Abstract
Background: Ponatinib is a novel TKI efficacious in relapsed refractory patients (pts) with CML and in those with T315I mutation. Despite the achievement of deep early responses in most pts observed in the frontline setting, the concern for arterio-thrombotic events led to the discontinuation (DC) of ponatinib frontline clinical trial. In this study, we have assessed the outcomes after DC of ponatinib of pts in a clinical trial of frontline ponatinib in CML-CP.
Methods: Fifty one pts with CML-CP were treated with frontline ponatinib in a single-arm, clinical trial between May 2012 and September 2013. Initial dose of ponatinib was 45 mg orally daily in 43 pts and, after amendment, 30 mg in 8 pts. All pts DC ponatinib therapy after June 2014 and were switched to another TKI. Patients were assessed for cause of DC, treatment received after ponatinib DC, response achieved/maintained on subsequent TKI, adverse events (AE) and survival after ponatinib DC. Survival was calculated from the time of ponatinib DC to the time of last follow up.
Results: All 51 patients DC ponatinib: 38 per FDA recommendation and 13 due to AE. Median duration of ponatinib therapy was 13.2 months (range-2.1-25.4). At the time of DC, 47/51 (92%) pts were in complete cytogenetic response (CCyR) and 4 (8%) in partial cytogenetic response (PCyR); 1 pt DC before 3-mo evaluation. Forty (78%) pts were in MMR and 26 (51%) in molecular response 4.5-log (MR4.5). Thirty-six (70%) pts were switched to dasatinib, 7 (14%) to imatinib, and 4 (8%) each to nilotinib and bosutinib. After switching to another TKI, with a median of 13 months (range, 0.2 to 26.3) of follow-up, 2 pts have lost their cytogenetic response (both PCyR on ponatinib), 1 pt improved from PCyR to CCyR and one maintained PCyR; all 47 (92%) pts with CCyR on ponatinib maintained this response. Molecular responses improved in some pts: 6 improved from no MMR to MMR; 1 to MR4.5 (median time on ponatinib 4 months; median time on subsequent TKI 17 months); 11 from MMR to MR4.5 (median time on ponatinib 13 months; median time on subsequent TKI 17 months). One pt (treated with imatinib 400) lost MR4.5 to no MMR after 2 months. At last follow-up 37 pts (72%) had MR4.5 and 45 (90%) MMR.
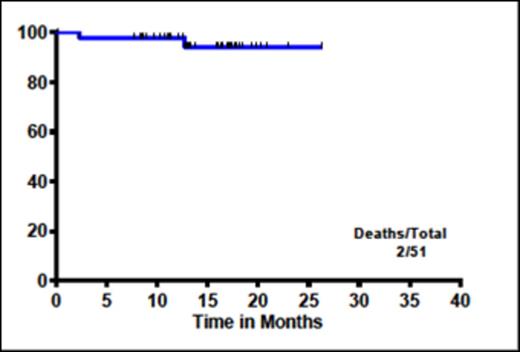
Two pts died after ponatinib DC. One pt was treated with imatinib 400 and developed grade-3 edema and recurrent lung cancer; the second was switched to dasatinib and had recurrent progressive peripheral arterial disease (PAD). Four pts had events (2 deaths, 1 secondary MDS with -7 and 1 pt lost major CyR). Median post ponatinib survival (Figure-1) and median post ponatinib event free survival (not shown) was not reached (1-year OS 98% and EFS 95%). Forty five pts continued on their first post-ponatinib TKIs, 5 required 2 post-ponatinib TKI and 1 pt received 3 different TKIs after DC. The most common cause for post-ponatinib TKI switch was toxicity (n=6).
Of the 36 pts switched to dasatinib, 5 discontinued: 4 due to pleural effusion and 1 with acute renal failure. 29/36 pts (81%) were in MMR before switch and all maintained MMR; 4 pts achieved MMR after switch to dasatinib. Four pts developed grade 3-4 non hematological vascular AEs (3 among pts with such events while on ponatinib and 1 new vascular event within 3 months of ponatinib DC). Of the 4 pts switched to nilotinib, 1 DC within 3 months due to grade-3 pancreatitis and also developed grade-1 pulmonary hypertension with 1 month of discontinuing ponatinib. This pt was then switched to bosutinib. The other 3 pts maintained MMR and had no vascular events. Of the 4 pts switched to bosutinib, one developed MDS, one was switched back to ponatinib off protocol (patient's choice), 1 lost cytogenetic response and one DC therapy after 1 month and maintains MR4.5 (this pt had TIA while on ponatinib, developed cerebral infarct within 1 month post ponatinib). Seven pts switched to imatinib. One developed a new PAD within 3 months of ponatinib DC (history of MI while on ponatinib). The other 6 pts maintained MMR on imatinib. Overall, 15 pts who had aggravated hypertension on ponatinib were under control after DC of ponatinib.
Conclusion: Treatment with 2nd generation TKIs and imatinib was effective and safe in pts who DC ponatinib, and most pts were able to maintain/improve the responses achieved on ponatinib. Ponatinib-associated hypertension was usually reversible after DC and most vascular events with subsequent TKIs occurred in patients with prior such events while on ponatinib.
Jabbour:pfizer: Research Funding; ariad: Research Funding; teva: Consultancy; teva: Research Funding; pfizer: Consultancy; bms: Consultancy; ariad: Consultancy. Estrov:incyte: Consultancy, Research Funding. Pemmaraju:Stemline: Research Funding; Incyte: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; LFB: Consultancy, Honoraria. Cortes:Pfizer: Consultancy, Research Funding; BerGenBio AS: Research Funding; Novartis: Consultancy, Research Funding; Teva: Research Funding; BMS: Consultancy, Research Funding; Ariad: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Ambit: Consultancy, Research Funding; Arog: Research Funding; Celator: Research Funding; Jenssen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.