Abstract
Background:Clinical trials exclusively focusing on pts with MDS/MPN are lacking.AZA is a DNA methyltransferase (DNMT) inhibitor approved for the therapy of MDS while RUX is a JAK inhibitor approved as therapy for myelofibrosis and polycythemia vera. RUX and AZA may target distinct clinical and pathological manifestations of MDS/MPNs.
Aim:To determine the efficacy and safety of RUX + AZA in pts with MDS/MPN requiring therapy including chronic myelomonocytic leukemia (CMML), atypical chronic myeloid leukemia BCR-ABL1 negative (aCML), and myelodysplastic/myeloproliferative neoplasm, unclassifiable (MDS/MPN-U)(ClinicalTrials.gov Identifier: NCT01787487).
Methods:A sequential approach with single-agent RUX 15 mg orally twice daily (if platelets 100-200) or 20 mg twice daily (if platelets >200) continuously (pts with platelets below 50 were not eligible) in 28-day cycles for the first 3 cycles followed by the addition of AZA 25 mg/m2 on days 1-5 of each 28-day cycle starting cycle 4 was adopted. The AZA dosage could be gradually increased to a maximum of 75 mg/m2. The AZA could be started earlier than cycle 4 and/or at a higher dose in pts with proliferative disease or elevated blasts.
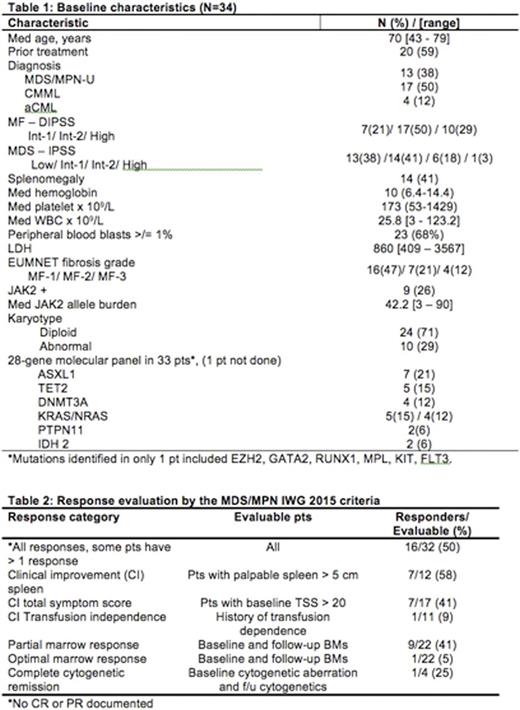
Results: 34 pts were enrolled between March 1, 2013 and June 30, 2016. Baseline characteristics are summarized in table 1. 21 pts remain alive after a median (med) follow-up of 14.2 months (range, 0.5-32.7+).
Responses were evaluated by the MDS/MPN IWG response criteria (Savona et al., Blood 2015, 125(12):1857-65). 32 pts were enrolled before April 1 2016 wereevaluable for response. Responses were noted in 16 (50%) pts. Details of responses are shown in table 2. Med time to IWG response was 1.8 mos (range, 0.7-11.0+) and the med duration of response is 7 mos (range, 2.3-31.8+). Seven (44%) of the IWG responses occurred after the addition of the AZA. There was a trend to higher IWG-responses in JAK2 mutated versus non-mutatedpts (7/8 versus 9/24 responses, P=0.18). Additionally, 9 pts had >5% pretreatment BM blasts and 7 achieved a reduction in blasts to <5% with a med time to blast reduction of 5.5 mos (range, 2.6-11.2+). Serial evaluation of bone marrow biopsies documented reduction in EUMNET fibrosis score in 5 of 20 (25%) evaluable pts after a med of 5.5 mos (range, 2.0-5.6+) on therapy. The reduction in fibrosis was by one grade in all 5 cases.
Oneptexperienced grade 3/4 non-hematological toxicity of limb edema. New onset grade 3/4anemiawas seen in 24 pts [71%; of which 7 (21%) had a >/=2+ grade change] and new onset grade 3/4 thrombocytopenia in 18 (53%) pts, respectively.
The AZA was started in cycle 4 in 14 pts (41%). The AZA was started earlier due to leukocytosis or increased blasts in 16 pts (47%): in cycle 1 (n=8), cycle 2 (n=5), and cycle 3 (n=3); 2 never started AZA due to low counts and 2 pts are too early to start AZA.23 (68%) pts have discontinued protocol therapy due to leukocytosis (n=7), progression to AML (n=5), stem cell transplant (n=3), lack of response (n=2), progressive thrombocytopenia (n=1), pneumothorax (n=1), concurrent T-cell neoplasm (n=1), and loss of insurance (n=1).
Thirteen (38%) pts have died: pneumonia (n=4), sepsis (n=4), progression to AML (n=4), and uncontrolled leukocytosis in oneptwith Ph- CML. The med overall survival (OS) for all 34 pts is 25.4mos+ (range, 1.0-32.7+). The MDS/MPN-U patients had a significantly better med OS (26.4+ months) as compared to the CMML (15.0+ months) andaCML(1.5+ months)pts, respectively (P=0.01).
Conclusion: Concomitant administration of RUX with AZA demonstrated an IWG-response rate of 50% in pts with MDS/MPNs, with expected myelosuppression as the only significant toxicity. The benefit seems to be more profound in pts with MDS/MPN-U and MDS/MPNpts with JAK2 mutations. This combination warrants further evaluation.
Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Verstovsek:Pfizer: Research Funding; Geron: Research Funding; Roche: Research Funding; Seattle Genetics: Research Funding; Celgene: Research Funding; Galena BioPharma: Research Funding; Promedior: Research Funding; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; Lilly Oncology: Research Funding; NS Pharma: Research Funding; Genentech: Research Funding; Gilead: Research Funding; AstraZeneca: Research Funding; Bristol-Myers Squibb: Research Funding; CTI BioPharma Corp: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.