coimmunoprecipitation (IP) was performed in Ba/F3-MPL cells using an anti-JAK2 antibody and blotted for the presence of HSP90 and MPL. Cell homogenates (H) show the amount of proteins before the IP. A quantification of both HSP90 and JAK2 in the IP fractions using ImageJ software (National Institutes of Health) was performed, and the histogram represents the HSP90/JAK2 ratios in AU. Blots shown were reproduced in 2 independent experiments.
Sensitivity of JAK2 R867Q and JAK2 S755R/R938Q mutants to JAK2 and HSP90 inhibitors. (A) Ba/F3-MPL cells expressing either JAK2 R867Q, JAK2 S755R/R938Q, or JAK2 V617F could be maintained in WEHI-supplemented medium but also exhibited cytokine-independent growth (Autonomous). Autonomous or WEHI-maintained cells were serum-starved for 6 hours and stimulated, or not (no TPO), with 5 ng/mL TPO for 15 minutes (+TPO). The constitutive phosphorylation level of each of the JAK2 constructs was analyzed by western blotting. The level of P-STAT5 was also detected, and expression of HSC70 served as a loading control. Blots are representative of a typical experiment. (B) Growth of autonomous Ba/F3-MPL cells expressing JAK2 V617F (×), R867Q (▽), and S755R/R938Q mutants (□), as well as WEHI-dependent Ba/F3-MPL expressing JAK2 WT (gray circle), was determined in response to treatment with various concentrations of INCB018424, TG101348, CYT-387, AZ960, (C) the PI3K inhibitor LY-294002, or (D) AUY922. A WST-1 proliferation assay was performed after 72 hours of exposure to the inhibitors, in the presence of 5 ng/mL TPO. Data (means ± SEM) were calculated as percentages of vehicle-treated cells and were conducted in duplicate in 4 independent experiments. Significant differences for R867Q and S755R/R938Q mutants compared with JAK2 WT were found for all inhibitors except for LY-294002 (P < .05 using the 2-tailed Student t test). (E) The 50% inhibitory concentration (IC50) values of cytokine-independent Ba/F3-MPL cells exposed to inhibitors for 72 hours were calculated using GraphPad PRISM software. (F) Coimmunoprecipitation (IP) was performed in Ba/F3-MPL cells using an anti-JAK2 antibody and blotted for the presence of HSP90 and MPL. Cell homogenates (H) show the amount of proteins before the IP. A quantification of both HSP90 and JAK2 in the IP fractions using ImageJ software (National Institutes of Health) was performed, and the histogram represents the HSP90/JAK2 ratios in AU. Blots shown were reproduced in 2 independent experiments.
The R867Q and S755R/R938Q JAK2 mutants presented similar sensitivity as JAK2 V617F to a PI3K inhibitor (LY-294002) (Figure 5C), suggesting that the 3 JAK2 mutants (R867Q, S755R/R938Q, and V617F) require downstream PI3K for Ba/F3-MPL cell proliferation. However, they were less sensitive than JAK2 V617F to an HSP90 inhibitor (AUY922), even at high doses (Figure 5D). Accordingly, HSP90 coimmunoprecipitated to a greater extent with JAK2 mutants (R867Q, S755R/R938Q) than with JAK2 V617F and WT (Figure 5F).
Altogether, these results show that the new JAK2 mutants are less sensitive than JAK2 V617F to both JAK2 and HSP90 inhibitors.
Effects of JAK2 mutants on MPL stability and MPL cell-surface localization
To understand the properties of these JAK2 mutants, we assessed their stability. We treated the different Ba/F3-MPL cell lines with cycloheximide (CHX) and performed a western blot analysis with anti-JAK2 and anti-MPL antibodies (Figure 6A). JAK2 S755R/R938Q mutant was twofold more stable than JAK2 WT, whereas JAK2 V617F was twofold less stable than JAK2 WT. JAK2 R867Q was found to be as stable as JAK2 WT. Moreover, JAK2 S755R/R938Q significantly increased the stability of cell-surface MPL compared with the other JAK2.
Effect of JAK2 mutations on protein stability and chaperone function for MPL cell-surface expression. (A) Ba/F3-MPL cells expressing either JAK2 WT, V617F, R867Q, or S755R/R938Q and maintained in WEHI-supplemented medium were treated with CHX (50 μg/mL) for 0, 0.5, 1, 2, 5, 8, and 24 hours. Levels of both total JAK2 and MPL proteins were examined by western blotting, and β-actin serves as loading control. Table shows means ± SEM of the half-lives (T1/2) of JAK2 WT and mutants and mature cell-surface MPL interpolated from y = 0.5 on the curves corresponding to half of the protein remaining in CHX-treated cells compared with CHX-untreated cells (n = 3). Significance compared with JAK2 WT T1/2 or cell-surface MPL T1/2 in presence of JAK2 WT was assessed using the 2-tailed Student t test. *P < .05; **P < .01; ***P < .001. (B) Ba/F3 cells expressing the FLAG-tagged MPL and transduced with the bicistronic retroviral pMIGR-IRES-GFP vector encoding either JAK2 WT, V617F, R867Q, S755R/R938Q, S755R, or R938Q were sorted for equal GFP levels and maintained in IL-3–supplemented medium. GFP expression allowed monitoring of JAK2 level in the various cell lines, and MPL cell-surface expression was assessed by flow cytometry using PE fluorescence labeling of the extracellular FLAG tag. Histogram shows the mean fluorescence intensities (MFIs) of PE-labeled cell-surface MPL. (C) Histogram shows means ± SEM of PE MFI in fold related to Ba/F3-MPL cells with no overexpression of JAK2 (Endog.) (n = 3). Significance compared with the Ba/F3–MPL–JAK2 WT condition was calculated using the 2-tailed Student t test. *P < .05.
Effect of JAK2 mutations on protein stability and chaperone function for MPL cell-surface expression. (A) Ba/F3-MPL cells expressing either JAK2 WT, V617F, R867Q, or S755R/R938Q and maintained in WEHI-supplemented medium were treated with CHX (50 μg/mL) for 0, 0.5, 1, 2, 5, 8, and 24 hours. Levels of both total JAK2 and MPL proteins were examined by western blotting, and β-actin serves as loading control. Table shows means ± SEM of the half-lives (T1/2) of JAK2 WT and mutants and mature cell-surface MPL interpolated from y = 0.5 on the curves corresponding to half of the protein remaining in CHX-treated cells compared with CHX-untreated cells (n = 3). Significance compared with JAK2 WT T1/2 or cell-surface MPL T1/2 in presence of JAK2 WT was assessed using the 2-tailed Student t test. *P < .05; **P < .01; ***P < .001. (B) Ba/F3 cells expressing the FLAG-tagged MPL and transduced with the bicistronic retroviral pMIGR-IRES-GFP vector encoding either JAK2 WT, V617F, R867Q, S755R/R938Q, S755R, or R938Q were sorted for equal GFP levels and maintained in IL-3–supplemented medium. GFP expression allowed monitoring of JAK2 level in the various cell lines, and MPL cell-surface expression was assessed by flow cytometry using PE fluorescence labeling of the extracellular FLAG tag. Histogram shows the mean fluorescence intensities (MFIs) of PE-labeled cell-surface MPL. (C) Histogram shows means ± SEM of PE MFI in fold related to Ba/F3-MPL cells with no overexpression of JAK2 (Endog.) (n = 3). Significance compared with the Ba/F3–MPL–JAK2 WT condition was calculated using the 2-tailed Student t test. *P < .05.
We next assessed the level of cell-surface MPL in the presence of the JAK2 mutants by flow cytometry using an anti-FLAG antibody. As previously described,25 overexpression of JAK2 WT led to increased MPL cell-surface expression, whereas JAK2 V617F induced its decrease. In good agreement with their stabilities, JAK2 S755R/R938Q allowed a twofold augmentation of MPL cell-surface expression, while JAK2 R867Q behaved similarly as JAK2 WT (Figure 6B-C). Thus, the half-life of the JAK2 mutants directly correlated with their ability to promote cell-surface localization of MPL.
In patients, JAK2 mutants specifically act on the megakaryocytic lineage
Four patients of family 1 displaying the JAK2 S755R/R938Q mutation (P1, P3, P7, and P8) and 2 patients of family 2 with the JAK2 R867Q mutation (P′2, P′3) were studied for hematopoietic progenitors and compared with control donors (n = 5). Clonogenic assays showed no growth of endogenous erythroid colonies or MK colonies in SCF plus IL-3 or SCF alone, respectively. There was no statistical difference compared with controls in the frequency of circulating BFU-E and CFU–granulocyte macrophage progenitors (Figure 7A-B). However, there was a slight but significant increase in CFU-MK colony frequency (Figure 7C-G) combined with larger colony size (number of MKs per colony) especially in the family with JAK2 S755R/R938Q (Figure 7H-I).
Assessment of myeloid progenitor amplification and signaling in platelets from JAK2-mutated patients from families 1 and 2. Mononuclear cells from R867Q (n = 2) patients, S755R/R938Q (n = 4) patients, or control donors (n = 5) were purified and plated (A-B) in methylcellulose with SCF/IL-3/EPO to study BFU-E progenitors or (C-D) in serum-free fibrin clot with SCF and increasing doses of TPO, (E) with 10 ng/mL TPO to study CFU-MK progenitors. Data represent means ± standard deviation for each patient plated in triplicate. (F-G) Histograms represent the means ± SEM of CFU-MK progenitors in the presence of SCF and TPO (10 ng/mL) for patients (n = 5 for S755R/938Q, n = 3 for R867Q) and controls (n = 4). Two-tailed Student t test: **P < .01. (H-I) The numbers of MKs per cluster were counted. (J-K) Platelets were isolated from R867Q (n = 2) patients, S755R/R938Q (n = 4) patients, or control donors (n = 3); washed in phosphate-buffered saline; and stimulated or not with TPO (5 ng/mL). Platelets were lysed, and the phosphorylation status of JAK2, STAT1, STAT3, STAT5, AKT, and ERK1/2 was examined by western blotting with the respective anti-phospho specific antibodies, as indicated. Expressions of HSC70 and of total STAT proteins, AKT, and ERK in the samples were used as loading controls. Blots show representative results in 2 S755R/R938Q patients (P1, P3) (J) and 2 R867Q patients (P′2, P′3) (K).
Assessment of myeloid progenitor amplification and signaling in platelets from JAK2-mutated patients from families 1 and 2. Mononuclear cells from R867Q (n = 2) patients, S755R/R938Q (n = 4) patients, or control donors (n = 5) were purified and plated (A-B) in methylcellulose with SCF/IL-3/EPO to study BFU-E progenitors or (C-D) in serum-free fibrin clot with SCF and increasing doses of TPO, (E) with 10 ng/mL TPO to study CFU-MK progenitors. Data represent means ± standard deviation for each patient plated in triplicate. (F-G) Histograms represent the means ± SEM of CFU-MK progenitors in the presence of SCF and TPO (10 ng/mL) for patients (n = 5 for S755R/938Q, n = 3 for R867Q) and controls (n = 4). Two-tailed Student t test: **P < .01. (H-I) The numbers of MKs per cluster were counted. (J-K) Platelets were isolated from R867Q (n = 2) patients, S755R/R938Q (n = 4) patients, or control donors (n = 3); washed in phosphate-buffered saline; and stimulated or not with TPO (5 ng/mL). Platelets were lysed, and the phosphorylation status of JAK2, STAT1, STAT3, STAT5, AKT, and ERK1/2 was examined by western blotting with the respective anti-phospho specific antibodies, as indicated. Expressions of HSC70 and of total STAT proteins, AKT, and ERK in the samples were used as loading controls. Blots show representative results in 2 S755R/R938Q patients (P1, P3) (J) and 2 R867Q patients (P′2, P′3) (K).
Finally, signaling studies on platelets were performed by western blot analysis. TPO induced the phosphorylation of JAK2, STAT1, STAT3, STAT5, AKT, and ERK (Figure 7J-K). As expected from Ba/F3-MPL cell lines, we observed a weak spontaneous phosphorylation of STAT1 in JAK2 S755R/R938Q patients (Figure 7J and supplemental Figure 3). Moreover, TPO-induced phosphorylation of STAT3 and STAT5 was reduced in the JAK2 R867Q patients compared with the control, whereas STAT1 phosphorylation was stimulated by TPO equally well in patients and in the control (Figure 7K).
Altogether, these results show that JAK2 R867Q and JAK2 S755R/R938Q both stimulate moderately but specifically MK proliferation, which correlates with STAT1-mediated signaling.
Discussion
In this study, we identified 2 families with hereditary thrombocytosis carrying 3 so far unidentified heterozygous JAK2 germ-line mutations in the kinase and pseudokinase domains. One family displayed a single point mutation in the JAK2 kinase domain (R867Q), and the other one 2 mutations in the same JAK2 allele affecting the pseudokinase (S755R) and the kinase (R938Q) domains. This is the first report of inherited mutations in the kinase domain of JAK2 associated with a thrombocytosis phenotype, while JAK2 R867Q was initially identified in a pediatric acute lymphoblastic leukemia.29 Two novel germ-line mutations in the pseudokinase domain of JAK2 (V617I and R564Q) have recently been observed in hereditary thrombocytosis.9,11 Thrombotic complications appear to be rare in the patients of these 2 families, suggesting that familial thrombocytosis related to these molecular variants could be underestimated.
JAK2 R867Q and JAK2 S755R/R938Q are gain-of-function mutations with weak activities compared with JAK2 V617F and are specifically dependent on the presence of MPL in both families, as demonstrated using the Ba/F3-MPL cell line and primary cells from patients. JAK2 R867Q or JAK2 S755R/R938Q overexpression induces TPO independency and a spontaneous phosphorylation of signaling molecules such as STAT1, STAT3, STAT5, ERK, and AKT in Ba/F3-MPL cells, whereas no or little effect was found in Ba/F3-EPOR cells. Both the absence of spontaneous CFU-MK in primary cells from patients and the preferential use of MPL to induce constitutive signaling suggest that the 2 new JAK2 mutants have a significantly weaker constitutive activity than JAK2 V617F. This is reminiscent of JAK2 V617I mutant shown to be weaker than JAK2 V617F on the growth of cytokine-independent progenitors.10,11,19 Signaling induced by JAK2 S755R/R938Q may result from the unusually long half-life of this mutant correlating with MPL cell-surface expression and stability. One major difference between MPL and EPOR is the extended stability of the mature cell-surface MPL, whereas EPOR is highly unstable.30 We suppose that the high stability of both MPL and the new JAK2 S755R/R938Q mutant induces a weak but persistent signal that will be sufficient to promote thrombocytosis, but not erythrocytosis. On the other hand, our results suggest a different mechanism for the constitutive activation of JAK2 R867Q. Significantly increased levels of autophosphorylation of this JAK2 mutant were detected compared with the other JAK2 mutants, possibly indicating an impaired regulation by phosphatases.
Interestingly, detailed studies in γ2A cells where we examined the ability of JAK2 mutants to activate STAT1, STAT3, and STAT5 via MPL in heterozygous situations showed that they essentially activate STAT1. These results are in line with the observation that these mutations specifically activate STAT1 in platelets from patients and in Ba/F3-MPL cells. A previous study showed that the JAK2 V617F–induced high phosphorylation level of STAT1 is associated with an ET-like phenotype without change in the number of red cells.31 In the Ba/F3-MPL cell model, overexpression of JAK2 V617F mimics the homozygous state of the mutation, characteristic of polycythemia vera, and did not induce spontaneous STAT1 phosphorylation. In contrast, the MPL-expressing γ2A cell model in which JAK2 V617F and JAK2 WT were cotransfected reproduces the heterozygous JAK2 V617F configuration found in ET patients, and JAK2 V617F stimulated the TPO-independent phosphorylation of STAT1.
Finally, we studied the respective functions of the individual JAK2 S755R and JAK2 R938Q mutants in Ba/F3 cells. We found that JAK2 R938Q was the key activating mutation because it induced constitutive signaling and the growth of Ba/F3-MPL cells like JAK2 S755R/R938Q. In contrast, JAK2 S755R activity was comparable to JAK2 WT except at high doses of TPO. This subtle difference might be important for the double mutant to escape a senescence-like phenotype because JAK2 WT was shown to induce senescence and antiproliferative effects at high levels of TPO.24,25 Presumably, the heterozygous expression of JAK2 R938Q in patients might not be sufficient to induce a thrombocytosis, whereas R938Q may synergize with S755R in the double JAK2 mutant. In favor of this hypothesis, we found that none of the single mutants were able to clearly increase MPL cell-surface expression in contrast to JAK2 S755R/R938Q (Figure 6B).
The R867Q and S755R/R938Q mutations conferred resistance to several JAK2 enzymatic inhibitors such as ruxolitinib, TG101348, CYT-387, and AZ960. In particular, ruxolitinib and CYT-387 failed to induce a complete inhibition of Ba/F3-MPL cell growth at high doses. It was reported that synthetic mutants of the kinase domain of JAK2 V617F, generated by random mutagenesis, were less sensitive to these inhibitors.27,28 The resistance of JAK2 R867Q and S755R/R938Q mutants to type I ATP-competitive inhibitors of JAK2 is likely to reflect allosteric effects on the ATP-binding pocket induced by the disruption of kinase domain and pseudokinase domain salt bridges (R867-D869 and D635-K752). Furthermore, R867Q and R938Q mutations are in proximity of residues that were previously shown to generate resistant mutants (E864K and Y931C), thus indicating that the allosteric effects on the ATP-binding pocket can be induced by several mutations in those regions.27,28,32
Recently, some studies have focused their interest on HSP90, an essential chaperone for JAK2 stability. Inhibition of HSP90 promotes the degradation of both JAK2 WT and JAK2 V617F. Weigert et al reported that their synthetic mutants of JAK2, which were resistant to JAK2 inhibitors, remained sensitive to HSP90 inhibition.28 In contrast, our data showed that JAK2 R867Q–expressing and JAK2 S755R/R938Q–expressing Ba/F3-MPL cells were also less sensitive to the HSP90 inhibitor AUY922 than cells harboring JAK2 V617F. Resistance to AUY922 is probably attributable to a more stable interaction between HSP90 and JAK2 S755R/R938Q or R867Q. JAK2 V617F was the least stable mutant and also the best target for both ATP-competitive JAK2 inhibitors and HSP90 inhibitors. These natural mutants of JAK2 that are less sensitive to the current JAK2 and HSP90 inhibitors could be used as models to screen new small molecules that could benefit MPN patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the physicians, Michel Blanc, Selim Corm, Valérie Ugo, and Unité de Recherche Clinique de l'est Parisien who contributed to the recruitment of patients and to the collection of biological samples. We wish to gratefully acknowledge the 2 families for participating in the study. We thank Gwendoline Leroy.
This work was supported by grants from Ligue Nationale Contre le Cancer “Equipe labellisée 2012,” Association pour la Recherche sur le Cancer (projet libre), Agence Nationale de la Recherche (thrombocytose), Agence Nationale de la Recherche, programme Jeunes Chercheuses et Jeunes Chercheurs, Programme Hospitalier de Recherche Clinique (AOR07014) (S.G.), Association pour la Recherche sur la Moelle Osseuse, and Institut National de la Santé et de la Recherche Médicale. C. Marty was funded by a postdoctoral fellowship from Agence Nationale de la Recherche Blanc and supported by a Laboratory of Excellence Globule Rouge-Excellence fellowship. R.K. received funding from the Austrian Science Fund (P23257-B12) and the MPN Research Foundation. Laboratory of Excellence Globule Rouge-Excellence (I.P., W.V.) is funded by the program “Investissements d’avenir.” Support to S.N.C. is acknowledged from the Fonds de la Recherche Scientifique-Fonds National de Recherche Scientifique and Fonds de la Recherche Scientifique Médicale, the Salus Sanguinis Foundation, the Association pour la Recherche sur le Cancer MEXP31C1 and Agence pour la Recherche sur le Cancer 10/15-027 program of the Université Catholique de Louvain, the Fondation contre le Cancer, the Belgium Interuniversity Attraction Poles Program, the St Luc Foundation, and the Ludwig Institute for Cancer Research. C.P. was supported by Fonds de la Recherche Scientifique-Fonds National de Recherche Scientifique, and E.L. by Industrial and Agricultural Research Fund's PhD fellowship of the FRS-FNRS Belgium.
Authorship
Contribution: I.P. and C.B.-C. designed and performed research, analyzed data, prepared figures, and wrote the paper; W.V. conducted research, analyzed data, and wrote the paper; C. Marty performed research, analyzed data, prepared figures, and wrote the paper; C.S.-M. performed research and analyzed data; A.N. conducted the study; S.N.C. conducted the study (luciferase tests, in silico modeling) and gave intellectual input; R.K. conducted the study on allele-sharing SNP genotyping analysis (SEGEX) analysis; A.S.H. performed allele-sharing SNP genotyping analysis analysis; E.L., C. Mouton, and C.P. performed experiments (luciferase tests, in silico modeling); E.S. discussed data and wrote the manuscript; R.F. did the MPL sequencing and TPO measurements; J.-F.A. was the clinician in charge of the families; J.S. performed semisolid experiments on primary cells; S.G. performed molecular biology and signaling studies; A.T. performed clonality analysis; and all authors contributed to writing and editing the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christine Bellanné-Chantelot, Hôpital Pitié-Salpétrière, 47/83 Bd de l’Hôpital, 75013 Paris, France; e-mail: christine.bellanne-chantelot@psl.aphp.fr; and Isabelle Plo, INSERM UMR1009, Institut Gustave Roussy, 114 rue Edouard Vaillant, 94805 Villejuif, France; e-mail: isabelle.plo@igr.fr.
References
Author notes
I.P. and C.B.-C. contributed equally to this study.

![Figure 2. Scheme and in silico modeling of the JAK2 mutants. (A) Scheme and location of germ-line JAK2 mutations. (B) Superimposition of the crystal structures of the active JH1 kinase and JH2 pseudokinase domains of JAK2 wild type (WT) based on 2 radiograph crystal structure studies.20,21 On the left in blue: coordinate of active JH1 (protein data bank [PDB] code: 2B7A). On the right in green: coordinate of active JH2 (PDB code: 4FVQ). Two of the mutations, R938Q and R867Q, are located in the JH1, whereas the S755R mutation is located in the JH2 (pink residues). The germ-line JAK2 S755R/R938Q mutant therefore contains mutations in both JH2 and JH1. The activation loop is represented in red in both JH1 and JH2. The glycine-rich loop is in yellow, and the catalytic loop is in orange. The salt bridge between D869 and R867 is only formed in the inactive JH1, which is the coordinate represented on the small square on the left. The coordinate of inactive JH1 is PDB code 3UGC. The salt bridge between K752 and D635 is formed in active JH2 (WT and V617F). The coordinate shown here in the small square on the right is the active JH2 WT (PDB code: 4FVQ).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/9/10.1182_blood-2013-05-504555/4/m_1372f2.jpeg?Expires=1769901287&Signature=Qy6H6ghLEw1PTfL8aeGTjQvgqrdM6O~5AohxIraxVLl89yExAcm5vXeKlrvUuizfyDG5VhpD0XLQIbgxKribi8za9e5K6sha1m7vwlHht43vTc6SQPiDodTGk6ZkTLPU~Jf25rKOKWdTKr5zwm6fSJqqRf6Gsre5A4mEvwHgASBoH-KiNskcGzfud5VZVwrKgKUaYBpg0BPEkPQuyBtOtbU6PFBiunt4Xu9vIAk6bYwHFZRrfgy2gMKlqZqwhhg7eT1a48GpBHthm91mC3QlZ1Q~1pdmymSWC0dkadOxlkk-I7kJrSTFg6rtrNZQHqU862KCfmcCSfTNol2O7cTxdQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. Signaling studies in Ba/F3-EPOR and Ba/F3-MPL cells and STAT activities in γ2A cells. (A) Ba/F3-EPOR or (B) Ba/F3-MPL cells expressing the different JAK2 constructs were serum- and cytokine-starved for 6 hours prior to a 15-minute stimulation with (A) 1 U/mL EPO and (B) 0.05 and 5 ng/mL TPO at 37°C, as indicated. Cells were lysed, and the phosphorylation status of STAT1, STAT3, STAT5, AKT, and ERK1/2 was examined by western blotting with the respective anti-phospho specific antibodies, as indicated. Expression of HSC70 in the samples was used as loading control and was consistent with expression of total AKT, ERK1/2, and the individual STAT isoforms. Blots shown were reproduced in 2 independent experiments. The quantification of the phospho-STAT5 (P-STAT5) blots for unstimulated Ba/F3-EPOR and Ba/F3-MPL were shown as P-STAT5/HSC70 ratios (arbitrary units [AU]) and displayed below the figures. (C) JAK2-deficient γ2A cells were transfected to express the various JAK2 mutants in the presence of equal amounts of JAK2 WT and MPL. STAT1-, STAT3-, or STAT5-dependent transcriptional activity was measured 24 hours after transfection by the dual firefly (pGRR5 reporter responding to STATs) and renilla (pRL-TK reporter with constitutive expression) luciferase system. Shown are averages ± SEM of 9 experiments in triplicate. Two-tailed Student t test: *P < .05; **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/123/9/10.1182_blood-2013-05-504555/4/m_1372f4.jpeg?Expires=1769901287&Signature=rkkZK3lvKPG9URQmoLmGg3vOsU9OTnSedWgNBZz3AxQMWTQjeMEv3DJJJMbXJ~gZigFmDrMK6dOkiPiepiXA4u6i~PajNIadwtDBzdH~Jm1ceC~gj30P310GNdrvI4lJc-d2yeWmraOD06fSYWmzjIKWx2GFo1dIXV3P-l66W1PCqz0jm8wi6OnnKO5rOzF3rWgh0bEhIp8oZLHv9VuE~JPOM2VPBLbDg8MuR5MyepOsVY1M3SvvYQ14FkzDbHaZnnBQ7OheYLnvFmRgaeWJm06d-1ow1P6ijbM~Id~7zcv84J3BHhNKdt2OLPjap7KC8-MEc8jr1ZVXY39~B5WMKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)