Key Points
Establishing a novel Dll4hiDC-based programming approach that produces alloreactive T cells able to eliminate leukemic cells without GVHD.
Dll4 induction of T-cell IFN-γ limits the expansion of Dll4hiDC-induced T cells in GVHD target tissues and development of GVHD.
Abstract
Alloreactive T cells play a critical role in eliminating hematopoietic malignant cells but are also the mediators of graft-versus-host disease (GVHD), a major complication that subverts the success of allogeneic hematopoietic stem cell transplantation (HSCT). However, induction of alloreactive T cells does not necessarily lead to GVHD. Here we report the development of a cellular programming approach to render alloreactive T cells incapable of causing severe GVHD in both major histocompatibility complex (MHC)–mismatched and MHC-identical but minor histocompatibility antigen–mismatched mouse models. We established a novel platform that produced δ-like ligand 4–positive dendritic cells (Dll4hiDCs) from murine bone marrow using Flt3 ligand and Toll-like receptor agonists. Upon allogeneic Dll4hiDC stimulation, CD4+ naïve T cells underwent effector differentiation and produced high levels of interferon γ (IFN-γ) and interleukin-17 in vitro, depending on Dll4 activation of Notch signaling. Following transfer, allogeneic Dll4hiDC-induced T cells were unable to mediate severe GVHD but preserved antileukemic activity, significantly improving the survival of leukemic mice undergoing allogeneic HSCT. This effect of Dll4hiDC-induced T cells was associated with their impaired expansion in GVHD target tissues. IFN-γ was important for Dll4hiDC programming to reduce GVHD toxicities of alloreactive T cells. Absence of T-cell IFN-γ led to improved survival and expansion of Dll4hiDC-induced CD4+ T cells in transplant recipients and caused lethal GVHD. Our findings demonstrate that Dll4hiDC programming can overcome GVHD toxicity of donor T cells and produce leukemia-reactive T cells for effective immunotherapy.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is an effective cellular therapy for hematological malignancies. A primary barrier that limits its success is acute graft-versus-host disease (GVHD).1-5 GVHD is caused by donor T cells that recognize and react to histocompatibility differences between host and donor cells. GVHD is initiated by priming of donor T cells by host antigen-presenting cells and followed by robust proliferation and differentiation of alloreactive T cells that mediate tissue injury.4,5 Thus, modulation of alloreactive T-cell responses has been a main strategy to reduce GVHD.2-5
Interestingly, induction of alloreactive T cells does not necessarily lead to GVHD. For example, naturally occurring effector memory T cells (nTEMs) are unable to mediate GVHD.6,7 These cells responded to alloantigen and mediated graft-versus-leukemia (GVL) effect but showed impaired expansion in local tissues.6-9 This nTEM pool might have a less diverse T-cell receptor (TCR) repertoire than the naïve T-cell (TN) pool7 ; however, even host antigen-sensitized TEMs showed reduced ability to trigger GVHD.10,11 These host-reactive T cells responded to the antigen but died faster than TNs, suggesting that cell-intrinsic properties independent of the TCR repertoire account for decreased ability of TEMs to mediate GVHD.11 Thus, induction of qualitative changes in donor T cells can reduce their antihost toxicities.
Notch signaling is critical for GVH responses.12-16 Notch receptors interact with Notch ligands of the δ-like and Jagged families,17-19 triggering the release of intracellular Notch (ICN) that activates Notch target genes.17-19 Inhibiting pan-Notch signaling in donor T cells reduced their production of interferon γ (IFN-γ) and interleukin-17 (IL-17).15 Notch ligand Dll4 mediates a dominant role for activating Notch signaling in alloreactive T cells.14 We previously identified inflammatory dendritic cells (DCs) that expressed high levels of Dll4 (Dll4hiDCs).13 They occurred in mice early during GVHD induction and had a greater ability than Dll4-negative DCs to induce IFN-γ and IL-17 in alloantigen-activated T cells.13 Differentiated effector T cells have reportedly reduced capacity to proliferate and persist in vivo20-23 ; therefore, we reasoned that in vitro priming with Dll4hiDCs could allow the induction of alloreactive effector T cells with reduced GVHD toxicity.
Materials and methods
Mice
C57BL/6 (B6, H-2b), BALB/c (H-2d), and B6xDBA/2 F1 (BDF1, H-2b/d) mice were from Taconic (Rockville, MD). Ifng-deficient (Ifng−/−) mice and C3H.SW mice were from The Jackson Laboratory. B6 mice conditionally expressing dominant-negative Mastermind-like 1 (DN-MAML) were crossed to CD4-cre transgenic mice.24,25 Experimental protocols were approved by the University of Michigan’s and Temple University’s Committee on Use and Care of Animals.
DC production
For induction of Dll4hiDCs, mouse bone marrow (BM) was cultured in RPMI 1640 medium containing 10% fetal bovine serum and recombinant mouse Flt3L (50 ng/mL). Eight days later, CD11c+ immature DCs were magnetically sorted and incubated with lipopolysaccharide (LPS, 100 ng/mL) and R848 (resiquimod, 100 ng/mL), which activate Toll-like receptor 4 and 7/8 signaling, respectively.26 Cells were harvested at day 9 for experiments. BM DCs produced from granulocyte-macrophage (GM) colony-stimulating factor–based cultures were also stimulated with LPS + R848 and named GM-DCs.27
GVHD and GVL response
Mice underwent bone marrow transplantation as described.28 GVHD score and severity were graded by clinical parameters and histopathological analysis.29,30 To induce GVHD, we irradiated BALB/c recipients to 800 cGray from a 137Cs source followed by transplantation with donor B6 T-cell depleted (TCD)–BM with or without B6 CD4+ T cells. GVHD in BDF1 mice was induced by transfer of donor B6 CD4+ plus CD8+ T cells.31 In the C3H.SW anti-B6 mouse model of GVHD directed against minor histocompatibility antigens, B6 recipients were irradiated using 1000 cGray from an radiograph source, followed by transfer of C3H.SW TCD-BM with or without CH3.SW CD8+ T cells. In GVL experiments, we injected A20TGL cells (1 × 106) 2 hours before HSCT, which reflects the residual disease in human transplant recipients as described,32,33 and monitored leukemic growth using bioluminescence imaging.15 In some experiments, C1498 myeloid leukemic cells (1.5 × 104 per mouse) and mastocytoma P815 cells (2 × 103 per mouse) were used to induce leukemia and tumor.
Other assays
Our data regarding antibodies (Ab’s), flow cytometry analysis, T-cell preparation and culture, real-time reverse transcription polymerase chain reaction and cytotoxic T-lymphocyte assay, and statistical analysis are detailed in the supplemental Methods (available on the Blood Web site).
Results
Generation of murine Dll4hiDCs
We have demonstrated that an average of 0.03 × 105 Dll4hiDCs were recovered from a single mouse undergoing HSCT.13 Furthermore, only ∼5% of DCs derived from normal mice expressed Dll4.13 To provide adequate numbers of Dll4hiDCs for therapeutic use, we developed a culture system capable of generating sufficient numbers of Dll4hiDCs. We previously identified phenotypic similarities between Dll4hiDCs and plasmacytoid DCs (pDCs),13 the latter of which can be induced using Flt3L.34 Culture of murine BM with Flt3L induced CD11c+ DCs (named Flt3L-DCs) that were Dll4 negative (Figure 1A-B). Overnight incubation with LPS, R848, or LPS + R848 induced Dll4 expression on the surface of Flt3L-DCs. Concurrent stimulation with LPS and R848 induced the highest level of Dll4 (Figure 1B) and was therefore used for all subsequent experiments. These Dll4-expressing Flt3L-DCs are henceforth referred to as Dll4hiDCs. An average of 2.5 × 106 Dll4hiDCs were generated in cultures from 1 mouse BM, and >60% of them expressed high levels of Dll4.
In vitro generation of Dll4hiDCs. Flt3L-DCs were generated by incubating BALB/c mouse BM mononuclear cells in cultures with Flt3L. GM-DCs were induced by culturing c-kit+ hematopoietic progenitor cells in the presence of GM colony-stimulating factor, IL-4, and stem cell factor. After 8 days in culture, cells were collected and cultured in medium containing indicated stimuli for additional 24 hours. (A) Graphs show the number of CD11c+ cells (mean ± standard deviation [SD] of triplicates). (B) Histograms and a graph show the percentage of Dll4 on the surface of Flt3L-DCs (mean ± SD of triplicates). (C, E) Real-time reverse transcription polymerase chain reaction analysis revealed the relative expression of indicated genes in DCs generated in cultures (mean ± SD of triplicates). (D) Histograms show the expression of tested markers on the surface of DCs with or without stimulation of LPS+R848. Representative results of 3 independent experiments are shown. ** P < .01; *** P < .001.
In vitro generation of Dll4hiDCs. Flt3L-DCs were generated by incubating BALB/c mouse BM mononuclear cells in cultures with Flt3L. GM-DCs were induced by culturing c-kit+ hematopoietic progenitor cells in the presence of GM colony-stimulating factor, IL-4, and stem cell factor. After 8 days in culture, cells were collected and cultured in medium containing indicated stimuli for additional 24 hours. (A) Graphs show the number of CD11c+ cells (mean ± standard deviation [SD] of triplicates). (B) Histograms and a graph show the percentage of Dll4 on the surface of Flt3L-DCs (mean ± SD of triplicates). (C, E) Real-time reverse transcription polymerase chain reaction analysis revealed the relative expression of indicated genes in DCs generated in cultures (mean ± SD of triplicates). (D) Histograms show the expression of tested markers on the surface of DCs with or without stimulation of LPS+R848. Representative results of 3 independent experiments are shown. ** P < .01; *** P < .001.
Flt3L-DCs stimulated with LPS and R848 showed a dramatic decrease in messenger RNA transcripts encoding inflammatory cytokines (eg, Ifna, Ifnb, Il12, Il4, and Il6) (Figure 1C) but simultaneously upregulated molecules associated with DC maturation, including antigen-presenting molecules (Ia), costimulatory molecules (CD80, CD86, and CD40), CD103 (marker of migratory DCs), and CD11b (marker for conventional DCs)35 (Figure 1D). Thus, induction of Dll4 on Flt3L-DCs is associated with maturation. Immature Dll4hiDCs expressed B220 (33%) and Siglec H (49%) (Figure 1D), which are pDC markers,36 suggesting that Dll4hiDCs may originate from both pDCs and conventional DCs. In contrast, GM-DCs did not express Dll4 despite stimulation with LPS + R848 (Figure 1B-C). As compared with Dll4hiDCs, GM-DCs had lower levels of Ifngb, Il4, Il6, and Ido, expressed higher levels of iNOS and Arg1 (arginase I) and expressed no surface CD103 (Figure 1C-E).
Dll4hiDC-induced alloreactive T cells have reduced ability to cause GVHD
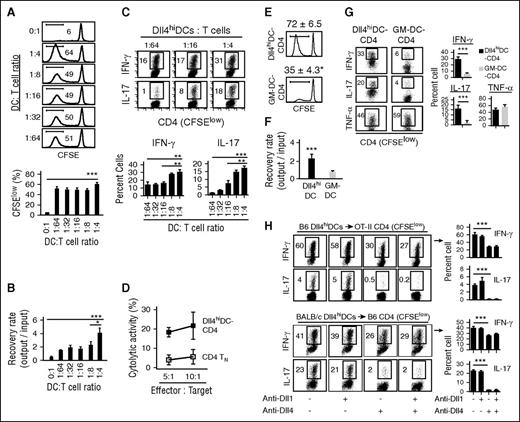
To determine if Dll4hiDCs could program TNs for reducing GVHD, we incubated B6 CD4+ TNs with or without escalating numbers of BALB/c Dll4hiDCs for 5 days. These Dll4hiDC-activated CD4+ T cells underwent extensive proliferation, as evidenced by low levels of carboxyfluorescein diacetate succinimidyl ester (CFSElow) (Figure 2A). As a result, Dll4hiDCs induced expansion of donor CD4+ T cells (Figure 2B) and production of high levels of IFN-γ and IL-17 (Figure 2C). These Dll4hiDC-induced T cells killed A20 leukemia cells in vitro (Figure 2D). As compared with Dll4hiDCs, GM-DCs were significantly less potent in promoting proliferation of allogeneic CD4+ T cells and their production of IFN-γ and IL-17 (Figure 2E-G), confirming the functional difference between Dll4hiDCs and GM-DCs.
Dll4hiDCs induce effector CD4+ T-cell differentiation. Dll4hiDCs or GM-DCs were generated from BM of BALB/c mice. (A-D) CD4+ TNs were purified from B6 mice, labeled with CFSE, and incubated with BALB/c Dll4hiDC at escalating DC/T-cell ratios. Five days later, T cells were collected to measure their proliferation (A) and recovery (B). In panel C, dot plots and graphs show the percentages of IFN-γ–producing and IL-17–producing cells among proliferating CD4+ T cells that expressed CFSElow (mean ± SD of triplicates). (D) Graph shows in vitro cytotoxic activity of B6 CD4+ TNs or BALB/c Dll4hiDCs activated B6 CD4+ T cells (DC/T-cell ratio of 1:4) against A20 leukemia cells. Representative data from 2 independent experiments are shown. (E-G) CFSE-labeled B6 CD4+ TNs were incubated with BALB/c Dll4hiDCs or GM-DCs with DC/T-cell ratio of 1:4. Five days later, T cells were collected to measure their proliferation (E) and recovery (F). (G) Dot plots and graphs show the percentages of IFN-γ, IL-17, and tumor necrosis factor α (TNF-α)–producing cells among proliferating CD4+ T cells that expressed CFSElow (mean ± SD of triplicates). (H) B6 Dll4hiDCs were cultured with CD4+ T cells specific to OT-II peptide (OVA232-239), which were isolated from TCR transgenic OT-II mice in the presence of OT-II peptides (top). BALB/c Dll4hiDCs were cultured with CD4+ T cells isolated from WT B6 mice (bottom). Dot plots and graphs show the percentages of IFN-γ and IL-17 in the different neutralizing Ab condition (mean ± SD of triplicates). *P < .05; **P < .01; ***P < .001.
Dll4hiDCs induce effector CD4+ T-cell differentiation. Dll4hiDCs or GM-DCs were generated from BM of BALB/c mice. (A-D) CD4+ TNs were purified from B6 mice, labeled with CFSE, and incubated with BALB/c Dll4hiDC at escalating DC/T-cell ratios. Five days later, T cells were collected to measure their proliferation (A) and recovery (B). In panel C, dot plots and graphs show the percentages of IFN-γ–producing and IL-17–producing cells among proliferating CD4+ T cells that expressed CFSElow (mean ± SD of triplicates). (D) Graph shows in vitro cytotoxic activity of B6 CD4+ TNs or BALB/c Dll4hiDCs activated B6 CD4+ T cells (DC/T-cell ratio of 1:4) against A20 leukemia cells. Representative data from 2 independent experiments are shown. (E-G) CFSE-labeled B6 CD4+ TNs were incubated with BALB/c Dll4hiDCs or GM-DCs with DC/T-cell ratio of 1:4. Five days later, T cells were collected to measure their proliferation (E) and recovery (F). (G) Dot plots and graphs show the percentages of IFN-γ, IL-17, and tumor necrosis factor α (TNF-α)–producing cells among proliferating CD4+ T cells that expressed CFSElow (mean ± SD of triplicates). (H) B6 Dll4hiDCs were cultured with CD4+ T cells specific to OT-II peptide (OVA232-239), which were isolated from TCR transgenic OT-II mice in the presence of OT-II peptides (top). BALB/c Dll4hiDCs were cultured with CD4+ T cells isolated from WT B6 mice (bottom). Dot plots and graphs show the percentages of IFN-γ and IL-17 in the different neutralizing Ab condition (mean ± SD of triplicates). *P < .05; **P < .01; ***P < .001.
Allogeneic mixed leukocyte reaction activates polyclonal T cells and is unable to model alloreactivity to a single alloantigen. To test it, we isolated CD4+ TNs specific to OT-II peptide (OVA232-239) from TCR transgenic OT-II mice and cultured them with syngeneic Dll4hiDCs pulsed by OT-II peptides. Addition of Dll4hiDCs and OT-II peptides induced vigorous proliferation of OT-II CD4+ TNs (supplemental Figure 1) and production of IFN-γ and IL-17 (Figure 2H). Blocking Dll4 but not Dll1 using neutralizing Ab’s reduced IFN-γ and IL-17 by CD4+ T cells (Figure 2H). Dll4 blockade also inhibited IFN-γ and IL-17 production by B6 CD4+ TNs stimulated with BALB/c Dll4hiDCs (Figure 2H). These findings demonstrate that antigenic peptides presented by Dll4hiDCs elicit specific T-cell responses and that Dll4 is critical for promoting IFN-γ and IL-17 production.
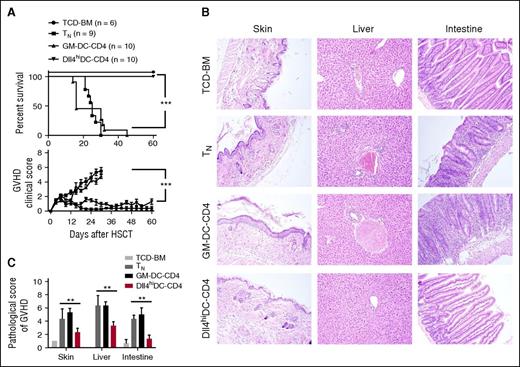
To test the ability of allogeneic Dll4hiDC-induced CD4+ T cells (Dll4hiDC-CD4) to mediate GVHD, we harvested primed T cells 5 days after stimulation and transferred them with TCD-BM into irradiated BALB/c mice. Transfer of donor CD4+ TNs caused lethal GVHD (Figure 3A-C). In contrast, BALB/c mice receiving Dll4hiDC-CD4+ T cells developed only minimal GVHD and complete survival (Figure 3A-C). Histological examination demonstrated that Dll4hiDC-CD4+ T cells caused less severe tissue inflammation compared with CD4+ TNs (Figure 3B-C). Notably, GM-DC-CD4+ T cells mediated severe GVHD, with all recipients dying from the disease (Figure 3A-C). Thus, culture with Dll4hiDCs rather than GM-DCs can reduce the GVHD activity of donor CD4+ TNs.
Dll4hiDC-induced alloreactive T cells have reduced ability to cause GVHD. Dll4hiDCs and GM-DCs were generated from BM of BALB/c mice. After 8 days in culture, DCs were stimulated by LPS and R848 for 24 hours. Then, both Dll4hiDCs and GM-DCs were cultured with allogeneic B6 CD4+ T cells for 5 days (DCs/T cells = 1:4). Lethally irradiated (8 Gy) BALB/c mice were injected with B6 TCD-BM (5.0 × 106) mixed with or without either naïve or in vitro activated allogeneic CD4+ T cells (1.0 × 106). (A) Survival and GVHD clinical score of the recipients were monitored over time (mean ± standard error of the mean). Data shown here are pooled from 2 independent experiments. (B) Representative images show the skin, liver, and small intestine from 1 of 6 recipients in each group at day 14 after transplantation. Photographs were obtained with an Olympus BX41 microscope (10/0.3 NA lens, ×200 magnification, digital DP70 camera). (C) Graphs show the pathological scores of GVHD 14 days after HSCT (6 mice per group). **P < .01; ***P < .001.
Dll4hiDC-induced alloreactive T cells have reduced ability to cause GVHD. Dll4hiDCs and GM-DCs were generated from BM of BALB/c mice. After 8 days in culture, DCs were stimulated by LPS and R848 for 24 hours. Then, both Dll4hiDCs and GM-DCs were cultured with allogeneic B6 CD4+ T cells for 5 days (DCs/T cells = 1:4). Lethally irradiated (8 Gy) BALB/c mice were injected with B6 TCD-BM (5.0 × 106) mixed with or without either naïve or in vitro activated allogeneic CD4+ T cells (1.0 × 106). (A) Survival and GVHD clinical score of the recipients were monitored over time (mean ± standard error of the mean). Data shown here are pooled from 2 independent experiments. (B) Representative images show the skin, liver, and small intestine from 1 of 6 recipients in each group at day 14 after transplantation. Photographs were obtained with an Olympus BX41 microscope (10/0.3 NA lens, ×200 magnification, digital DP70 camera). (C) Graphs show the pathological scores of GVHD 14 days after HSCT (6 mice per group). **P < .01; ***P < .001.
Dll4hiDC-CD4+ T cells retain antileukemia activity
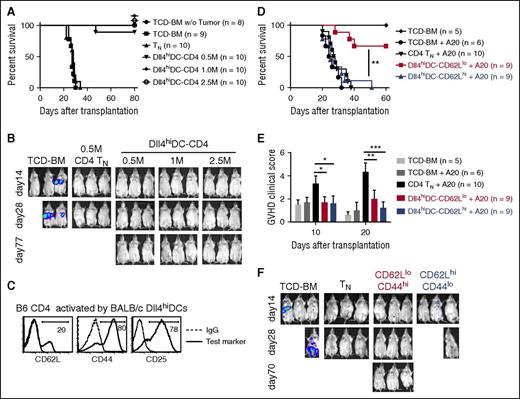
To determine whether these Dll4hiDC-CD4+ T cells preserved GVL activity, we transplanted B6 TCD-BM with or without titrated numbers of Dll4hiDC-CD4+ T cells into lethally irradiated BALB/c mice. A20TGL leukemia cells were injected into these recipients to induce leukemia. Mice receiving TCD-BM died of leukemia, whereas mice receiving TCD-BM plus CD4+ TNs (0.5 × 106 cells per mouse) eliminated leukemia cells but died of GVHD (Figure 4A-B). In contrast, transfer of up to 2.5 × 106 Dll4hiDC-CD4+ T cells did not induce severe GVHD but protected recipients against leukemic cell challenge (Figure 4A-B). This suggests that our strategy dramatically expands the safety range of infused donor T-cell dose by at least fivefold.
Dll4hiDC-induced T cells retain potent antileukemia activity. Dll4hiDCs were generated from BM of BALB/c mice. Then, the Dll4hiDCs were cultured with allogeneic B6 CD4+ T cells for 5 days (DCs/T cells = 1:4). Lethally irradiated (8 Gy) BALB/c mice were injected with B6 TCD-BM (5.0 × 106) mixed with in vitro activated allogeneic CD4+ T cells. In addition, A20TGL leukemia/lymphoma cells (1.0 × 106) were injected to these recipients 2 hours before transplantation to induce leukemia. (A, B) The numbers of Dll4hiDC-induced CD4+ T cells were titrated from 0.5 million (M) to 2.5 M. B6 TCD-BM with or without addition of unstimulated B6 CD4+ TNs were transferred to lethally irradiated BALB/c mice as controls. (A) Survival of the recipients was monitored over time. (B) Pictures show in vivo detection of luciferase activity at days 14, 28, and 77 after transplantation. Data shown here are pooled from 2 independent experiments. (C) Histograms show the expression of tested markers on the surface of Dll4hiDC-induced CD4+ T cells. (D-F) Dll4hiDC-induced CD4+ T cells were flow sorted into CD4+CD44hiCD62Llo cells (0.5 × 106) and CD4+CD44loCD62Lhi cells (0.5 × 106) and transferred together with TCD-BM into lethally irradiated leukemia BALB/c mice. (D) Survival of the recipients was monitored over time. (E) Histogram shows GVHD clinical scores at days 10 and 20. (F) In vivo images of the luciferase-positive leukemia cells at days 14, 28, and 70 were shown. Photographs were taken by Xenogen IVIS 100. Data shown here are pooled from 2 independent experiments. *P < .05; **P < .01; ***P < .001.
Dll4hiDC-induced T cells retain potent antileukemia activity. Dll4hiDCs were generated from BM of BALB/c mice. Then, the Dll4hiDCs were cultured with allogeneic B6 CD4+ T cells for 5 days (DCs/T cells = 1:4). Lethally irradiated (8 Gy) BALB/c mice were injected with B6 TCD-BM (5.0 × 106) mixed with in vitro activated allogeneic CD4+ T cells. In addition, A20TGL leukemia/lymphoma cells (1.0 × 106) were injected to these recipients 2 hours before transplantation to induce leukemia. (A, B) The numbers of Dll4hiDC-induced CD4+ T cells were titrated from 0.5 million (M) to 2.5 M. B6 TCD-BM with or without addition of unstimulated B6 CD4+ TNs were transferred to lethally irradiated BALB/c mice as controls. (A) Survival of the recipients was monitored over time. (B) Pictures show in vivo detection of luciferase activity at days 14, 28, and 77 after transplantation. Data shown here are pooled from 2 independent experiments. (C) Histograms show the expression of tested markers on the surface of Dll4hiDC-induced CD4+ T cells. (D-F) Dll4hiDC-induced CD4+ T cells were flow sorted into CD4+CD44hiCD62Llo cells (0.5 × 106) and CD4+CD44loCD62Lhi cells (0.5 × 106) and transferred together with TCD-BM into lethally irradiated leukemia BALB/c mice. (D) Survival of the recipients was monitored over time. (E) Histogram shows GVHD clinical scores at days 10 and 20. (F) In vivo images of the luciferase-positive leukemia cells at days 14, 28, and 70 were shown. Photographs were taken by Xenogen IVIS 100. Data shown here are pooled from 2 independent experiments. *P < .05; **P < .01; ***P < .001.
To define the relative difference between Dll4hiDC-CD4+ T cells and TNs in mediating GVL, we examined if there was a threshold for TNs that would cause less GVHD but preserved antileukemia activity. Transfer of 0.1 × 106 TNs induced delayed onset of lethal GVHD compared with transfer of 0.5 × 106 TNs. However, 5 of 9 mice receiving the lower dose of TNs died with leukemia (supplemental Figure 2). Thus, TNs have a limited dose window between mediating GVL and GVHD.
CD4+ T cells from Dll4hiDC-induced cultures contained at least 2 different subsets: CD44hiCD62Llo effector cells (80%) and CD44loCD62Lhi TN-like cells (20%) (Figure 4C). TNs have a greater ability than effector T cells to proliferate and survive after adoptive transfer.20,27,28 To exclude if these CD44loCD62Lhi TN-like cells might account for eliminating leukemic cells in HSCT recipients, we flow sorted Dll4hiDC-induced T cells into 2 subsets: CD44hiCD62Llo cells and CD44loCD62Lhi cells (supplemental Figure 3). Transfer of either CD44hiCD62Llo cells or CD44loCD62Lhi cells did not cause severe GVHD in these recipients (Figure 4D-E). Interestingly, all mice receiving CD44loCD62Lhi TN-like cells died of leukemia, whereas 6 of 9 mice receiving Dll4hiDC-induced CD44hiCD62Llo cells survived without leukemia and GVHD (Figure 4D-F). Thus, allogeneic Dll4hiDC-induced CD44hiCD62Llo effector T cells are responsible for controlling leukemia. In contrast, CD44loCD62Lhi cells that survived in Dll4hiDC cultures might contain alloreactive T cells lower than the threshold sufficient to mediate GVHD. Indeed, transfer of a higher dose of CD44loCD62Lhi cells (ie, 2.5 × 106) caused lethal GVHD (data not shown).
Upon BALB/c GM-DC activation, B6 CD4+ T cells upregulated CD25 but decreased CD62L, generating both CD62Lhi and CD62Llo cell subsets (supplemental Figure 4A). GM-DC–induced CD62Lhi CD4+ T cells were unable to mediate either GVHD or GVL, whereas transfer of GM-DC–induced CD62LloCD4+ T cells caused GVHD (supplemental Figure 4B-D), confirming that GM-DCs are not useful for reducing the capacity of donor CD4+ TNs to mediate GVHD.
Dll4hiDC stimulation reduces capacity of CD8+ T cells to mediate GVHD
To rule out the possibility of a model-specific effect, we first used B6 an anti-BDF1 mouse model to examine the GVH effect of Dll4hiDC-induced T cells. As compared with Dll4hiDC-CD4+ T cells, Dll4hiDC-CD8+ T cells produced higher levels of IFN-γ (supplemental Figure 5A). We transplanted both Dll4hiDC-CD4+ T cells and Dll4hiDC-CD8+ T cells, with B6 TCD-BM, into irradiated BDF1 mice that had been inoculated with mastocytoma P815 cells. Macroscopic examination of tumor in the lung and liver was performed to monitor tumor growth. Transfer of unstimulated T cells caused GVHD, with 5/8 mice dying from GVHD and 3/8 dying with tumor (supplemental Figure 5B-C). In contrast, BDF1 mice receiving Dll4hiDC-induced T cells did not develop GVHD (supplemental Figure 5B-D). These Dll4hiDC-induced T cells retained antitumor activities evidenced by significantly prolonged median survival time compared with mice receiving TCD-BM plus P815 cells (36 days vs 11 days; supplemental Figure 5B-C).
To determine the precise impact of Dll4hiDCs on CD8+ T-cell–mediated GVHD directed against minor histocompatibility antigens, we generated Dll4hiDCs from B6 mouse BM and added into cultures of C3H.SW CD8+ TNs. Five days later, Dll4hiDC-CD8+ T cells vigorously proliferated and produced high levels of IFN-γ and TNF-α, which were inhibited upon Dll4 blockade (supplemental Figure 6A-B). Transfer of Dll4hiDC-CD8+ T cells caused significantly less severe GVHD in B6 mice than did CD8+ TNs, as evidenced by improved overall survival (60% vs 10%, respectively) and reduced clinical scores (supplemental Figure 6C-D). Transfer of Dll4hiDC-CD8+ T cells markedly prolonged the mean survival time of B6 mice inoculated with C1498 leukemic cells compared with TCD-BM (40 days vs 23 days, supplemental Figure 6E-F). These data reveal a specific effect of Dll4 on the alloreactive CD8+ T-cell responses and suggest that Dll4hiDCs can be used for reducing GVHD toxicity mediated by CD8+ T cells.
Dll4hiDC-CD4+ T cells have impaired capacity to expand in vivo
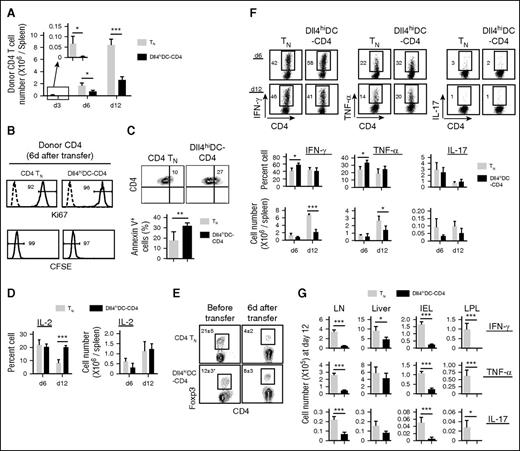
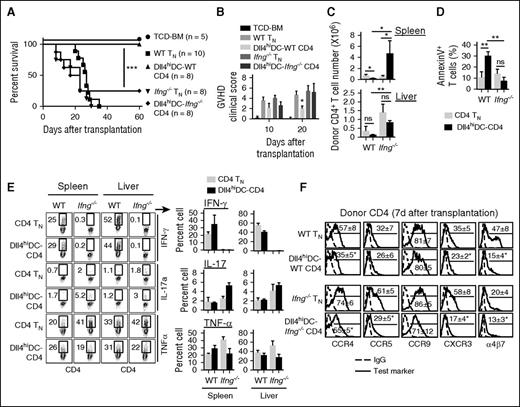
We determined the underlying mechanism rendering Dll4hiDC-CD4+ T cells ineffective in mediating GVHD. Dll4hiDC-CD4+ T cells and CD4+ TNs were transferred into irradiated allogeneic BALB/c mice. As compared with TN recipients, Dll4hiDC-CD4+ T-cell recipients showed significantly fewer donor T cells in the spleen at days 3, 6, and 12 (Figure 5A). Transferred Dll4hiDC-CD4+ T cells had undergone similar proliferation as measured by Ki67 and CFSE dilution 6 days after transplantation (Figure 5B) but had a 1.5-fold higher frequency of apoptotic cells (Figure 5C). Anergic T cells have decreased capacity to produce IL-2 and might contribute to impaired expansion of alloreactive T cells.37 Dll4hiDC-CD4+ T cells produced higher levels of IL-2 than CD4+ TNs (Figure 5D). FoxP3+ regulatory T cells (Treg) also affect GVH reactions.4,38 As compared with CD4+ TNs, Dll4hiDC-CD4+ T cells contained fewer number of Treg’s prior to transfer but showed slightly higher frequency of Treg’s without statistical significance 6 days after transfer (Figure 5E). These data suggest that impaired expansion of Dll4hiDC-T cells in vivo may be primarily attributable to increased apoptosis.
Dll4hiDC-T cells produced high levels of IFN-γ but have impaired capacity to expand in vivo. Dll4hiDC-induced CD4+ T cells (1.0 × 106) of B6/SJL origin (H-2b, CD45.1) were transplanted with TCD-BM (5.0 × 106) into lethally irradiated BALB/c mice (H-2d, CD45.2). Equal numbers of unstimulated B6/SJL CD4+ TNs were transferred to lethally irradiated BALB/c mice as controls. At days 3, 6, and 12 after transplantation, donor T cells were isolated from the spleens, lymph nodes (LN), liver, intestinal epithelial lymphocytes (IEL), and lamina propria lymphocytes (LPL) of recipient mice (3 mice per group). (A) Graphs show the numbers of donor (CD45.1+) CD4 T cells recovered from the spleens of recipient BALB/c mice at the indicated time points. (B) Histogram shows Ki67 and CFSE in donor CD4+ T cells 6 days after in vivo transfer. (C) Plots and graphs show the percentage of early apoptotic donor CD4+ T cells that were annexin V positive. (D) Graphs show the percentages and numbers of donor CD4+ T cells producing IL-2 in the spleen. (E) Plots and the graph show the percentage of donor FoxP3-positive Treg cells prior to transfer and 6 days after transfer. (F) Dot plots and graphs show the percentages and numbers of donor CD4+ T cells producing IFN-γ, TNF-α, and IL-17 in the spleen at day 6 and day 12 after transplantation. (G) Graphs show the number of donor CD4+ T cells producing IFN-γ, TNF-α, and IL-17 in GVHD target organs at day 12 after transplantation. Data show mean ± SD. Representative data from 2 independent experiments are shown. *P < .05; **P < .01; ***P < .001.
Dll4hiDC-T cells produced high levels of IFN-γ but have impaired capacity to expand in vivo. Dll4hiDC-induced CD4+ T cells (1.0 × 106) of B6/SJL origin (H-2b, CD45.1) were transplanted with TCD-BM (5.0 × 106) into lethally irradiated BALB/c mice (H-2d, CD45.2). Equal numbers of unstimulated B6/SJL CD4+ TNs were transferred to lethally irradiated BALB/c mice as controls. At days 3, 6, and 12 after transplantation, donor T cells were isolated from the spleens, lymph nodes (LN), liver, intestinal epithelial lymphocytes (IEL), and lamina propria lymphocytes (LPL) of recipient mice (3 mice per group). (A) Graphs show the numbers of donor (CD45.1+) CD4 T cells recovered from the spleens of recipient BALB/c mice at the indicated time points. (B) Histogram shows Ki67 and CFSE in donor CD4+ T cells 6 days after in vivo transfer. (C) Plots and graphs show the percentage of early apoptotic donor CD4+ T cells that were annexin V positive. (D) Graphs show the percentages and numbers of donor CD4+ T cells producing IL-2 in the spleen. (E) Plots and the graph show the percentage of donor FoxP3-positive Treg cells prior to transfer and 6 days after transfer. (F) Dot plots and graphs show the percentages and numbers of donor CD4+ T cells producing IFN-γ, TNF-α, and IL-17 in the spleen at day 6 and day 12 after transplantation. (G) Graphs show the number of donor CD4+ T cells producing IFN-γ, TNF-α, and IL-17 in GVHD target organs at day 12 after transplantation. Data show mean ± SD. Representative data from 2 independent experiments are shown. *P < .05; **P < .01; ***P < .001.
Interestingly, Dll4hiDC-CD4+ T cells produced higher levels of IFN-γ and TNF-α than CD4+ TN cells 6 days after transfer (Figure 5F). Twelve days after transplantation, Dll4hiDC-CD4+ T cells and CD4+ TNs produced similar levels of IFN-γ and TNF-α (Figure 5F). However, the impaired expansion of transferred Dll4hiDC-CD4+ T cells led to an overall reduction of IFN-γ–producing and TNF-α–producing alloreactive T cells in the spleen, lymph nodes, liver, and intestine compared with CD4 TNs (Figure 5F-G).
Whether T helper 17 (Th17) cells mediate GVHD remains controversial.39-41 Th17 cells were shown to induce GVHD, but donor T cells lacking IL-17 induce worse GVHD when compared with normal T cells.40 Interestingly, although Dll4hiDC-CD4+ T cells produced high levels of IL-17 prior to their transplantation, they did not sustain this capacity following transfer (Figure 5F-G). Th17 cells have a demonstrated plasticity of becoming Th1 cells.42 Thus, whether the decrease in production of IL-17 seen in Dll4hiDC-CD4+ T cells in vivo results from impaired survival and/or IFN-γ–mediated repression of IL-17 during GVH reaction seen by other studies43 has to be determined.
Collectively, our data suggest that impaired expansion capability of Dll4hiDC-CD4+ T cells in GVHD target organs may account for protection against GVHD.
T-cell IFN-γ is required for Dll4hiDC programming of T-cell alloreactivity
Previous studies suggested that administration of IFN-γ to allogeneic HSCT recipients at the time of transplantation repressed GVHD.44 T cells from Ifng−/− donors induced more severe systemic GVHD than wild-type (WT) donors.40,45-48 To determine whether IFN-γ was required for reducing the capacity of Dll4hiDC-induced CD4+ T cells to mediate GVHD, we cultured Ifng−/− B6 CD4+ T cells with BALB/c Dll4hiDCs. Ifng−/− CD4+ T cells did not produce IFN-γ, had similar levels of IL-4 and TNF-α, and produced more IL-17 (supplemental Figure 7). Thus, Ifng−/− CD4+ T cells can respond to allogeneic Dll4hiDCs.
As expected, Ifng−/− CD4+ TNs induced lethal GVHD in all BALB/c recipients (Figure 6A-B). Dll4hiDC-Ifng−/− CD4+ T cells also induced lethal GVHD in 6/8 of BALB/c recipients (Figure 6A-B). Similar to WT CD4+ TNs, both Dll4hiDC-Ifng−/− CD4+ T cells and Ifng−/− CD4+ TNs infiltrated the spleen and the liver, with threefold more donor T cells in the spleen of Dll4hiDC-Ifng−/− CD4+ T-cell recipients 6 days after transfer (Figure 6C). Loss of IFN-γ led to markedly decreased apoptosis of Dll4hiDC-Ifng−/− CD4+ T cells compared with their WT counterparts (Figure 6D). Despite Dll4hiDC induction, Ifng−/− CD4+ T cells retained the capacity to produce TNF-α and IL-17 during GVH reaction (Figure 6E). These results indicate that IFN-γ is important for enhanced apoptosis of Dll4hiDC-WT CD4+ T cells and their inability to mediate GVHD.
T-cell IFN-γ is required for Dll4hiDCs programming to reduce CD4+ T-cell–mediated GVHD. Unstimulated WT or Ifng−/− CD4+ TNs (0.5 × 106) and allogeneic Dll4hiDC-induced WT or Ifng−/−CD4+ T cells (0.5 × 106) of B6 background were separately transplanted with TCD-BM (5.0 × 106) into lethally irradiated BALB/c mice. Survival (A) and GVHD clinical score (B) of the recipients were monitored over time. Data shown here are pooled from 2 independent experiments. (C) Six days after transplantation, donor T cells were isolated from the spleens and livers. Graphs show the numbers of donor CD4+ T cells in recipient BALB/c mice (mean ± SD). (D) Graph shows the percentage of annexin V+ cells among donor CD4+ T cells. (E) Plots and graphs show the percentages of donor CD4+ T cells producing IFN-γ, IL-17, and TNF-α in the spleen and the liver. (F) Histograms show the expression of indicated chemokine receptors on the surface of unstimulated WT or Ifng−/− CD4+ TNs and Dll4hiDC-stimulated WT or Ifng−/− CD4+ T cells 7 days after in vivo transfer. Representative results of 2 independent experiments are shown. *P < .05; **P < .01; ***P < .001.
T-cell IFN-γ is required for Dll4hiDCs programming to reduce CD4+ T-cell–mediated GVHD. Unstimulated WT or Ifng−/− CD4+ TNs (0.5 × 106) and allogeneic Dll4hiDC-induced WT or Ifng−/−CD4+ T cells (0.5 × 106) of B6 background were separately transplanted with TCD-BM (5.0 × 106) into lethally irradiated BALB/c mice. Survival (A) and GVHD clinical score (B) of the recipients were monitored over time. Data shown here are pooled from 2 independent experiments. (C) Six days after transplantation, donor T cells were isolated from the spleens and livers. Graphs show the numbers of donor CD4+ T cells in recipient BALB/c mice (mean ± SD). (D) Graph shows the percentage of annexin V+ cells among donor CD4+ T cells. (E) Plots and graphs show the percentages of donor CD4+ T cells producing IFN-γ, IL-17, and TNF-α in the spleen and the liver. (F) Histograms show the expression of indicated chemokine receptors on the surface of unstimulated WT or Ifng−/− CD4+ TNs and Dll4hiDC-stimulated WT or Ifng−/− CD4+ T cells 7 days after in vivo transfer. Representative results of 2 independent experiments are shown. *P < .05; **P < .01; ***P < .001.
The observation that mice receiving Dll4hiDC-CD4+ T cells had relatively fewer donor T cells in the intestine compared with the spleen suggested the possibility of impaired migration (Figure 5F-G). To test this, we assessed Dll4hiDC-CD4+ T-cell expression of chemokine receptors (eg, CCR4, CCR5, CCR9, and CXCR3) and adhesion molecule integrin-α4β7. These molecules are important for the migration of alloreactive T cells into GVHD target tissues, including skin, intestine, and liver.4,49 We observed that as compared with donor WT CD4+ TNs, donor Dll4hiDC-CD4+ T cells had lower levels of CCR4, CXCR3, and α4β7 (Figure 6F). Interestingly, Ifng−/− CD4+ T cells, which are known to induce less severe GVHD in the gut than their WT counterparts,48 also expressed a lower percentage of α4β7 (Figure 6F). Thus, we do not rule out a possible contribution of decreased expression of α4β7, CCR4, and CXCR3 to the impairment of Dll4hiDC-CD4+ T cells in GVHD induction.
Dll4hiDCs promote effector differentiation via a Notch-dependent mechanism
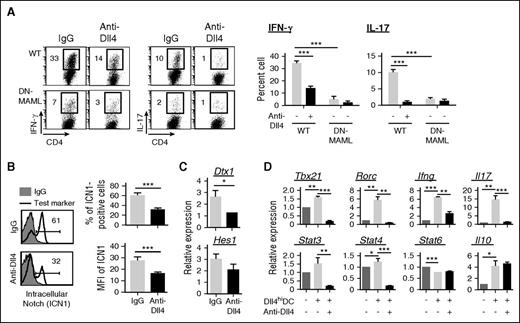
To determine the role of Notch in Dll4hiDC induction of alloreactive T cells, we used T cells expressing DN-MAML, a specific pan-Notch inhibitor.50 Upon allogeneic Dll4hiDC activation, DN-MAML CD4+ T cells produced 5 times fewer Th1 cells in cultures compared with WT CD4+ T cells (Figure 7A). Addition of anti-Dll4 Ab did not further decrease Th1 cells in cultures of DN-MAML CD4+ T cells (Figure 7A), suggesting the importance of Notch in Dll4hiDC-mediated Th1 differentiation.
Dll4 activation of Notch signaling is critical for Dll4hiDCs to induce IFN-γ production in CD4+ T cells. BALB/c Dll4hiDCs were cultured for 5 days with B6 WT CD4+ TNs or DN-MAML CD4+ TNs. (A) Dot plots and graphs show the percentages of IFN-γ–producing and IL-17–producing cells cultured with or without addition of anti-Dll4 Ab. (B) Histograms and graphs show the percentages and mean fluorescence intensity (MFI) of cleaved ICN1 in WT CD4+ T cells 72 hours after Dll4hiDC activation, with or without addition of neutralizing anti-Dll4 Ab. (C) Graphs show the relative expression of Notch target genes in WT CD4+ T cells after 5 days in culture. (D) Graphs show the relative expression of indicated genes in WT CD4+ T cells after 5 days in culture. Representative data (mean ± SD of triplicates) from 2 independent experiments are shown. *P < .05; **P < .01; ***P < .001.
Dll4 activation of Notch signaling is critical for Dll4hiDCs to induce IFN-γ production in CD4+ T cells. BALB/c Dll4hiDCs were cultured for 5 days with B6 WT CD4+ TNs or DN-MAML CD4+ TNs. (A) Dot plots and graphs show the percentages of IFN-γ–producing and IL-17–producing cells cultured with or without addition of anti-Dll4 Ab. (B) Histograms and graphs show the percentages and mean fluorescence intensity (MFI) of cleaved ICN1 in WT CD4+ T cells 72 hours after Dll4hiDC activation, with or without addition of neutralizing anti-Dll4 Ab. (C) Graphs show the relative expression of Notch target genes in WT CD4+ T cells after 5 days in culture. (D) Graphs show the relative expression of indicated genes in WT CD4+ T cells after 5 days in culture. Representative data (mean ± SD of triplicates) from 2 independent experiments are shown. *P < .05; **P < .01; ***P < .001.
Dll4 is a known activator of Notch1 signaling14,19 ; therefore, we hypothesized that Dll4hiDC activation induced ICN1 in alloreactive T cells. Indeed, 61% of Dll4hiDC-CD4+ T cells produced high levels of ICN1. Blocking Dll4 led to significant reduction of ICN1 (Figure 7B) and its target genes (eg, Dtx1 and Hes1) in WT CD4+ T cells stimulated with Dll4hiDCs (Figure 7C), indicating the activation of Notch1 in these T cells. Transcription factors T-bet and Stat4 are essential for inducing Ifng transcription in Th1 cells.51 We found that addition of anti-Dll4 Ab reduced the expression of Ifng, Tbx21, and Stat4 in CD4+ T cells activated by allogeneic Dll4hiDCs (Figure 7D). Thus, Dll4 activation of Notch signaling is important for the expression of transcription factors critical for Th1 differentiation.
Dll4hiDC-CD4+ T cells demonstrate greater in vivo expansion compared with nTEMs
Previous studies have shown that nTEMs caused less GVHD but retained GVL effect in mice.6,10,11,52 We confirmed in mice that CD4+ nTEMs showed potent antileukemia effects without severe GVHD by titrating donor T-cell doses: only 1 of 5 mice receiving low-dose CD4+ nTEMs died of A20 leukemia (supplemental Figure 8A). To compare the differences in expansion and IFN-γ production between Dll4hiDC-CD4+ T cells and nTEMs, we cotransferred equal numbers of Dll4hiDC-CD4+ T cells (B6, CD45.2+H2b+) and nTEMs (B6/SJL, CD45.1+H2b+) into lethally irradiated BALB/c mice (H2b+). This competitive experiment allowed us to assess their differences in response to alloantigens (supplemental Figure 8B). There were ∼3-fold and 10-fold higher fractions of donor cells derived from Dll4hiDC-CD4+ T cells than that of nTEMs in the spleen and BM at days 7 and 10 after transfer, respectively (supplemental Figure 8C). As compared with nTEM progeny, Dll4hiDC-CD4+ T cells contained significantly more progeny undergoing proliferation (Ki67+ cells, supplemental Figure 8D) and producing IFN-γ (supplemental Figure 8E). Thus, Dll4hiDC-CD4+ T cells demonstrate greater capability than nTEMs to expand and produce IFN-γ in allogeneic recipients.
Discussion
This study presents a novel cellular programming approach that produces alloreactive effector T cells incapable of causing severe GVHD but retaining GVL effects. To facilitate this strategy, we developed a platform that produces Dll4hiDCs from murine BM. Upon in vitro stimulation by allogeneic Dll4hiDCs, donor CD4+ TNs became alloreactive effector cells that secreted high levels of IFN-γ and IL-17. Adoptive transfer of these Dll4hiDC-induced T cells eliminated leukemic cells without causing severe GVHD, leading to significantly improved survival of leukemic mice undergoing allogeneic HSCT. This strategy has several potential advantages compared with current and developing methods for the modification of donor grafts to reduce GVHD,3,6,7,10,53 including no requirement for T-cell subset selection and a dramatically expanded safety range of infused donor T-cell dose. Importantly, our platform does not require transfection with viral vectors, which has limitations of safety and efficiency. Thus, Dll4hiDC programming can overcome GVHD toxicity of donor T cells and produce leukemia-reactive T cells for effective immunotherapy.
The reduction of Dll4hiDC-induced T cells in mediating GVHD may be primarily explained by their impaired expansion capability in vivo. We found that Dll4hiDC-induced CD4+ T cells underwent enhanced apoptosis early after transfer compared with unstimulated CD4+ TNs, leading to an overall reduction of donor T cells in GVHD target organs. Effector differentiation is known to be associated with reduced proliferation and expansion of antigen-specific T cells.20 The intrinsic defect of antigen-sensitized CD4+ TEMs in mediating GVHD was linked to their limited expansion in target tissues.11 However, the underlying molecular mechanisms remain elusive.
IFN-γ plays paradoxical roles in regulating GVH reactivity against lymphohematopoietic cells vs nonhematopoietic tissues.40,45-48 IFN-γ prevented severe GVHD early after allogeneic HSCT in mice and repressed the damage to the skin and lung.45-47 IFN-γ promoted apoptosis in activated T cells23,45,46,54 and upregulated expression of PD-L1, an inhibitory immune checkpoint molecule, in lung parenchyma, leading to reduced expansion of alloreactive T cells and protection against lung GVHD.40 However, IFN-γ has also been implicated in mediating GVH reactions against hematopoietic tissues45,46 and gastrointestinal tract.48 We found that as compared with Dll4hiDC-induced WT CD4+ T cells, Dll4hiDC-induced Ifng−/− CD4+ T cells had greater ability to expand in the spleen and accumulate in the liver and caused more severe GVHD after transplantation. Thus, T-cell IFN-γ is required to limit the expansion of Dll4hiDC-induced T cells in GVHD target tissues and development of GVHD. Further studies will investigate why Dll4hiDC-CD4+ T cells produce high levels of IFN-γ but have reduced ability to mediate gut GVHD.
Notch regulates the genetic programs important for effector differentiation independently of cytokine signals.16,55-57 Notch binds to the regulatory regions of genes encoding transcription factors (eg, Tbx21, Rorc, and Gata3) and cytokines (eg, Ifng, Il17, and Il4),50,55-57 thereby amplifying signals for Th differentiation.55 We found that Dll4hiDC-derived Dll4 was critical for activating Notch signaling in alloreactive T cells. Inhibition of Dll4 led to dramatically reduced expression of Tbx21 and Stat4 in CD4+ T cells stimulated by allogeneic Dll4hiDCs. Furthermore, blocking Dll4 decreased ICN1 in CD4+ T cells activated by Dll4hiDCs. Thus, Dll4 derived from Dll4hiDCs regulates Th1 differentiation through a Notch-dependent pathway.
The differential effects of GM-DCs and Dll4hiDCs on eliciting alloreactive T-cell responses may involve a complex mechanism. First, activation of T-cell Notch signaling may explain their difference in mediating GVHD. Dll4hiDC-induced T cells have undergone a greater extent of proliferation and effector differentiation than GM-DC-induced T cells. This is in agreement with the finding that Notch signaling has a broad impact on promoting the production of multiple lineages of effector T cells.14,15,55 Because IFN-γ produced by Dll4hiDC-CD4+ T cells was critical for limiting their expansion in GVHD target tissues, the inferior capacity of GM-DCs relative to Dll4hiDCs to induce IFN-γ–producing T cells could account for their difference in mediating GVHD. Second, GM-DCs and Dll4hiDCs displayed significant differences in expression of genes associated with inflammatory cytokines (eg, Ifna, Ifnb, Il4, and Il6) and immune modulatory molecules (eg, iNos, Arg1, and Ido). Because these molecules are reportedly important for regulating T-cell immune responses,4 they might have significant impact on DC programming of alloreactive T-cell responses.
We demonstrated that Dll4hiDC-induced alloreactive effector T cells had acquired the capability of killing leukemic cells. This may potentially improve the antileukemic response early after HSCT and overcome some barriers to the GVL response such as high disease burden and pharmacologic immunosuppression. Importantly, following transfer, these Dll4hiDC-induced T cells continually produced high levels of IFN-γ and TNF-α and elicited GVL activity with a wider safety range of infused T-cell numbers without causing severe GVHD than their naïve counterparts. Given the fact that DC activation of TNs allows priming them with antigens, we propose that Dll4hiDCs loaded with leukemia-associated antigens may facilitate the selection and expansion of leukemic cell–reactive T cells that mediate antileukemia activity. Importantly, we have recently identified human Dll4hiDCs that possess great ability to promote allogeneic CD4+ TNs to become effector cells producing high levels of IFN-γ.58 If sufficient numbers of human Dll4hiDCs can be produced, a novel strategy can be potentially devised for human patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Warren Pear (University of Pennsylvania) and Ivan Maillard (University of Michigan) for kindly providing DN-MAML mice, and Marcel van den Brink (Memorial Sloan Kettering Cancer Center) for providing A20-TGL cells.
This work was supported by a Damon Runyon-Rachleff Innovation Award (Yi Zhang), the American Cancer Society (Yi Zhang), the Department of Defense (Yi Zhang), and National Cancer Institute, National Institutes of Health (grants CA172106 [Yi Zhang], CA72669 [B.R.B.], and CA178202 [R.R.]).
Authorship
Contribution: K.M. and Yi Zhang conceived and designed the project; K.M., L.M., I.M., Q.T., S.H., Y.L., J.P., H.F., M.R.Z., and Yi Zhang performed experiments and analyzed and interpreted the data; Yanyun Zhang, B.R.B., H.Y., and S.M. analyzed and interpreted the data; K.M., L.M., and Yi Zhang wrote the manuscript; and K.M., L.M., R.R., B.R.B., S.M., and Yi Zhang edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for K.M. and I.M. is Department of Pediatric Oncology, Fukushima Medical University, Fukushima, Japan.
Correspondence: Yi Zhang, Fels Institute for Cancer Research and Molecular Biology, Department of Microbiology and Immunology, Temple University School of Medicine, Philadelphia, PA 19140; e-mail: yi.zhang@temple.edu.
References
Author notes
K.M. and L.M. contributed equally to this study.

![Figure 1. In vitro generation of Dll4hiDCs. Flt3L-DCs were generated by incubating BALB/c mouse BM mononuclear cells in cultures with Flt3L. GM-DCs were induced by culturing c-kit+ hematopoietic progenitor cells in the presence of GM colony-stimulating factor, IL-4, and stem cell factor. After 8 days in culture, cells were collected and cultured in medium containing indicated stimuli for additional 24 hours. (A) Graphs show the number of CD11c+ cells (mean ± standard deviation [SD] of triplicates). (B) Histograms and a graph show the percentage of Dll4 on the surface of Flt3L-DCs (mean ± SD of triplicates). (C, E) Real-time reverse transcription polymerase chain reaction analysis revealed the relative expression of indicated genes in DCs generated in cultures (mean ± SD of triplicates). (D) Histograms show the expression of tested markers on the surface of DCs with or without stimulation of LPS+R848. Representative results of 3 independent experiments are shown. ** P < .01; *** P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/25/10.1182_blood-2015-05-644476/4/m_3270f1.jpeg?Expires=1771023115&Signature=v9gbHJKNJKtVtMFVHjjkWz0ZctKlMdiy0ziDMq7hw8DnU~TOSBBerkconSM9tyEW3GxI-z5ilfRxJSzKxJRZw~hvOkUkNivcgUmmMxOovSZYkkubvA9V1u7O4bmdxv2Jxu9-BjtMOl0xK9T0yzd~vfTGXXMQUMGMuBw8Z1Umf9I2PcX1uf6UaC5YQXL5dC9vLUJXVEmql7CG6EQT9tJqsh6Z5nmkPECYFnxX80WQ8EvAqVkZlnQcWTnx9Sx2Un6MigZxHyIkIy8PS1UT8joWT8smy2Q1vPgCWW8GYha5osIu0tsWq-HYSM5L63NgbpUs~7y4mSr2VspA58xUSjHRoA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)