Abstract
Background:
Therapy-related myeloid neoplasms develop after cytotoxic chemotherapy or radiation therapy. The available data on the outcome of pts with t-de novo AML without antecedent history of myelodysplastic syndrome (MDS) is limited.
Methods:
We reviewed the records of pts with newly diagnosed AML who presented to our tertiary care center from 1/2000 to 1/2014. t-de novo AML was defined as having at least 20% blasts in bone marrow with a history of any previous cytotoxic chemotherapy or radiation therapy, and without an antecedent history of MDS. Leukemia-free survival (LFS) was defined as time from achieving complete response (CR) to relapse or death. The overall survival (OS) and LFS in pts with t-de novo AML were compared to those of with de novo AML with normal karyotype (NK) and complex karyotype (CK).
Results:
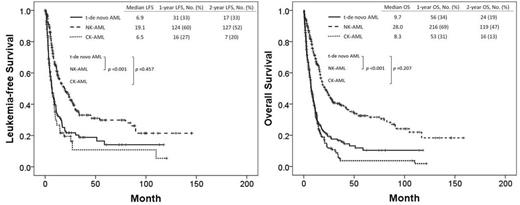
Among 1677 pts with newly diagnosed AML, 383 had de novo NK-AML, 218 had de novo CK-AML, and 187 had t-de novo AML. The median follow-up was 9.3 months (range; 0.2-161.0). Pt characteristics and outcomes are described in Table 1. Among the 187 pts, 69 had a history of lymphoma; 63 pts breast cancer (Ca); 10 pts colon Ca; 10 pts sarcoma; 8 pts prostate; 7 pts bladder Ca; 6 pts uterine Ca; 5 pts lung Ca; 5 pts head and neck Ca; 30 pts other type of Ca. Among the pts with t-de novo AML, 15 pts (8%) had a favorable-risk karyotype by WHO, 53 pts (28%) intermediate-risk karyotype, and 119 pts (64%) poor-risk karyotype. The median LFS duration in t-de novo AML, NK-AML, and CK-AML were 7 months (95% confidence interval [CI]; 5.1-8.7), 19 months (95% CI; 13.0-25.2), and 6 months (95% CI; 9.0-13.5) (p<.001), respectively. The median OS duration in t-de novo AML, NK-AML, and CK-AML were 7 months (95% CI; 5.9-8.9), 21 months (95% CI; 16.2-25.5), and 12 months (95%CI; 10.6-13.5) (p<.001), respectively. The results of univariate (UVA) and multivariate analysis (MVA) associated with OS were summarize in Table 1. MVA identified age over 60, white blood cell count (WBC) over 10 x103/µL, thrombocytopenia below 30 x103/µL, non-favorable cytogenetic abnormalities, positive RAS mutation, and the absence of CR or CR with incomplete platelet recovery (CRp) as poor prognostic features related to OS.
Conclusion:
LFS and OS were shorter in patients with t-de novo AML than in those with NK-AML but did not differ significantly from patients with CK-AML.
Patient Characteristics and Outcomes
| . | t-de novo AML [n= 187] . | de novo AML with NK [n= 383] . | de novo AML with CK [n= 218] . | P . | |
|---|---|---|---|---|---|
| Age at diagnosis, median (years) | 64 (21-89] | 63 (17-90) | 67 (18-87) | ||
| Prior radiation therapy, No. (%) | 101 (54) | 0 | 0 | ||
| Prior chemotherapy, No. (%) | 186 (99) | 0 | 0 | ||
| White blood cell count at diagnosis, median (x103/µL) | 3.2 (0.2-191) | 4.3 (0.2-390.0) | 2.9 (0.5-278.2) | ||
| Hemoglobin at diagnosis, median (g/dL) | 9.1 (4.5-12.9) | 9.1 (4-14.6) | 9.0 (2.5-14.2) | ||
| Platelet count at diagnosis, median (x103/µL) | 34 (4-454) | 51 (3-469) | 42 (2-319) | ||
| LDH at diagnosis, median (IU/L) | 1359 (210-22090) | 1189 (200-42000) | 1274 (231-20572) | ||
| Peripheral blood blast percent at diagnosis, median (%) | 8 (0-98) | 9.5 (0-98) | 10 (0-98) | ||
| Bone marrow blast percent at diagnosis, median (%) | 41 (0-96) | 44 (0-96) | 33 (0-97) | ||
| Molecular genetic abnormalities at diagnosis, No. (%) | |||||
| FLT3-ITD | 17 (9) | 96 (25) | 5 (2) | ||
| FLT3-D835 | 6 (3) | 23 (6) | 1 (1) | ||
| NPM1 | 7 (4) | 104 (27) | 4 (2) | ||
| JAK2 | 3 (2) | 6 (2) | 8 (4) | ||
| RAS | 17 (9) | 50 (13) | 8 (4) | ||
| RUNX1-RUNX1T1 | 4 (2) | 0 | 0 | ||
| CBFb-MYH | 6 (3) | 0 | 0 | ||
| Response, No. (%) | <0.001 | ||||
| Complete response | 89 (48) | 237 (62) | 76 (35) | ||
| Complete response without platelet recovery | 15 (8) | 1 (0) | 15 (7) | ||
| 1-year LFS, (%) | 33 | 60 | 27 | <0.001 | |
| 2-year LFS, (%) | 33 | 52 | 20 | <0.001 | |
| 1-year OS, (%) | 34 | 68 | 30 | <0.001 | |
| 2-year OS, (%) | 24 | 46 | 13 | <0.001 | |
| UVA and MVA of OS in t-de novo AML | |||||
| UVA | MVA | Hazard ratio | 95% CI | ||
| Age at diagnosis | |||||
| Age =< 60 years | - | ||||
| Age >60 years | < .001 | .001 | 2.238 | 1.417-3.534 | |
| White blood cell count (WBC) (x103/µL) | |||||
| WBC =< 10.0 | - | ||||
| WBC > 10.0 | .002 | .037 | 1.617 | 1.030-2.540 | |
| Hemoglobin (Hgb) (g/dL) | |||||
| Hgb >= 8 | - | ||||
| Hgb < 8 | .749 | ||||
| Platelet count (Plt) (x103/µL) | |||||
| Plt >= 30 | - | ||||
| Plt < 30 | .008 | .004 | 1.852 | 1.224-2.803 | |
| LDH (IU/L) | |||||
| LDH =<1000 | - | ||||
| LDH > 1000 | .640 | ||||
| Peripheral blood blast percent (PB blast)(%) | |||||
| PB blast =< 10% | - | ||||
| PB blast >10% | .178 | ||||
| Bone marrow blast percent (BM blast) (%) | |||||
| BM blast =<40% | - | ||||
| BM blast >40% | .393 | ||||
| Cytogenetic abnormality | |||||
| Favorable | - | ||||
| Non-Favorable | .002 | .019 | 5.836 | 1.342-25.370 | |
| FLT3-ITD | |||||
| Negative | - | ||||
| Positive | .768 | ||||
| RAS | |||||
| Negative | - | ||||
| Positive | .047 | .003 | 2.576 | 1.367-4.856 | |
| Response | |||||
| CR or CRp | - | ||||
| Non-CR or CRp | <.001 | .009 | .331 | 0.145-0.757 | |
| CR | - | ||||
| Non-CR | <.001 | 0.903 | .950 | 0.418-2.160 | |
| . | t-de novo AML [n= 187] . | de novo AML with NK [n= 383] . | de novo AML with CK [n= 218] . | P . | |
|---|---|---|---|---|---|
| Age at diagnosis, median (years) | 64 (21-89] | 63 (17-90) | 67 (18-87) | ||
| Prior radiation therapy, No. (%) | 101 (54) | 0 | 0 | ||
| Prior chemotherapy, No. (%) | 186 (99) | 0 | 0 | ||
| White blood cell count at diagnosis, median (x103/µL) | 3.2 (0.2-191) | 4.3 (0.2-390.0) | 2.9 (0.5-278.2) | ||
| Hemoglobin at diagnosis, median (g/dL) | 9.1 (4.5-12.9) | 9.1 (4-14.6) | 9.0 (2.5-14.2) | ||
| Platelet count at diagnosis, median (x103/µL) | 34 (4-454) | 51 (3-469) | 42 (2-319) | ||
| LDH at diagnosis, median (IU/L) | 1359 (210-22090) | 1189 (200-42000) | 1274 (231-20572) | ||
| Peripheral blood blast percent at diagnosis, median (%) | 8 (0-98) | 9.5 (0-98) | 10 (0-98) | ||
| Bone marrow blast percent at diagnosis, median (%) | 41 (0-96) | 44 (0-96) | 33 (0-97) | ||
| Molecular genetic abnormalities at diagnosis, No. (%) | |||||
| FLT3-ITD | 17 (9) | 96 (25) | 5 (2) | ||
| FLT3-D835 | 6 (3) | 23 (6) | 1 (1) | ||
| NPM1 | 7 (4) | 104 (27) | 4 (2) | ||
| JAK2 | 3 (2) | 6 (2) | 8 (4) | ||
| RAS | 17 (9) | 50 (13) | 8 (4) | ||
| RUNX1-RUNX1T1 | 4 (2) | 0 | 0 | ||
| CBFb-MYH | 6 (3) | 0 | 0 | ||
| Response, No. (%) | <0.001 | ||||
| Complete response | 89 (48) | 237 (62) | 76 (35) | ||
| Complete response without platelet recovery | 15 (8) | 1 (0) | 15 (7) | ||
| 1-year LFS, (%) | 33 | 60 | 27 | <0.001 | |
| 2-year LFS, (%) | 33 | 52 | 20 | <0.001 | |
| 1-year OS, (%) | 34 | 68 | 30 | <0.001 | |
| 2-year OS, (%) | 24 | 46 | 13 | <0.001 | |
| UVA and MVA of OS in t-de novo AML | |||||
| UVA | MVA | Hazard ratio | 95% CI | ||
| Age at diagnosis | |||||
| Age =< 60 years | - | ||||
| Age >60 years | < .001 | .001 | 2.238 | 1.417-3.534 | |
| White blood cell count (WBC) (x103/µL) | |||||
| WBC =< 10.0 | - | ||||
| WBC > 10.0 | .002 | .037 | 1.617 | 1.030-2.540 | |
| Hemoglobin (Hgb) (g/dL) | |||||
| Hgb >= 8 | - | ||||
| Hgb < 8 | .749 | ||||
| Platelet count (Plt) (x103/µL) | |||||
| Plt >= 30 | - | ||||
| Plt < 30 | .008 | .004 | 1.852 | 1.224-2.803 | |
| LDH (IU/L) | |||||
| LDH =<1000 | - | ||||
| LDH > 1000 | .640 | ||||
| Peripheral blood blast percent (PB blast)(%) | |||||
| PB blast =< 10% | - | ||||
| PB blast >10% | .178 | ||||
| Bone marrow blast percent (BM blast) (%) | |||||
| BM blast =<40% | - | ||||
| BM blast >40% | .393 | ||||
| Cytogenetic abnormality | |||||
| Favorable | - | ||||
| Non-Favorable | .002 | .019 | 5.836 | 1.342-25.370 | |
| FLT3-ITD | |||||
| Negative | - | ||||
| Positive | .768 | ||||
| RAS | |||||
| Negative | - | ||||
| Positive | .047 | .003 | 2.576 | 1.367-4.856 | |
| Response | |||||
| CR or CRp | - | ||||
| Non-CR or CRp | <.001 | .009 | .331 | 0.145-0.757 | |
| CR | - | ||||
| Non-CR | <.001 | 0.903 | .950 | 0.418-2.160 | |
Kantarjian:ARIAD, Pfizer, Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.