Abstract
Over the past decade the development of safer reduced-intensity conditioning regimens, expanded donor pools, advances in supportive care, and prevention/management of graft-versus-host disease have expanded stem cell transplantation (SCT) availability for chronic lymphocytic leukemia (CLL) patients. However, there are now increasingly active treatment options available for CLL patients with favorable toxicity profiles and convenient administration schedules. This raises the critical issue of whether or not attainment of cure remains a necessary goal. It is now less clear that treatment with curative intention and with significant toxicity is required for long-term survival in CLL. In addition, the demonstrated safety and activity of genetically modified chimeric antigen receptor (CAR) T cells present the opportunity of harnessing the power of the immune system to kill CLL cells without the need for SCT. We attempt to define the role of SCT in the era of targeted therapies and discuss questions that remain to be answered. Furthermore, we highlight the potential for exciting new cellular therapy using genetically modified anti-CD19 CAR T cells and discuss its potential to alter treatment paradigms for CLL.
Introduction
Allogeneic stem cell transplantation (SCT) is considered potentially curative for some patients with chronic lymphocytic leukemia (CLL).1-4 However, there is an ongoing transformation in CLL management with a plethora of new or soon-to-be-available promising experimental treatment options with remarkable activities, favorable toxicity profiles, and convenient administration schedules.5
Conventional immunochemotherapy combinations such as fludarabine, cyclophosphamide, and rituximab remain the standard of care for CLL patients with a good performance requiring treatment.6-8 However, patients with poor-risk features and older patients have inferior outcomes following immunochemotherapy and treatment options in the relapse-refractory settings have been quite limited until recently.
Although the newest approved therapies for CLL such as ibrutinib or idelalisib rarely result in complete remissions (CRs), their abilities to partially overcome poor-risk prognostic features highlight why new therapies call into question the goals of treatment of CLL.9-11 As was the case more than a decade earlier with the introduction of the tyrosine kinase inhibitor (TKI) imatinib for chronic myelogenous leukemia (CML),12 it is now less clear that treatment with curative intention but with high treatment-related morbidity and mortality is required for long-term survival in CLL; the role for transplant in the new treatment era has recently been nicely reviewed.2 In addition, the suggestion of durable activity of genetically modified chimeric antigen receptor (CAR) T cells presents the opportunity to harness the power of cellular therapy for CLL without the need for SCT. Though experience remains limited and follow-up relatively short, it is no longer taboo or a great stretch of the imagination to think we will develop new safer immunotherapies for CLL that may indeed have curative activity.
Indications for allogeneic SCT in CLL in the era of kinase inhibitors
In 2007, the Society for European Bone Marrow Transplantation (EBMT) proposed guidelines for allogeneic SCT in CLL based on a review of the literature and consensus of experts.1 The group was charged with evaluating the weight of evidence supporting graft-versus-leukemia (GVL) activity in CLL, assessing the overall efficacy of SCT in CLL with a focus on patients with poor prognostic features and defining a prognostic risk profile that justifies SCT given the significant nonrelapse mortality (NRM) of this approach. Poor-risk CLL was defined as “patients who can expect a significant reduction of life expectancy under alternative therapies.” Furthermore, outcomes for standard therapies in poor-risk patients were defined as resulting in “a median overall survival of less than 1-2 years and a 4 year OS of less than 20%.” The consensus opinion was that allogeneic SCT should be considered for relapsed CLL with poor-risk features (defined as primary refractory disease, early relapse within 12 months following purine-analog therapy, relapse within 24 months after purine-analog–based therapy, or treatment of similar efficacy) and patients with p53 mutation/deletion requiring treatment (including following frontline induction therapy).1
In support of these criteria, a retrospective donor vs no donor matched comparison of poor-risk patients was performed that demonstrated a 2-year survival advantage for patients for whom a compatible donor could be identified within 3 months vs no donor available (78% vs 55%, hazard ratio .38, P = .01).13 Additionally, a matched case-control retrospective analysis was performed that studied 37 patients (36% del17p, 52% fludarabine-refractory, 35% with progressive disease [PD] at the time of SCT) who underwent reduced-intensity conditioning (RIC) SCT compared with 43 transplant eligible matched controls who did not undergo SCT that demonstrated a survival advantage for SCT group (133 months from SCT vs 85 months from time of diagnosis).14 Similar results were obtained when comparing life expectancy for relapsed-refractory CLL patients who underwent RIC SCT as compared with those who were treated with conventional chemoimmunotherapy using pooled estimates in a Markov decision analysis.15
Therefore, although these data support consideration of transplant for at least some patients with poor-risk CLL, the treatment paradigm for CLL has substantially changed and it is critically important to try to interpret historical guidelines in light of recent developments, particularly for these patients with poor-risk disease (purine-analog refractory, presence of p53 mutation/17p deletion). The main questions to address include: are newer agents active enough in poor-risk patients that the need for transplantation is completely negated or at least pushed back further in their treatment algorithm, and if SCT is reserved for kinase inhibitor failures, are outcomes compromised or enhanced?
At this time, several targeted therapies have been approved or are near approval for CLL in the relapse or refractory and frontline settings.16 Promising classes of agents include Bruton tyrosine kinase inhibitors, phosphatidylinositol-4,5-bisphosphate-3-kinase inhibitors, and BCL2 inhibitors.17-19 The patient cohorts in whom these agents were studied were by nature enriched for poor-risk features. Based on published inclusion/exclusion criteria, one can conclude that the vast majority of patients who participated in landmark studies leading to US Food and Drug Administration (FDA) approvals of ibrutinib and idelalisib did not undergo RIC SCT prior to enrollment though a few subjects were censored following exposure to either ibrutinib or idelalisib for SCT.9-11 Therefore, although attractive to consider using these agents to debulk disease prior to SCT or as a maintenance therapy following SCT, at this time, there is a paucity of data to guide use of kinase inhibitors prior to or following RIC SCT. However, intriguing work presented by the Stanford group suggests patients can be salvaged with ibrutinib after transplant. The response rate was 87.5% (24-month progression-free survival [PFS] 77%) in 16 patients who relapsed after allogeneic SCT of whom 13 had a del17p or del11q.20 Interestingly, ibrutinib exposure may have the ability to salvage the graft and induce GVL when administered following SCT promoting full donor chimerism, chronic graft-versus-host disease (cGVHD) resolution, and minimal residual disease (MRD)–negative disease.21 It should be emphasized, however, that by transplanting only, patients refractory to TKIs and other available therapies may limit their posttransplant efficacy.
A major issue is that the definition of “poor risk” may change with the development of new therapies and we need to begin to try to reinterpret how risk is considered for SCT candidacy and the timing of SCT for these patients.1,9-11,22-24 For example, it has been suggested that the presence of poor-risk features such as del17p does not impact response rates or PFS in CLL patients treated with the combination of idelalisib and rituximab.25 Similarly, of 144 CLL patients with del17p treated with ibrutinib for relapsed-refractory disease, 79.3% were alive and progression free at 12 months.24 Therefore, if 17p deletion no longer heralds rapid relapse with refractory disease, current guidelines recommending early transplant will need to be reconsidered.1 Before altering guidelines, however, longer follow-up and data on long-term toxicities are needed. Although del17p may not impact response rate (68% overall response rate [ORR] in del 17p disease), it may impact durability of response compared with patients without del17p (26 months PFS and overall survival [OS] 57% and 70%, respectively).11
Other prognostic factors may need to be taken into account when considering new guidelines. For instance, outcomes are inferior in patients treated with ibrutinib who have a complex (≥3 abnormalities) karyotype.26 In addition, unlike CML where there is extensive literature on resistance mechanisms to TKI therapy and agents which can overcome resistant clones, we need to better understand resistance mechanisms to kinase inhibitor therapy in CLL, particularly in patients who progress on idelalisib; more data on the potential of cross-resistance after failing 1 agent and starting another are also needed.26-28 Two recent series composed of patients who discontinued ibrutinib due to toxicity, transformation, SCT, or progression highlight the difficulty in salvaging patients after ibrutinib failure, especially in the setting of disease transformation.29,30 These results question the ability to salvage ibrutinib failures and therefore the feasibility of RIC SCT following ibrutinib discontinuation.29 Dreger at al have recently provided their perspective on management of high-risk CLL weighing the data for SCT vs novel agents and highlighting unanswered questions.2 Ultimately, these decisions are likely to be very personalized to a patient’s physical and disease status, prior therapies, and genetic risk profiles and we believe current treatment algorithms are likely to evolve over time.
The evidence for reduced-intensity allogeneic SCT in CLL
Although earlier reports of allogeneic SCT focused on myeloablative conditioning regimens, this approach is often impractical as the benefit is more than offset by unacceptably high NRM from infection, organ failure, GVHD, and high rates of relapse.31,32 Therefore, the majority of allogeneic SCT performed in the United States and Europe for CLL are performed using RIC regimens.
RIC SCT techniques were developed in the 1990s to minimize toxicity and expand the availability of donor SCT for patients who are generally older or heavily pretreated. Therefore, CLL patients may be an ideal group to use this approach. More recently, the donor pool for transplant has been further expanded through additional stem cell sources such as umbilical cord and haploidentical donors.33,34
Many centers have reported their experiences utilizing RIC SCT in CLL, often in patients with refractory disease or with poor-risk features in first CR.35-47 The ability of SCT to induce MRD-negative remissions, correlation of the presence of cGVHD with durable responses, increased risk of relapse when grafts are T-cell depleted, and the ability of donor lymphocyte infusions (DLIs) to induce remissions for posttransplant relapse support the presence of a GVL effect in CLL. However, the evidence for a perceived survival advantage resulting from RIC SCT in CLL is still considered circumstantial as the literature draws on historical comparisons and lacks randomized studies comparing SCT to standard CLL treatments. Several larger series with available long-term follow-up can be used to highlight the potential efficacy and toxicity of RIC SCT in CLL and can help guide discussions about the role of transplant for CLL. A summary of demographics and outcomes from these selected trials is outlined in Table 1.
To be most useful, transplant studies need to have long-term follow-up. Performing truly nonmyeloablative SCT with 2 Gy total body irradiation (TBI; or 2 Gy TBI and fludarabine with unrelated donors), the Seattle group described outcomes of 82 patients, 64 with 5-year follow-up transplanted for fludarabine-refractory CLL.35,48 As shown in Table 1, CR rate at 5 years after SCT was 55% and 5-year NRM and PFS were 23% and 39%, respectively. As with most series of RIC SCT, the 2 most common causes of NRM were infection and GVHD. The presence of fludarabine-refractory disease or poor-risk cytogenetics did not influence outcomes. However, lymphadenopathy ≥5 cm at the time of SCT and hematopoietic cell transplant comorbidity index (HCT CI) scores ≥1 were independent predictors of PFS and OS.49 These factors were used to stratify patients into 4 risk groups (3-year OS ranging from 27% to 78%). These results strongly support the presence of a potent GVL reaction in CLL and show that long-term disease control is possible at least for a subset of patients.
In further support of these findings, results from the German CLL3X trial included 90 poor-risk CLL patients who were 65 years of age or younger treated with RIC SCT (median follow-up 72 months).36 Poor risk was defined by purine-analog refractory disease, relapse after autologous SCT, or progression with high-risk genetic features. Outcomes are shown in Table 1 and include event-free survival (EFS) of 38% and OS at 6 years of 58% though with NRM of 23%.50 A unique feature of this report was MRD sampling at 12 months that predicted EFS and relapse. TP53, SF3B1, and Notch1 mutations did not influence MRD status at 12 months.50 One-third of patients were MRD negative after DLI for overt relapse or MRD-positive disease, providing support for GVL activity and suggesting that sequential MRD monitoring can identify patients who may benefit from early immune intervention.36 The presence of del17p did not impact outcomes, however, refractory disease at SCT or alemtuzumab use prior to SCT negatively impacted EFS, OS, and NRM. Additionally, the presence of TP53 mutation, SF3B1 mutation and Notch1 mutation did not influence OS, PFS, or EFS.50 In a subsequent article, outcomes were reported for 44 patients with del17p who underwent RIC SCT.51 In this group, 3-year OS and PFS were 44% and 37% with 4 year NRM of 32%,51 findings again supporting GVL and long-term remissions at the expense of significant toxicity.
Comparable outcomes were noted in a third series of 76 similarly high-risk CLL patients.47 With a median follow-up of 5.1 years, the OS, PFS, NRM, and relapse rate were reported as 63%, 43%, 16%, and 40%. Independent predictors of OS were age, sirolimus containing GVHD prophylaxis, lymph nodes >5 cm, bone marrow involvement, and year of SCT. Independent predictors of PFS were patient age, stable/progressive disease (SD/PD) at SCT, bone marrow involvement, elevated lymphocyte count, elevated lactate dehydrogenase, and increased HCT CI score. Patients were stratified patients into 4 separate groups with a 5-year PFS ranging from 6% to 83% and a 5-year OS ranging from 22% to 91%.
The MD Anderson group incorporated rituximab into their RIC SCT conditioning regimen (fludarabine, cyclophosphamide, rituximab) with a defined posttransplant immunomanipulation regimen and has reported outcomes with long-term follow-up for 86 poor-risk patients treated between 1996 and 2007.52,53 With a median follow-up of 37.2 months, 5-year PFS and OS estimates were 36% and 51%, respectively, and 1-year NRM was 17.4%. Fifty percent of patients required posttransplant immunomanipulation in the setting of SD/PD leading to a CR rate of 47% in that subset. Predictors of CR following immunomanipulation included a peripheral blood cell graft (vs bone marrow), ≥90% donor chimerism 90 days following SCT and being HLA-A1 positive/HLA-A2 negative/HLA-B44 negative. A model including these factors (score 0-4) resulted in CR to immunomanipulation ranging from 9% to 91%.
Taken together, these studies highlight that RIC SCT can induce long-term remissions for a subset of patients with poor-risk CLL. CLL can be quite susceptible to GVL activity of allogeneic SCT. Outcomes in these reports are remarkably similar with disease-free survival (DFS) 36% to 43% and OS 50% to 63%, suggesting that these are realistic estimates of outcomes and not simply related to patient selection at individual institutions. Numerous prognostic factors for outcome after transplant are identified but it is difficult to compare conclusions between studies. Nevertheless, bulky disease, disease status at transplant, patient functional status as well as a number of other factors may influence outcomes and need to be taken into account when making treatment decisions or choosing between transplant and alternative therapies.
The role of MRD monitoring following SCT
Two approaches have been developed for MRD monitoring in CLL that use either multicolor flow cytometry or polymerase chain reaction (PCR)-based assays using clonal rearrangement of the hypervariable region of the immunoglobulin heavy chain. Multicolor flow cytometry is more commonly used and is now standardized and readily available, and is less labor intensive than PCR-based techniques.54,55
The use of MRD status as a prognostic marker following conventional therapy as well as SCT is well established.56-58 What is unique about cellular-based therapies is that the therapy itself is a living, dynamic product which can respond to immune modulation techniques in the setting of perceived failure. Therefore, as Ritgen et al describe, longitudinal quantitative MRD monitoring following a cellular-based therapy offers both prognostic information and an opportunity for intervention in MRD-positive patients who are thought to be at high risk for overt clinical relapse.59 The vast majority of patients who are MRD negative at 1 year remain MRD negative on sequential testing, suggesting that SCT is capable of eradicating the CLL clone and therefore has curative potential.
Genetically modified T cells in CLL
The ability to achieve long-term disease control at least in some very high-risk patients with RIC SCT is a testament to the potency of cellular therapy against CLL. Nevertheless, despite all the modern advances, we believe that application of allogeneic SCT for CLL will remain limited due to still high rates of NRM as well as high relapse rates. Compared with allogeneic cell therapy, the use of autologous T cells to target CLL has several potential advantages; there is no risk for GVHD, long-term immunosuppression is not needed, and autologous cells can survive for long periods of time and provide ongoing protection against relapse.
CAR-modified T cells have been developed to target CD19 on CLL and other malignancies.60-62 CD19 is an excellent tumor target; it is expressed throughout B-cell development, is expressed on almost all B-cell malignancies, but is not on hematopoietic stem cells, and historically it is clear that patients can survive despite B-cell aplasia.
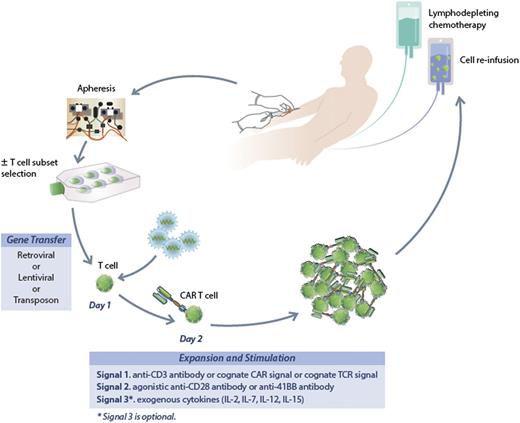
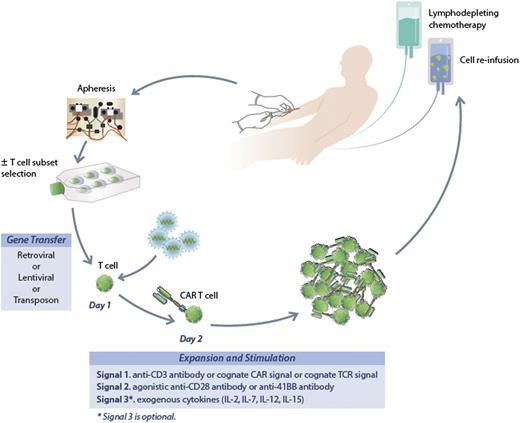
A fairly general process to generate CAR-modified T cells is illustrated in Figure 1. Patients undergo steady-state leukapheresis for T-cell collection. Varied approaches are used to generate gene-modified T cells at different centers, but all take a similar approach.63 We use a lentiviral vector to transfer the new genetic material encoding the CAR. Retroviral vectors and electroporation have also been used for gene transfer.64,65 Whether the method of delivery of the new genetic material will impact activity is not known. Modified cells undergo short-term culture for expansion and activation, and after 12 to 14 days cells are harvested for infusion. Patients typically receive lymphodepleting chemotherapy prior to T-cell infusion designed to enhance homeostatic proliferation of the infused T cells. Typically, standard CLL-directed chemotherapy has been used for this purpose though it is not known if there is an ideal regimen.
The process to generate CAR-modified T cells. Patients undergo steady-state leukapheresis for T-cell collection. Lentiviral vector is used to infect the T cells and transfer the new genetic material encoding the CAR. Modified cells undergo short-term culture for expansion and activation, and after 12 to 14 days are harvested for infusion. Patients typically receive lymphodepleting chemotherapy prior to T-cell infusion.
The process to generate CAR-modified T cells. Patients undergo steady-state leukapheresis for T-cell collection. Lentiviral vector is used to infect the T cells and transfer the new genetic material encoding the CAR. Modified cells undergo short-term culture for expansion and activation, and after 12 to 14 days are harvested for infusion. Patients typically receive lymphodepleting chemotherapy prior to T-cell infusion.
The CAR molecule used at the University of Pennsylvania contains the CD3ζ activation domain and CD137 costimulatory domain (CTL019 cells).66 Similar CD19-directed products at other centers use retrovirus gene transfer and CARs that contain the CD28 costimulatory domain.67,68 The costimulatory domain is likely critical for activity and persistence, though the ideal costimulatory molecule has not been defined. Preclinical data have suggested that CD137 may provide a more potent signal enhancing not only T-cell proliferation but also T-cell survival69 ; whether this translates into clinical efficacy is not known and will ultimately require comparative trials.
Clinical data on the application of anti-CD19 CAR T-cell therapy for CLL are actually quite limited and are summarized in Table 2. These studies show significant responses in small numbers of patients; responses have occurred even in patients who have relapsed after prior allogeneic SCT.67,68,70-72
At the University of Pennsylvania, we have shown that treatment of bulky, relapsed refractory, and high-risk CLL with anti-CD19 CAR-modified T cells (referred to as CTL019) can result in sustained remissions in small numbers of patients.70 All 3 of the initial patients treated had marked responses including 2 patients who achieved a CR. Both of these patients remain alive in remission >4 years after CTL019 infusion (D.L.P., manuscript submitted May 2015). Kochenderfer et al described complete remission in 3 of 4 patients with advanced CLL ongoing at 14 to 23 months at the time of publication.73 Taken together, these reports raise hopes that this approach, like transplant, has the potential to induce durable responses.
We have since treated over 45 patients with CLL with CTL019 and several conclusions can be drawn.74 ORRs in our studies have been ∼45%. No obvious patient or disease characteristics have yet been identified to predict which patients respond. When effective, CTL019 cells can undergo marked in vivo expansion and result in rapid elimination of tumor, leading to long-term remissions even in patients with bulky disease. Furthermore, we have now observed long-term persistence of the genetically modified T cells with ongoing functional activity lasting beyond 3 years.74,75 CAR T-cell persistence in other trials for CLL has been more limited68,73 though it remains unknown whether long-term CAR T-cell survival is necessary for durable remissions.
In our studies, patients who have persistent CAR T cells also develop B-cell aplasia and hypogammaglobulinemia, considered both “on-target” toxicity and a measure of functional persistence. The most significant and unique toxicity from CAR T-cell therapy is cytokine release syndrome (CRS).76,77 A similar syndrome has been described in patients with acute lymphoblastic leukemia (ALL); however, in our experience, the incidence and severity of CRS appears to be lower in CLL than ALL for reasons that are not well defined despite CLL patients having very high tumor burdens. The symptoms and severity of CRS can be quite variable but 1 consistent hallmark is escalating fevers that typically can exceed 40°C with associated myalgias, arthralgias, nausea, vomiting, and diarrhea. CRS can evolve with life-threatening complications that include hypotension, capillary leak, and hypoxia, necessitating intensive care-level supportive care. Interleukin-6 appears to be central to the development of CRS, and anti-interleukin-6 directed therapies have resulted in rapid reversal of CRS signs and symptoms in a number of cases.76,77 Several strategies to mitigate CRS severity can be considered including administering cells at the time of low tumor burden,77 including a “suicide switch” to allow modulation of T-cell activity,78 or altering dose and schedule of the administered T cells.
Certainly, CAR T-cell therapy will become more attractive by developing methods that minimize toxicity and increase response rates, and clinical trials to address both issues will be performed. Approaches in development to enhance response may include combining CAR T cells with immune checkpoint inhibitors or perhaps by additional engineering to include cytokine transgenes that might protect the T cell from the inhibitory tumor microenvironment.79 Alternatively, selecting specific T-cell subsets (such as central memory T cells) for genetic modification may enhance persistence and activity, and clinical trials using this approach have just started.80,81
Although most experience with CAR T-cell therapy for CLL has been directed against CD19, other approaches are possible. Data using an anti-CD20 CAR have shown limited activity in patients with lymphoma82 but future iterations of this approach could be applied in CLL. Other potential antigens could be targeted in CLL, and promising preclinical data targeting CD23 and R0R1 have been reported.83,84
Conclusions, unanswered questions, and future outlook
Allogeneic SCT can result in long-term disease control for CLL. When one is considering SCT for a particular patient, literature and experience provide guidance to aid in appropriate patient selection. The prognostic factors and outcomes models proposed by the larger series with long-term follow-up may help identify SCT candidates who are unlikely to benefit from SCT sparing them the morbidity and mortality associated with this procedure, while at the same time identifying patients with the best chance of disease control with SCT or subsequent response to GVL induction with DLI. In addition, the literature supports the clinical value of post-SCT monitoring for the presence of MRD using either flow cytometry of PCR methods to identify a patient subset at high risk for failure with the potential for meaningful intervention and long-term DFS. Posttransplant maintenance may be another strategy to optimize long-term outcomes.
Although there is now increased access and improved safety with SCT, these advances come as we embark on an era where the treatment paradigm is shifting from one of potential cure at high risk to one of sequential therapies or novel combinations and long-term disease control. Recent data suggest that both ibrutinib and idelalisib are active and result in durable responses in poor-risk patients who would traditionally have been considered earlier as candidates for RIC SCT. Furthermore, CAR T cells may emerge as a therapy that can result in long-term DFS after a 1-time infusion and, at least in a subset of patients, preclude the need for SCT.
Given the myriad of novel therapies, there is likely to be little enthusiasm for randomized trials of transplant vs novel therapy for relapsed-refractory CLL or younger patients with del17p in first CR. In the absence of such randomized data, trying to decide between an approach focused on sequential therapies or SCT remains difficult. Of course, the decision to start a targeted therapy is not mutually exclusive of consideration of SCT. Assuming most SCT candidates will see 1 or more novel agent before SCT referral, it will be important to determine impact of prior targeted therapy on transplant outcome and to reevaluate risk models in this new era. It will also be important to determine whether targeted agents improve outcomes of transplant by improving responses, reducing toxicity such as GVHD, or minimizing risk of relapse. Although the number of patients treated is small at this time, we also believe CAR T-cell therapy will have a powerful role, at least for some patients with relapsed and refractory or high-risk CLL. Ultimately, the availability of these more targeted and potent therapies will increasingly limit the need for transplant for many patients who in the past had no other reasonable option.
Authorship
Contribution: A.M. and D.L.P. both contributed equally to writing this manuscript.
Conflict-of-interest disclosure: A.M. received research funding from Celgene, Pharmacyclics, Gilead, and Abbvie, and provided consultancy services to Pharmacyclics, Gilead, Celgene, and Genentech. D.L.P. received research funding, royalty, and IP interest in CTL019 technology from Novartis, and has a spouse employed by Genentech.
Correspondence: David L. Porter, Division of Hematology/Oncology, University of Pennsylvania, 3400 Civic Center Blvd, 2 PCAM West, Philadelphia, PA 19104; e-mail: david.porter@uphs.upenn.edu.