Abstract
INTRODUCTION: Pure erythroid leukemia (PEL) is a rare form of acute leukemia characterized by a neoplastic proliferation of immature erythroblasts with an extremely aggressive clinical course. Although there are previous reports describing its association with a complex karyotype and chromosome 5 and 7 abnormalities, there is no knowledge on the mutational landscape of this disease. Also, further evaluation of the optimal treatment strategy for this subset of patients is still required.
METHODS: We retrospectively evaluated all patients with pure erythroid leukemia treated at The University of Texas MD Anderson Cancer Center from 1980 to 2016. Cytogenetic analysis was reported following the ISCN 2013 nomenclature. Sequencing data was obtained by use of a 28-gene targeted PCR-based next generation sequencing (NGS) platform. Clinical and demographic data was obtained from clinical records. Response was defined following 2003 IWG criteria. Generalized linear models were used to study the association of overall response (OR), complete response (CR) and risk factors. Kaplan-Meier produce limit method was used to estimate the median overall survival (OS) and leukemia-free survival (LFS).
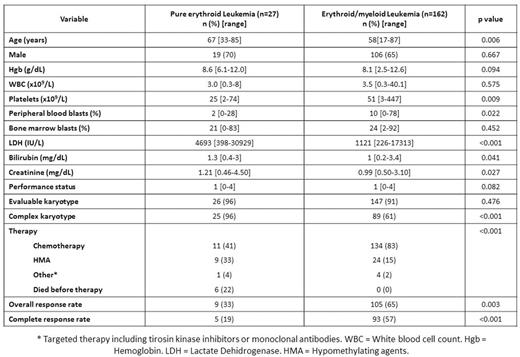
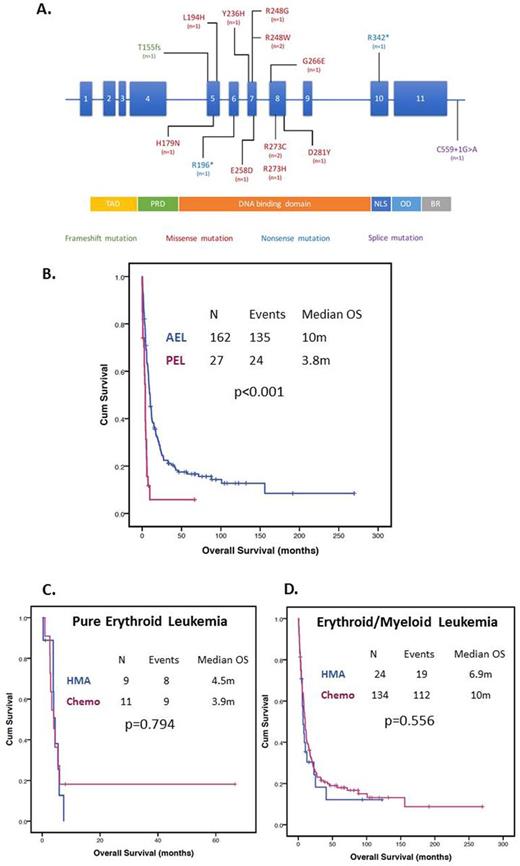
RESULTS: A total of 27 patients had PEL. Patient characteristics are shown in Table 1. Median age at diagnosis was 67 years (range 33-65). Eleven (41%) patients had therapy related disease, 10 (37%) evolved from a prior myelodysplastic syndrome and 1 (4%) from chronic myeloid leukemia. Presence of a complex karyotype was observed in 96% (25/26) patients. A total of 13 (48%) patients with PEL had mutation analysis available. Twelve (92%) had at least one detectable mutation. Median number of mutations was 1 (range 0-3). TP53 mutations were found in 11/12 (92%) patients, and ASXL1, PTPN11 and DNMT3A each found in 1 (8%) patient. Type and frequency of TP53 mutations is shown in Figure 1A. Fifteen (58%) patients with PEL had chromosome 17 abnormalities including monosomy 17, del(17p) and add(17)(p11.2). Five (50%) patients with cytogenetic and mutation data available had both TP53 mutation and chromosome 17 abnormalities. Nine (27%) patients were treated with hypomethylating agent based therapy, 11 (41%) with intensive chemotherapy, 1 (4%) with targeted therapy and 6 (22%) died before receiving any form of therapy. We compared clinical characteristics and outcomes of patients with PEL with that of 162 patients with acute erythroid/myeloid leukemia (AEL) treated at MDACC during the same time period. Patients with PEL tended to be older (p=0.006), have lower platelet count (p=0.009) and lower peripheral blast percentage (p=0.022), higher LDH levels (p<0.001) and a higher frequency of a complex karyotype (96 vs 61%, p<0.001) (Table 1). Patients with PEL were less frequently treated with intensive chemotherapy (p<0.001) and had lower CR rates compared to patients with AEL treated with chemotherapy (OR 0.23, 95% CI 0.58-0.90, p=0.051). Median follow up was 8 months (range 0-269 months). The median OS for patients with PEL was 3.8 months compared to 10 months for AEL (HR 2.895, 95% CI 1.843-4.55, p<0.001) (Figure 1B). No differences in survival were observed with chemotherapy compared to hypomethylating agents for both patients with PEL (median OS 4.4 months vs 3.9 respectively, p=0.794) (Figure 1C) or AEL (median OS 10 vs 6.9 months respectively, p=0.556) (Figure 1D).
CONCLUSION: Pure erythroid leukemia represents 14% of cases of all erythroid leukemia. We establish, in the largest series to date, PEL is characterized by a very complex karyotype. There is also a high prevalence of chromosome 17 abnormalities and mutations in TP53; interestingly, in several patients, there was co-occurrence of both, or double TP53 mutations. Our data demonstrates that severely impaired and completely lost of TP53 function, resulting in genomic instability, is a strong influential factor for PEL pathogenesis in particular, and is consistent with its associated dismal prognosis irrespective of currently available therapies. In view of PEL outcomes and older age at diagnosis, enrollment in clinical trials targeting or circumventing mutant p53, and less intensive approaches is the current optimal strategy for this subset of patients.
Ravandi:BMS: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. Daver:Sunesis: Consultancy, Research Funding; Ariad: Research Funding; Kiromic: Research Funding; Otsuka: Consultancy, Honoraria; BMS: Research Funding; Pfizer: Consultancy, Research Funding; Karyopharm: Honoraria, Research Funding. DiNardo:Daiichi Sankyo: Other: advisory board, Research Funding; Novartis: Other: advisory board, Research Funding; Abbvie: Research Funding; Celgene: Research Funding; Agios: Other: advisory board, Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Konopleva:Reata Pharmaceuticals: Equity Ownership; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Stemline: Consultancy, Research Funding; Eli Lilly: Research Funding; Cellectis: Research Funding; Calithera: Research Funding. Kantarjian:Amgen: Research Funding; Bristol-Myers Squibb: Research Funding; ARIAD: Research Funding; Pfizer Inc: Research Funding; Delta-Fly Pharma: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.