Key Points
The outcome of HSCT in this large SCN cohort is acceptable.
Given the TRM, a careful selection of HSCT candidates should be undertaken.
Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative treatment of severe congenital neutropenia (SCN), but data on outcome are scarce. We report on the outcome of 136 SCN patients who underwent HSCT between 1990 and 2012 in European and Middle East centers. The 3-year overall survival (OS) was 82%, and transplant-related mortality (TRM) was 17%. In multivariate analysis, transplants performed under the age of 10 years, in recent years, and from HLA-matched related or unrelated donors were associated with a significantly better OS. Frequency of graft failure was 10%. Cumulative incidence (day +90) of acute graft-versus-host disease (GVHD) grade 2-4 was 21%. In multivariate analysis, HLA-matched related donor and prophylaxis with cyclosporine A and methotrexate were associated with lower occurrence of acute GVHD. Cumulative incidence (1 year) of chronic GVHD was 20%. No secondary malignancies occurred after a median follow-up of 4.6 years. These data show that the outcome of HSCT for SCN from HLA-matched donors, performed in recent years, in patients younger than 10 years is acceptable. Nevertheless, given the TRM, a careful selection of HSCT candidates should be undertaken.
Medscape Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1970.
Disclosures
The authors, Associate Editor Catherine M. Bollard, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Describe outcomes of hematopoietic stem cell transplantation (HSCT) in patients with severe congenital neutropenia (SCN).
Determine predictors of outcomes of HSCT in patients with SCN.
Assess the clinical implications of the findings from this study of 136 patients with SCN treated between 1990 and 2012 in European and Middle East centers.
Release date: October 15, 2015; Expiration date: October 15, 2016
Introduction
The severe congenital neutropenias (SCNs) are a group of congenital disorders characterized by a persistent absolute neutrophil count (ANC) <0.5 × 109/L because of a lack of promyelocyte maturation and by dependency on granulocyte–colony-stimulating factor (G-CSF) therapy.1 A mutation is found in almost 60% of patients; ELANE and HAX1 are the genes most commonly involved in the sporadic/autosomal dominant and recessive forms.2-4 Other genes, such as GFI1, WASP, and G6PC3, are less frequently mutated.5-7 SCN patients are at risk of developing potentially lethal infections. Before the introduction of G-CSF, SCN was associated with a mortality risk of up to 50% in the first year of life because of bacterial infections. After the introduction of G-CSF, the prognosis of SCN has dramatically improved,8 with long-term survival up to 95%.9 SCN patients are also at considerable risk of transforming events such as myelodysplastic syndrome (MDS) and/or acute leukemia (AL) with a cumulative incidence of 22% and 11% according to the Severe Chronic Neutropenia International Registry and the Severe Chronic Neutropenia French Registry, respectively.9,10 In these patients, the only curative treatment is hematopoietic stem cell transplantation (HSCT). Indications for HSCT and transplant modalities are based on single case reports, small cohorts of patients,11-27 and national group guidelines.28 Information derived by these heterogeneous sources report survival after HSCT at ∼80% and suggest that the presence of MDS/acute myeloid leukemia at HSCT and the use of matched unrelated donors (MUDs) have a negative impact on the outcome.12-17 The databases of the Severe Aplastic Anemia, the Inborn Error and the Pediatric Disease Working Parties of the European Society for Blood and Marrow Transplantation (EBMT) and of Stem Cell Transplant for Immunodeficiencies in Europe (SCETIDE) contain data on a large number of SCN patients who underwent HSCT, thus providing a unique opportunity to obtain evidence supporting treatment decision making. The aim of the present cooperative study was to evaluate the outcome of HSCT in SCN patients reported to the previously mentioned databases and to identify risk factors for outcome.
Patients and methods
Data sources
This is a multicenter retrospective study conducted in collaboration with the Severe Aplastic Anemia, the Inborn Error and the Pediatric Disease Working Parties of the EBMT and the SCETIDE. These groups collect data from patients with diseases eligible for HSCT by using standard data collection forms. In the case of SCN, specific items that were not included in the EBMT and SCETIDE data collection forms were requested through a specifically designed questionnaire sent to all participating centers. This questionnaire required data on molecular characteristics of the disease, pre-HSCT treatment, pretransplant infections, hospitalizations, and clinical status at time of transplant. All data were carefully monitored, and institutions were contacted in case of inconsistencies. Informed written consent was obtained according to the Helsinki Declaration. All data, including those collected through SCETIDE, were incorporated into the EBMT data set.
Inclusion criteria
All patients affected with SCN who underwent any type of first HSCT and who were reported to the EBMT and SCETIDE Registries between 1990 and 2012 entered this study. Poor G-CSF response, poor control of infections, and clonal transformation were indications for HSCT in 72% of the patients. In the remaining 28%, the decision to perform HSCT was either based on individual center policy or not reported.
Definitions
Overall survival (OS) was calculated from the date of HSCT to the date of last follow-up or to the date of death from any cause. Event-free survival (EFS) was calculated from the date of HSCT to the date of graft failure, secondary graft loss, second transplant, or death, which were considered as events. HLA matching was defined by transplant centers as matched or mismatched. Definition of matching was time dependent because the number of tested HLA antigens changed over time. In general, a mismatched donor was considered as having 1 or more mismatched HLA antigens. It was assumed that for transplants performed from the early 2000s onward allele matching was adopted. Engraftment was defined as the achievement of an ANC of ≥0.5 × 109/L for at least 3 consecutive days. Primary graft failure was defined as a neutrophil count never reaching ≥0.5 × 109/L. Secondary graft failure was defined as a neutrophil of ≥0.5 × 109/L for at least 2 consecutive days and subsequently decreasing to a lower level. Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were defined according to published criteria.29,30 The type of conditioning regimens were defined as reduced-intensity conditioning (RIC) and myeloablative according to the Center for International Blood and Marrow Transplant Research definition.31 MDS and AL were defined according to the classical definition. Second malignancies were reported as late events.
Statistical methods
OS and EFS probabilities were estimated by the Kaplan-Meier method, and the log-rank test was used to assess differences in univariate analysis. The cumulative incidence of engraftment, aGVHD, and cGVHD were estimated by the proper nonparametric estimator taking competing risks into account, and differences were assessed by the Gray test. Multivariate analyses were performed by Cox regression for OS, EFS, aGVHD, and cGVHD, considering age at transplantation, calendar year, donor type, source of cells, type of conditioning regimen, GVHD prophylaxis, in vivo T-cell depletion, type of infection at transplant, and presence of MDS/AL. Factors that were significant at a 20% level in univariate analysis were candidates to be included in the models. The functional form of the effect of age and calendar year considered as continuous variables was checked by the analysis of martingale residuals. The variable donor was included as categorical with 3 levels. However, in view of the nonrelevant difference between matched-related donor and MUD in the models for OS and EFS in univariate analysis, these 2 categories were combined together in the matched category. All tests reported were 2-sided, and the confidence intervals (CIs) were computed at 95% confidence level.
Results
Characteristics of the cohort
From 1990 to 2012, 136 patients with SCN underwent HSCT from an HLA-matched related donor (N = 61), HLA-MUD (N = 61), and mismatched donor (N = 14, 4 unrelated and 10 related). Seventy percent of patients originated from Western Europe and 30% from Eastern Europe/Middle East area (Russia, Iran, Israel, Saudi Arabia, and Turkey). There were similar numbers of males and females in each group. Fifteen percent of patients were aged >15 years, and 6% were older than 20 years at transplant. Median age at diagnosis was 0.4 years (range, 0-35.5), and the median age at HSCT was 4.7 years (range, 0.23-43.1). Median follow-up was 4.6 years (range, 2 months to 18 years). Twenty-two (16%) patients were transplanted before or during the year 2000 and the remaining 114 (84%) after the year 2000. Stem cell source was bone marrow (BM) in 58% of patients, cord blood (CB) in 18%, and peripheral blood (PB) in 24% of cases. Further patient characteristics are reported in Table 1.
Disease status before HSCT
Unfortunately, information regarding the status of SCN disease before HSCT was incomplete with an average of ∼30% missing data. As shown in Table 1, percentages were calculated on the available data. Based on this, 71% of the patients carried a known mutation (mainly ELANE and HAX1), 79% of patients were on a G-CSF dose higher than the usual 5 mcg/kg per day, and 89% had some infections prior to HSCT while on G-CSF treatment. Infections were grouped as severe (37%) including sepsis, deep infections of gastrointestinal tract and liver, osteomyelitis, and meningitis; skin/subcutaneous and mouth infections (27%); and respiratory tract infections, otitis, and otomastoiditis (35%). Eighty-four percent of patients had had at least 1 hospitalization for infection. These infections were not active at the time of transplant. Sixteen percent of patients had transformed to MDS/AL before HSCT. No further details on the subtype of MDS or on AL remission status were available. Information on transformation was available in 64% of patients.
Graft failure
Probability of graft failure after 90 days from HSCT was 10%. Graft failure was mostly primary (65%) and more rarely secondary (35%) and was not associated with a specific type of infection before HSCT, nor with any specific mutation or presence/absence of MDS/AL. Probability of engraftment was 92% in HLA-matched (related and unrelated) and 72% in mismatched donor (P = .094) HSCT. Differences observed according to stem cell source (BM, 89%; CB, 68%; PB, 84%; P = .274) and to the type of conditioning regimen (RIC, 94% vs myeloablative regimen, 82%; P = .29) were not significant. Among the 12 patients with graft failure, 6 underwent a second HSCT that was successful in 5. The 6 patients who did not receive a second transplant died.
GVHD
The 90-day cumulative incidence of aGVHD grade 2 to 4 was 21%. In univariate analysis, only 2 factors affected aGVHD. These were (1) the use of an HLA-matched related donor, associated with a significantly lower rate (9%) of GVHD compared with an HLA-MUD (31%, P = .006); and (2) prophylaxis with CSA plus methotrexate, associated with a significantly lower incidence of GVHD compared with CSA alone (11% vs 31%; P = .006) (Table 2). These 2 factors were also significant in multivariate analysis.
Cumulative incidence of cGVHD was 20%, 26%, and 28% at 12, 36, and 120 months after HSCT, respectively. The incidence of cGVHD at 12 months was higher in patients older than 10 years (42%) compared with those younger than 10 years (11%; P = .001) and in patients who received PB rather than CB or BM cells (Gray test; P = .006). The use of ATG was not significantly associated with the incidence of cGVHD (P = .239; Table 2). In multivariate analysis, age >10 years was confirmed to negatively affect the incidence of cGVHD, whereas the use of PB showed a trend toward a negative association without reaching significance.
OS
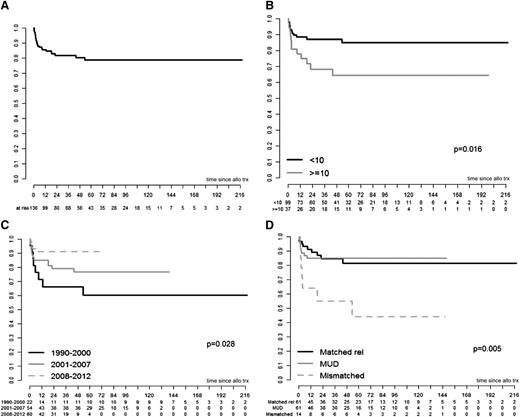
The 3-year probability of OS following HSCT was 82% (Figure 1A). Twenty-three patients died of transplant-related causes, thus accounting for a transplant-related mortality (TRM) rate of 17%, and 2 died because of disease progression or infection. Specific causes of death were aGVHD and cGVHD (n = 8, 32%), infections (n = 5, 20%), organ damage (n = 4, 16%) and other combined causes related to transplant (n = 6, 24%; Table 3). For patients who died, the median time from transplantation to death was 3 months. Eighty percent of deaths occurred within 12 months after transplantation. No significant reduction in the overall mortality was observed in recent years (data not shown).
OS curves. (A) OS. (B) OS by age. (C) OS by calendar period. (D) OS by donor type. The curves in panels C and D are shown for the maximal follow-up and were not statistically analyzed past the point of <5 patients. Mismatched includes both related and unrelated donors.
OS curves. (A) OS. (B) OS by age. (C) OS by calendar period. (D) OS by donor type. The curves in panels C and D are shown for the maximal follow-up and were not statistically analyzed past the point of <5 patients. Mismatched includes both related and unrelated donors.
In univariate analysis (Table 4), 3-year OS was significantly higher in transplants performed before the age of 10 years vs those performed after this age (87% vs 68%; P = .016; Figure 1B), in HSCTs carried out in more recent years over those performed in the past decades (66% in 1990-2000, 79% in 2001-2007, 91% in 2008-2012; P = .028; Figure 1C), and in HSCTs performed from HLA-matched donors, related or unrelated) vs HLA mismatched donors (85% vs 85% vs 55%; P = .005; Figure 1D). GVHD prophylaxis with CSA and methotrexate was associated with significantly better OS than with CSA alone (91% vs 79%; P = .038). Regarding stem cell source, the OS appeared to be higher with CB and BM (92% and 82%, respectively) compared with PB (75%), without reaching significance (P = .128). The remaining tested factors (presence of MDS/AL at transplant, type of infectious episodes at transplant, type of conditioning regimen, and use of ATG) were not significantly associated with OS in univariate analysis (Table 4). In multivariate analysis, only age at transplant <10 years, transplant in more recent years, and the use of an HLA-matched donor were positive prognostic factors (Table 5).
EFS
The EFS probability at 3 years after transplantation was 71%. Sixty-seven percent of events occurred within 1 year after transplantation. In univariate analysis (Table 4), EFS was significantly better in patients transplanted from HLA-matched related (74%) or unrelated (71%) compared with HLA-mismatched donors (40%; P = .02). There was a trend in favor of improved EFS over time (53% in patients transplanted between 1990 and 2000, 70% in those transplanted between 2001 and 2007, and 79% between 2008 and 2012), but this did not reach statistical significance (P = .06). Similar to OS, EFS looked higher in patients transplanted with BM vs CB vs PB (76%, 70%, and 59%, respectively), although this difference was not significant (P = .130). All other tested factors (presence of MDS/AL at transplant, type of infections, type of conditioning regimen, and use of ATG) were not significantly associated with EFS. In multivariate analysis, transplant in more recent years and use of an HLA-matched donor were favorably associated with EFS (Table 5).
Late malignancies
No secondary malignancies occurring after HSCT have been reported in the study population after a median follow-up of 4.6 years (range, 2 months to 18 years).
Discussion
SCN is a rare disorder mostly observed by pediatric hematologists that, given the improvement in long-term survival, may occasionally be managed also by general adult hematologists, as indicated by the relatively high proportion (15%) of patients in our cohort who were older than 15 years. The existing literature on HSCT in SCN is based only on short series or case reports. The present study describes the outcome of HSCT for SCN in the largest ever reported cohort of 136 patients. Consistent with literature recommendations,28,32,33 the main reasons for HSCT were the need for high doses of G-CSF (>5 mcg/kg per day), a high rate and poorly manageable infections or transformation to MDS/AL. In our study 16% of patients received HSCT because of MDS/AL. The high number of missing data may render this figure difficult to interpret, but this rate appears to be in line with the cumulative incidence reported in the literature.9,10,32
Our study shows that 3-year OS and EFS are 82% and 71%, respectively, and that TRM, mainly because of GVHD and infections, is 17%. These data suggest that HSCT is a good option for SCN patients who are no longer eligible to G-CSF treatment because of high-dose requirement, high infection rate, and signs of transformation to MDS/AL.
Better OS and EFS were observed in patients who were transplanted in the most recent years and in subjects younger than 10 years. The first finding, also reported in other diseases, may be because of high resolution HLA-matching, the use of less toxic conditioning regimens, and improved supportive care.34-37 The positive effect of younger age at time of HSCT may be explained by a lower exposure to infections. Not surprisingly, OS and EFS were better when an HLA-matched donor, either sibling or unrelated, was used over a mismatched donor.
As for the source of stem cells, BM was most frequently used and was associated with a very good OS of 82% and EFS of 76%. The 2 other stem cell sources, CB and PB, provided a lower EFS, although the difference was not significant. Of note, the use of PB was associated with a significantly higher rate of cGVHD in univariate analysis. However, in multivariate analysis, this association was not significant. Because in our cohort GVHD was the most frequent cause of death, it would be reasonable, as with other marrow failure disorders,35-38 to consider BM as the preferred source of transplantable cells in SCN.
The presence of MDS/AL at transplant was not associated with a significantly reduced OS. This is in contrast to other reports where survival was significantly worsened by this factor13,39 and needs to be carefully evaluated. Indeed, because of missing data regarding this item in general and particularly on MDS subtype and on remission status of AL, we cannot draw definitive conclusions on the impact of MDS/AL on the outcome of HSCT.
Successful engraftment occurred in 90% of patients. Myeloablative conditioning regimens did not confer a higher engraftment rate compared with RIC. However, this finding should be interpreted with caution because of the lack of data on chimerism. Indeed, in a precancer disease like SCN, full donor chimerism is very important to achieve in order to prevent the reemergence of predisposed clones in the recipient marrow. Our study is not conclusive regarding the recommended conditioning regimen to use in HSCT for SCN, for which comparative data on chimerism after RIC vs myeloablative regimen are required. Engraftment seemed to be higher in HLA-matched (related and unrelated) over mismatched donor HSCT, although this difference did not achieve significance (P = .094). This lack of significance may be because of the limited number of mismatched donor HSCTs, and therefore, our findings cannot provide conclusive answers regarding the effect of the HLA matching on engraftment.
The incidence of aGVHD grade 2-4 was higher than in other nonmalignant diseases.33-36 The combination of CSA and methotrexate favorably affected not only aGVHD but also OS and EFS over methotrexate alone. The latter finding is in keeping with the fact that GVHD was the most frequent cause of death and points to this combination as the recommended regimen for GVHD prophylaxis in SCN transplants. The only factor strongly associated with an increased the risk of cGVHD was age at transplant under 10 years. The use of PB as source of stem cell negatively affected cGVHD occurrence in univariate analysis and showed a negative trend (not significant) in multivariate analysis. This finding may support the decision not to use PB as the stem cell source in SCN transplants.
Our work has strengths and limitations. The strengths include the large number of patients registered in European and non-European countries, the streamlined study design, and the detailed statistical analysis, enabling us to produce evidence to support the treatment decision-making processes. This is of major relevance in a rare disease like SCN, which entails the innate difficulty of performing large controlled studies and that may occasionally be seen by general hematologists.
Limitations are mainly related to the retrospective nature of the study, the heterogeneity of conditioning regimens, changes in HSCT procedures during the study period, and incompleteness of the data set regarding pre-HSCT variables. These aspects are, however, inherent in all registry studies but do not undermine the main messages of our work.
In summary, our study shows that HSCT performed from a matched donor has a good outcome in SCN patients who pre-HSCT require high doses of G-CSF and have a high infection rate, although the TRM rate of 17% indicates a very careful selection of HSCT candidates. The preferred source of stem cells should be BM, and the optimal GVHD prophylaxis regimen the combination of CSA and methotrexate.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all centers, including those who contributed data of <3 patients.
ERG s.p.a., Rimorchiatori Riuniti-Genova, Cambiaso & Risso Marine-Genova, and Saar Depositi Oleari Portuali-Genova are acknowledged for financial support of the activity of the Clinical & Experimental Unit of G. Gaslini Children's Hospital, Genoa.
This paper was also made possible thanks to the collaboration of the Inborn Error, the Pediatric Disease Working Parties of the EBMT and the SCETIDE.
Authorship
Contribution: C.D., F.F., and J.M. conceived and designed the study; A.v.B. and N.v.V.-T. collected materials; S.I. and L.d.W. performed the statistical analysis; B.G., P.A., J.D., M.A., C.P., S.M.-M., A.A.S., A.Y., A.F., M.B., G.O., O.S., P.L., R.P.D., A.G., B.A., R.W., K.K., and J.S.d.T.C. provided materials; F.F., C.D., B.M., S.I., and M.C. analyzed and interpreted the data; F.F. and C.D. wrote the manuscript; and S.I., A.v.B., B.G., P.A., J.D., M.A., C.P., M.C., S.M.-M., G.M., N.v.V.-T., L.d.W., A.A.S., A.Y., A.F., M.B., G.O., R.O., O.S., P.V., P.L., R.P.D., A.G., B.A., R.W., K.K., and J.M. approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of members of the Severe Aplastic Anemia Working Party appears in “Appendix.”
Correspondence: Francesca Fioredda, Clinical and Experimental Hematology, Giannina Gaslini Children’s Hospital, Largo G Gaslini 5, 16147 Genova, Italy; e-mail francescafioredda@ospedale-gaslini.ge.it.
Appendix: study group members
The members of the Severe Aplastic Anemia Working Party are: Mahmoud Aljurf, King Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia; Mutlu Arat, Florence Nightingale Sisli Hospital, Istanbul, Turkey (994); Andrea Bacigalupo, Ospedale San Martino, Genova, Italy; Fabian Beier, University Hospital Aachen, Aachen, Germany; Nana Benson-Quarm, King’s College Hospital, London, United Kingdom; Marc Bierings, University Hospital for Children, Utrecht, The Netherlands; Tim Brummendorf, University Hospital Aachen, Aachen, Germany; Helen Campbell, Alder Hey Children’s, Liverpool, United Kingdom; Simone Cesaro, Azienda Ospedaliera Integrata Verona, Verona, Italy; Fabio Ciceri, Hospital San Raffaele, Milano, Italy; Raynier Devillier, Institus Paoli Calmettes, Marseille, France; Carlo Dufour, Institute G. Gaslini, Genova, Italy; Francesca Fioredda, Institute G. Gaslini, Genova, Italy; Stijn Halkes, Leiden University Medical Centre, Leiden, The Netherlands; Britta Höchsmann, University of Ulm, Ulm, Germany; Cynthia Huisman, AMC, Amsterdam, The Netherlands; Ayad Ahmed Hussein, King Hussein Cancer Center, Jordan; Edgar Jost, University Hospital Aachen, Aachen, Germany; Austin Kulasekararaj, King’s College Hospital, London, United Kingdom; Per Ljungman, Karolinska University, Stockholm, Sweden; Anna Locasciulli, Ospedale San Camillo Circonvallazione, Roma, Italy; Judith Marsh, King’s College Hospital, London, United Kingdom; Sébastien Maury, Hopital Henri Mondor, Créteil, France; Paul Miller, Antony Nolan, London, United Kingdom; Andrea Delia Moicean, Funeni University Institute, Bucharest, Romania; José Moraleda, Murcia University, Murcia, Spain; Simona Pagliuca, Hopital St. Louis, Paris, France; Jakob Passweg, Basel University Hospital, Basel, Switzerland; Régis Peffault de Latour, Hopital St. Louis, Paris, France; Jouni Pesola, Kuopio Univeristy Hospital, Kuopio, Finland; Andrea Piccin, Ospedale San Marizio, Bolzano, Italy; Marta Pillon, Clinica di Oncoematologia Pediatrica, Padova, Italy; Maria Beatrice Pinazzi, Ospedale San Camillo Circonvallazione, Roma, Italy; Antonio Risitano, University of Napoli, Napoli, Italy; Alicia Rovo, Basel University Hospital, Basel, Switzerland; Elena Salomou, Patras University Medical School, Patras, Greece; Sujith Samarasinghe, Great Ormond Street Hospital for Children, London, United Kingdom; Hubert Schrezenmeier, University of Ulm, Ulm, Germany; John Snowden, Royal Hallamshire Hospital, Sheffield, United Kingdom; Gérard Socié, Hopital St. Louis, Paris, France; Brigitte Strahm, Children Hospital University of Freiburg, Freiburg, Germany; Argiris Symeonidis, Patras University Medical School, Patras, Greece; Louis Terriou, Hopital Huriez, Lille, France; André Tichelli, Basel University Hospital, Basel, Switzerland; Carlos Vallejo, Hospital Universitario Donostia, San Sebastian Gipuzkoa, Spain; Maria Teresa van Lint, Ospedale San Martino, Genova, Italy; Ayami Yoshimi, Children Hospital University of Freiburg, Freiburg, Germany.