Abstract
Background:
The mini-hyper-CVD regimen is being developed as a frontline treatment for older (≥60 years) patients with acute lymphoblastic leukemia (ALL). This regimen utilizes reduced doses of conventional cytotoxic chemotherapy in comparison to the traditional hyper-CVAD (cyclophosphamide and dexamethasone 50%, methotrexate 75%, cytarabine 83% dose reductions, and anthracycline omitted) and adds a novel antibody drug conjugate, inotuzumab ozogamicin (InO). Given that this regimen utilizes 75% less methotrexate than the traditional hyper-CVAD and that therapeutic drug monitoring is a standard of care for patients receiving high-dose methotrexate, here we aim to compare the clearance of methotrexate in patients receiving the mini-hyper-CVD regimen to those receiving traditional hyper-CVAD.
Methods:
Patients treated with frontline mini-hyper-CVD or hyper-CVAD at our institution from August 2011 to February 2016 were included in this single-center retrospective cohort analysis. Methotrexate levels from the first of four methotrexate cycles were obtained at the end of the continuous infusion (0-hour), at 24 and 48 hours post-infusion completion, or as clinically indicated. In some patients, methotrexate levels were not obtained at 48 hours if the 24-hour level demonstrated sufficient clearance. Delayed clearance was defined as methotrexate levels >20 µmol/L at 0 hour, >1 µmol/L at 24 hours, or >0.1 µmol/L at 48 hours. Identical supportive care interventions to facilitate methotrexate elimination (e.g., urinary alkalization) and leucovorin rescue dose and schedules were used for both mini-hyper-CVD and traditional hyper-CVAD regimens.
Results:
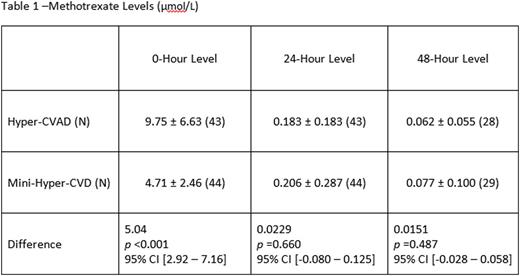
A total of 87 patients treated with mini-hyper-CVD (n=44) and traditional hyper-CVAD (n=43) were included in this analysis. The patient population was predominately male (n=26) in both groups. The hyper-CVAD methotrexate levels at the 0-hour were 9.75 ± 6.63 µmol/L, and the mini-hyper-CVD methotrexate levels were 4.71 ± 2.46 µmol/L. There was a significant mean difference of 5.04 µmol/L between the two regimens at the 0-hour measurement (p <0.001; 95% CI [2.92 - 7.16]). Measurements at 24 hours and 48 hours were similar between the 2 regimens: the hyper-CVAD methotrexate levels were 0.183 ± 0.183 µmol/L at 24 hours and 0.062 ± 0.055 µmol/L at 48 hours, and the mini-Hyper-CVD methotrexate levels were 0.206 ± 0.287 µmol/L at 24 hours and 0.077 ± 0.100 µmol/L at 48 hours. Delayed clearance at 0-hour was observed in 9% (n=4) of patients treated with the hyper-CVAD regimen versus none of the patients treated with the mini-hyper-CVD regimen (p=0.0554). At 48 hours, 11% (n=5) of the mini-hyper-CVD patients and 7% (n=3) of the hyper-CVAD patients had delayed clearance. All patients with delayed 48-hour clearance had repeat measurements indicating clearance at 72 hours. Methotrexate-related adverse effects were uncommon and did not differ between the two regimens.
Conclusions:
Significant differences in 0-hour methotrexate levels were observed between the two regimens with the mini-hyper-CVD being 48% lower than those with the hyper-CVAD. There was no difference in 24- or 48-hour clearance between the regimens. This suggests that further studies evaluating simplified methotrexate clearance supportive-care strategies with mini-hyper-CVD regimen are warranted.
O'Brien:Janssen: Consultancy, Honoraria; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. Konopleva:Reata Pharmaceuticals: Equity Ownership; Abbvie: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Stemline: Consultancy, Research Funding; Eli Lilly: Research Funding; Cellectis: Research Funding; Calithera: Research Funding. Jain:Servier: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Novimmune: Consultancy, Honoraria; Infinity: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Seattle Genetics: Research Funding; Celgene: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Genentech: Research Funding; BMS: Research Funding; Abbvie: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; Incyte: Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.