Key Points
Key biological features of MDSs are explained by NLRP3 inflammasome activation, which drives pyroptotic cell death and β-catenin activation.
Alarmin signals and founder gene mutations license this redox-sensitive inflammasome platform.
Abstract
Despite genetic heterogeneity, myelodysplastic syndromes (MDSs) share features of cytological dysplasia and ineffective hematopoiesis. We report that a hallmark of MDSs is activation of the NLRP3 inflammasome, which drives clonal expansion and pyroptotic cell death. Independent of genotype, MDS hematopoietic stem and progenitor cells (HSPCs) overexpress inflammasome proteins and manifest activated NLRP3 complexes that direct activation of caspase-1, generation of interleukin-1β (IL-1β) and IL-18, and pyroptotic cell death. Mechanistically, pyroptosis is triggered by the alarmin S100A9 that is found in excess in MDS HSPCs and bone marrow plasma. Further, like somatic gene mutations, S100A9-induced signaling activates NADPH oxidase (NOX), increasing levels of reactive oxygen species (ROS) that initiate cation influx, cell swelling, and β-catenin activation. Notably, knockdown of NLRP3 or caspase-1, neutralization of S100A9, and pharmacologic inhibition of NLRP3 or NOX suppress pyroptosis, ROS generation, and nuclear β-catenin in MDSs and are sufficient to restore effective hematopoiesis. Thus, alarmins and founder gene mutations in MDSs license a common redox-sensitive inflammasome circuit, which suggests new avenues for therapeutic intervention.
Introduction
Myelodysplastic syndromes (MDSs) are hematopoietic stem cell malignancies characterized by dysplastic and ineffective hematopoiesis. MDS bone marrow (BM) precursors are typically larger in size with deregulated proliferation and maturation and accelerated attrition by programmed cell death.1-3 Despite these shared phenotypes, MDSs harbor a spectrum of clonal chromosome abnormalities and somatic gene mutations.4,5 How such diverse genetic alterations initiate a common MDS phenotype is unexplained. Apoptosis, a noninflammatory cell death, has been implicated in the ineffective hematopoiesis in MDSs based upon membrane externalization of phosphatidylserine, mitochondrial depolarization, and DNA fragmentation.6-8 However, the cytokine profile and cellular milieu instead implicate aberrant innate immune activation.9 Indeed, inflammatory cytokines such as interleukin-1β (IL-1β), tumor necrosis factor α, transforming growth factor β, IL-6, and others are generated in excess in MDSs, accompanied by BM expansion of hematopoietic-inhibitory, myeloid-derived suppressor cells (MDSCs) activated by the danger-associated molecular pattern (DAMP) S100A9, a Toll-like receptor 4 (TLR4) and CD33 ligand.10-13 Furthermore, MDS hematopoietic stem and progenitor cells (HSPCs) overexpress TLRs, which is accompanied by activation of respective signaling intermediates and has been implicated in the aberrant proliferation of HSPCs and the pathogenesis of peripheral blood cytopenias.14-16
Recent studies have shown that activation of TLRs by select DAMPs can trigger pyroptosis, a novel caspase-1–dependent proinflammatory, lytic cell death3-15,17 Pyroptosis is mediated by the formation of inflammasome complexes, which are cytosolic heptameric oligomers composed of nucleotide-binding domain and leucine-rich repeat (NLR) pattern recognition receptors. The best-characterized NLR, NLRP3, is a redox-sensitive cytosolic sensor that recruits the ASC (apoptosis-associated speck-like protein containing a caspase-recruitment domain) adaptor protein. This interaction in turn triggers ASC polymerization and nucleation of large cytoplasmic aggregates referred to as ASC specks, permitting docking and activation of pro–caspase-1 that processes pro–IL-1β and pro–IL-18 to their active forms.18,19 Inflammasome activation begins with NF-κB–induced transcriptional priming of inflammasome proteins, followed by cation channel activation, cell volume expansion, and inflammasome component assembly.17,18,20,21 NLRP3 is activated by diverse DAMP signals, including S100A9 homodimers and S100A8/9 heterodimers that function as alarmins that converge upon NADPH oxidase to generate reactive oxygen species (ROS).21-23
Here, we show that S100A9 and ROS, generated in response to NLRP3 inflammasome activation or somatic gene mutations, serve as DAMP signaling intermediates responsible for inflammasome-mediated pyroptosis and β-catenin activation in MDSs. Remarkably, disabling this inflammasome circuit restores effective hematopoiesis in MDSs. Collectively, these findings define key biological effectors of the MDS phenotype and suggest novel strategies for therapeutic intervention.
Methods
Experimental methods and procedures can be viewed in supplemental Materials (available on the Blood Web site).
Results
MDS HSPCs manifest inflammasome activation and pyroptosis
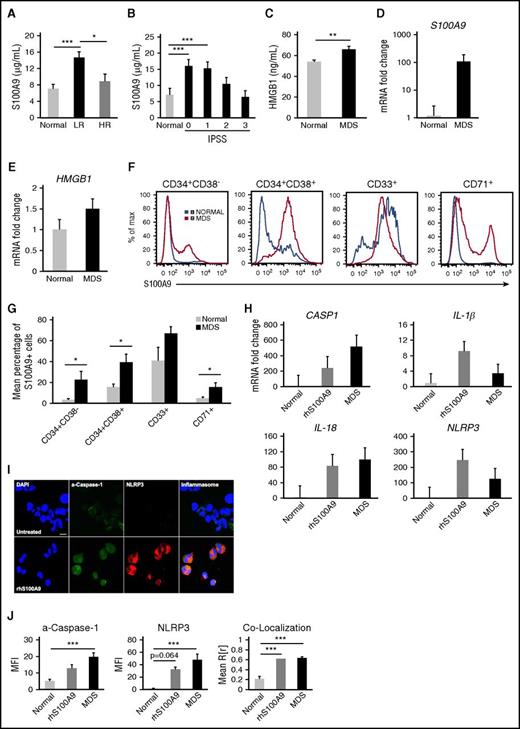
To determine if pyroptosis is primed in MDSs, expression of genes encoding inflammasome proteins was evaluated in BM mononuclear cells (MNCs) isolated from MDS patients (n = 10) and compared with age-matched normal controls (n = 5). MDS specimens displayed marked upregulation of inflammasome transcripts (Figure 1A), including caspase-1 (CASP1; 209-fold) and NLRP3 (48.1-fold), whereas levels of caspase-3 (CASP3) messenger RNA (mRNA), the canonical apoptotic caspase, were similar to normal BM-MNCs. Further, mRNAs encoding IL-1β and IL-18 were increased 3.7-fold and 29.6-fold in lower-risk (LR) MDS (n = 5) vs normal donors (n = 5), whereas expression was only increased 1.1-fold and 9.2-fold in higher-risk (HR) MDS specimens (n = 5).
Fulminant pyroptosis is manifest in HSPCs and progeny in MDSs. (A) Quantitative polymerase chain reaction (qPCR) analyses of expression of pyroptosis-associated genes in BM-MNCs isolated from MDS patient specimens (n = 10 total, n = 5 LR-MDS, and n = 5 HR-MDS) compared with normal BM-MNCs (n = 5). (B) Representative confocal fluorescence micrograph (original magnification ×2520, 7.5 µm scale) of a–caspase-1 and NLRP3 expression in MDS vs normal BM-MNCs (DAPI, blue; a–caspase-1, green; and NLRP3, red; merged images show inflammasome formation). (C) Quantitative analysis of confocal images of BM-MNCs isolated from MDS patients (n = 7 LR-MDS, n = 3 HR-MDS) and normal donors (n = 6). (D) Binding of ASC to NLRP3 in LR-MDS BM-MNCs compared with normal donors (immunoprecipitation [IP], NLRP3; immunoblot [IB], NLRP3, ASC). Data are representative of 3 independent experiments. (E) Immunoblot following chemical crosslinking of BM-MNC lysates derived from normal donors (n = 3) and LR-MDS patients (n = 3). (F) Quantitation of inflammasome activation based on ASC oligomerization in BM-MNCs from LR-MDS (n = 5) vs normal BM-MNCs (n = 3). (G) Mean percentage of ASC specks and speck MFI in the BM plasma of LR-MDS specimens (n = 6) compared with normal BM plasma (n = 3). (H) The mean percentage of pyroptotic cells by hematopoietic lineage in LR-MDS (n = 8) vs normal donors (n = 8). (I-J) Mean percentage of (I) total a–caspase-1+ and (J) a–caspase-3/7+ cells assessed by hematopoietic lineage in LR-MDS (n = 8) and normal donors (n = 5). (K) Comparison of the mean percentage of pyroptotic vs apoptotic cells by hematopoietic lineage in LR-MDS specimens (n = 8). (L) Comparison of the mean percentage of a–caspase-1+ vs a–caspase-3/7+ cells in the same LR-MDS patients (n = 8). (M) Mean percentage of pyroptotic cells following knockdown of NLRP3, CASP1, and CASP3 by short hairpin RNA–directed silencing of LR-MDS BM-MNCs (n = 4 NLRP3, n = 3 CASP1 and CASP3). Error bars represent standard error (SE). *P < .05, ** P < .01, and ***P < .001. See also supplemental Figures 1 and 2.
Fulminant pyroptosis is manifest in HSPCs and progeny in MDSs. (A) Quantitative polymerase chain reaction (qPCR) analyses of expression of pyroptosis-associated genes in BM-MNCs isolated from MDS patient specimens (n = 10 total, n = 5 LR-MDS, and n = 5 HR-MDS) compared with normal BM-MNCs (n = 5). (B) Representative confocal fluorescence micrograph (original magnification ×2520, 7.5 µm scale) of a–caspase-1 and NLRP3 expression in MDS vs normal BM-MNCs (DAPI, blue; a–caspase-1, green; and NLRP3, red; merged images show inflammasome formation). (C) Quantitative analysis of confocal images of BM-MNCs isolated from MDS patients (n = 7 LR-MDS, n = 3 HR-MDS) and normal donors (n = 6). (D) Binding of ASC to NLRP3 in LR-MDS BM-MNCs compared with normal donors (immunoprecipitation [IP], NLRP3; immunoblot [IB], NLRP3, ASC). Data are representative of 3 independent experiments. (E) Immunoblot following chemical crosslinking of BM-MNC lysates derived from normal donors (n = 3) and LR-MDS patients (n = 3). (F) Quantitation of inflammasome activation based on ASC oligomerization in BM-MNCs from LR-MDS (n = 5) vs normal BM-MNCs (n = 3). (G) Mean percentage of ASC specks and speck MFI in the BM plasma of LR-MDS specimens (n = 6) compared with normal BM plasma (n = 3). (H) The mean percentage of pyroptotic cells by hematopoietic lineage in LR-MDS (n = 8) vs normal donors (n = 8). (I-J) Mean percentage of (I) total a–caspase-1+ and (J) a–caspase-3/7+ cells assessed by hematopoietic lineage in LR-MDS (n = 8) and normal donors (n = 5). (K) Comparison of the mean percentage of pyroptotic vs apoptotic cells by hematopoietic lineage in LR-MDS specimens (n = 8). (L) Comparison of the mean percentage of a–caspase-1+ vs a–caspase-3/7+ cells in the same LR-MDS patients (n = 8). (M) Mean percentage of pyroptotic cells following knockdown of NLRP3, CASP1, and CASP3 by short hairpin RNA–directed silencing of LR-MDS BM-MNCs (n = 4 NLRP3, n = 3 CASP1 and CASP3). Error bars represent standard error (SE). *P < .05, ** P < .01, and ***P < .001. See also supplemental Figures 1 and 2.
Confocal fluorescence microscopy confirmed activation of NLRP3 inflammasome complexes in MDS specimens vs age-matched controls, evidenced by colocalization and increased active caspase-1 (a–caspase-1) (mean fluorescence intensity [MFI], 3.7-fold increase in LR-MDS [P = 7.1 × 10−3] and 4.1-fold in HR-MDS [P = 6.0 × 10−3]) and NLRP3 (MFI, 69.1-fold increase in LR-MDS [P = .013] and 68.2-fold in HR-MDS [P = 5.1 × 10−3]) (Figure 1B-C). NLRP3 oligomerization and activation was confirmed by ASC binding to NLRP3 in MDS compared with normal donors (Figure 1D) and by formation of NLRP3-dependent ASC monomers and oligomers, which are indispensable for inflammasome activity (Figure 1E). MDS BM-MNCs also displayed increased pro– and a–caspase-1 and pro–IL-1β and a–IL-1β, as well as robust expression of ASC monomers and higher-order complexes compared with normal BM-MNCs (n = 3) (Figure 1E). Finally, inflammasome formation was confirmed by flow cytometry assessment of ASC oligomerization, where ASC polymerization in inflammasome complexes can be detected by changes in fluorescence pulse height and area (Figure 1F).24
MDS specimens also displayed significantly greater inflammasome assembly compared with controls, irrespective of International Prognostic Scoring System (IPSS) risk group (Figure 1C). NLRP3 inflammasome assembly was increased 2.9-fold in LR-MDS patients (P = 3.9 × 10−5) and 3.1-fold in HR-MDS patients (P = 7.1 × 10−5). Catalytically active ASC specks are released into the extracellular space following cytolytic execution of pyroptosis.25 Notably, analysis of ASC specks in BM plasma from LR-MDS specimens (n = 6) confirmed a profound increase in the percentage and MFI of ASC specks in MDS vs normal BM plasma (n = 3) (mean, 36.2 ± 1.4 vs 6.0 ± 8.4; Figure 1G). Finally, immunofluorescence and flow cytometry analyses of other hematologic malignancies suggest that inflammasome activation is specific for MDSs (supplemental Figure 1).
Caspase-1 activation by the inflammasome is followed by mitochondrial depolarization and caspase-3 activation as late events in pyroptosis. To specifically distinguish pyroptosis from apoptosis, the percentage of pyroptotic cells, defined as a–caspase-1+/a–caspase-3/7+/annexin V+ cells, was determined in phenotypically distinct hematopoietic lineages by flow cytometry. Normal (n = 5) and LR-MDS BM-MNCs (n = 8) were incubated with autologous BM plasma for 24 hours prior to analysis. MDS HSPCs demonstrated a profound increase in the fraction of pyroptotic cells (4.1-fold in CD34+CD38− stem cells [P = .035], 4.9-fold in progenitor cells [CD34+CD38+; P = 6.8 × 10−3], 6.6-fold in immature myeloid cells [CD33+; P = 1.5 × 10−3), and 7.3-fold in erythroid cells [CD71+; P = 2.8 × 10−3]) compared with normal controls (Figure 1H). Additionally, the percentage of a–caspase-1+ cells was increased 14.2-fold in the stem cell fraction (P = 8.0 × 10−3), 13.2-fold in progenitors, 12.9-fold in immature myeloid cells (P = 1.3 × 10−4), and 13.0-fold in CD71+ erythroid precursors (P = 7.7 × 10−3) (Figure 1I). A–caspase-1 MFI directly correlated with NLRP3 MFI, inflammasome assembly, and the percentage of pyroptotic stem cells. Notably, the latter was directly associated with the percentage of a–caspase-1+ CD33+ myeloid progenitors (supplemental Figure 2). In contrast, there were no significant differences in the fraction of apoptotic cells (ie, a–caspase-3/7+/a–caspase-1−/annexin V+) in LR-MDS (n = 8) vs normal progenitors in any of the 4 hematopoietic lineages investigated (Figure 1J). Indeed, the pyroptotic cell fraction was 14.4-fold (P = 9.7 × 10−3), 9.7-fold (P = 2.3 × 10−3), 21.9-fold (P = 9.5 × 10−4), and 12.1-fold (P = 1.6 × 10−3) increased in stem cells, progenitor cells, immature myeloid cells, and erythroid cells when compared with the apoptotic cell fraction, respectively (Figure 1K). Finally, the fraction of a–caspase-1+ cells was significantly greater than the corresponding a–caspase-3/7+ cell fraction, confirming that caspase-1 activation (pyroptosis) exceeds isolated caspase-3 activation (apoptosis) in MDSs (Figure 1L).
To confirm that NLRP3 inflammasome activation and caspase-1 are essential for hematopoietic cell death in MDSs, short hairpin RNA–directed knockdown of NLRP3, CASP1, and CASP3 was performed by lentivirus transfection in LR-MDS BM-MNCs (NLRP3, n = 4; CASP1 and CASP3, n = 3) (Figure 1M). Protein levels of NLRP3 were reduced ∼54%, whereas expression of CASP1 and CASP3 were reduced 34% and 40%, respectively. Knockdown of NLRP3 and caspase-1 significantly decreased the fraction of pyroptotic cells vs scrambled transfected controls (P = 5.7 × 10−3 and .038, respectively) (Figure 1M). In contrast, knockdown of caspase-3 had no discernible effect (Figure 1M), confirming selective NLRP3 and caspase-1 dependence.
The alarmin S100A9 initiates pyroptosis
As we previously reported,11 BM plasma concentration of S100A9 was significantly higher in LR-MDS patient specimens (n = 33) than in controls (n = 12; P = 1.5 × 10−4) (Figure 2A). Analysis of S100A9 BM plasma concentration by IPSS risk category showed a 2.3- and 2.2-fold increase in low-risk (n = 10, P = 2.3 × 10−5) and intermediate I–risk MDSs (n = 23, P = 1.0 × 10−3) compared with normal controls (n = 12), whereas there were no significant differences among controls and intermediate II–risk (n = 17) or high-risk (n = 10) disease (Figure 2B). Notably, BM plasma S100A9 concentrations were significantly higher in LR-MDS specimens than in HR-MDS specimens (P = .013) (Figure 2A), consistent with the reduced fraction of MDSCs and acquisition of survival signals in HR-MDS that mitigates cell death and DAMP elaboration.4,7
S100A9 initiates pyroptosis in MDS. (A) Enzyme-linked immunosorbent assay (ELISA) assessment of BM plasma concentration of S100A9 in normal donors (n = 12) vs MDS (n = 33 lower risk, n = 27 higher risk). (B) S100A9 BM plasma concentration analyzed according to IPSS risk score. (C) HMGB1 BM plasma concentration assessed by ELISA in normal donors (n = 11) and MDS patients (n = 55). (D) qPCR analysis of S100A9 mRNA levels in normal (n = 2) vs LR-MDS BM-MNCs (n = 8). (E) HMGB1 mRNA levels in normal (n = 6) vs MDS BM-MNCs (n = 10). (F) Representative histograms of intracellular levels of S100A9 by hematopoietic lineage in BM-MNCs isolated from MDS patients (n = 6) and normal donors (n = 5). (G) Mean percentage of S100A9+ cells by hematopoietic lineage. (H) qPCR analysis of untreated normal BM-MNCs (n = 3), normal BM-MNCs treated with 1 µg/mL rhS100A9 for 24 hours (n = 2), and MDS patient specimens (n = 5). (I) Representative micrograph (original magnification ×2520, 7.5 µm scale) depicting inflammasome formation in normal, untreated BM-MNC or normal BM-MNC treated with 5 µg/mL rhS100A9 for 24 hours (DAPI, blue; a–caspase-1, green; and NLRP3, red; merged images show inflammasome formation). (J) Quantitative analysis of confocal images of BM-MNCs from normal donors (n = 6), normal BM-MNCs treated with 5 µg/mL rhS100A9 (n = 2), and MDS patients (n = 10). Error bars represent SE. *P < .05, ** P < .01, and ***P < .001.
S100A9 initiates pyroptosis in MDS. (A) Enzyme-linked immunosorbent assay (ELISA) assessment of BM plasma concentration of S100A9 in normal donors (n = 12) vs MDS (n = 33 lower risk, n = 27 higher risk). (B) S100A9 BM plasma concentration analyzed according to IPSS risk score. (C) HMGB1 BM plasma concentration assessed by ELISA in normal donors (n = 11) and MDS patients (n = 55). (D) qPCR analysis of S100A9 mRNA levels in normal (n = 2) vs LR-MDS BM-MNCs (n = 8). (E) HMGB1 mRNA levels in normal (n = 6) vs MDS BM-MNCs (n = 10). (F) Representative histograms of intracellular levels of S100A9 by hematopoietic lineage in BM-MNCs isolated from MDS patients (n = 6) and normal donors (n = 5). (G) Mean percentage of S100A9+ cells by hematopoietic lineage. (H) qPCR analysis of untreated normal BM-MNCs (n = 3), normal BM-MNCs treated with 1 µg/mL rhS100A9 for 24 hours (n = 2), and MDS patient specimens (n = 5). (I) Representative micrograph (original magnification ×2520, 7.5 µm scale) depicting inflammasome formation in normal, untreated BM-MNC or normal BM-MNC treated with 5 µg/mL rhS100A9 for 24 hours (DAPI, blue; a–caspase-1, green; and NLRP3, red; merged images show inflammasome formation). (J) Quantitative analysis of confocal images of BM-MNCs from normal donors (n = 6), normal BM-MNCs treated with 5 µg/mL rhS100A9 (n = 2), and MDS patients (n = 10). Error bars represent SE. *P < .05, ** P < .01, and ***P < .001.
In addition, the BM plasma concentration of HMGB1, a nuclear DAMP and TLR4 ligand, was significantly increased in MDSs (n = 55) compared with controls (n = 11) (P = 2.6 × 10−3) (Figure 2C), consistent with intracellular DAMP release upon cytolysis.26,27 Moreover, S100A9 and HMGB1 transcripts were upregulated 104.5-fold and 1.5-fold in MDSs, respectively, compared with normal donors (Figure 2D-E). Further, flow cytometry analyses of phenotypically distinct hematopoietic lineages confirmed a corresponding increase in intracellular S100A9 in MDS stem cells and progeny (Figure 2F-G).
As TLRs and NLRs are sensors of DAMP signals, we tested if S100A9 would directly trigger pyroptosis in HSPCs. Normal BM-MNCs were treated with 1 µg/mL rhS100A9, and changes in gene expression were assessed by quantitative polymerase chain reaction. Expression of pyroptosis genes was significantly induced by rhS100A9 and exceeded that found in MDSs (Figure 2H). Accordingly, treatment of normal BM-MNCs with 5 µg/mL rhS100A9 was sufficient to induce a–caspase-1 and NLRP3 (Figure 2I) by 2.5-fold and 47.1-fold (P = .064), respectively, as well as NLRP3 inflammasome assembly (2.9-fold; P = 3.1 × 10−4) (Figure 2J). Although rhS100A9 induced inflammasome assembly and caspase-1 activation in normal BM-MNCs, MDS patient BM-MNCs displayed greater activation of these effectors. Notably, treatment of normal BM-MNCs with MDS-derived BM plasma did not induce pyroptosis, as measured by mean percentage of pyroptotic cells, a–caspase-1+ cells, or a–caspase-1 MFI (data not shown), indicating that MDS HSPCs are selectively primed for pyroptosis.
Inflammasome-activated cation channels increase the size of MDS progenitors
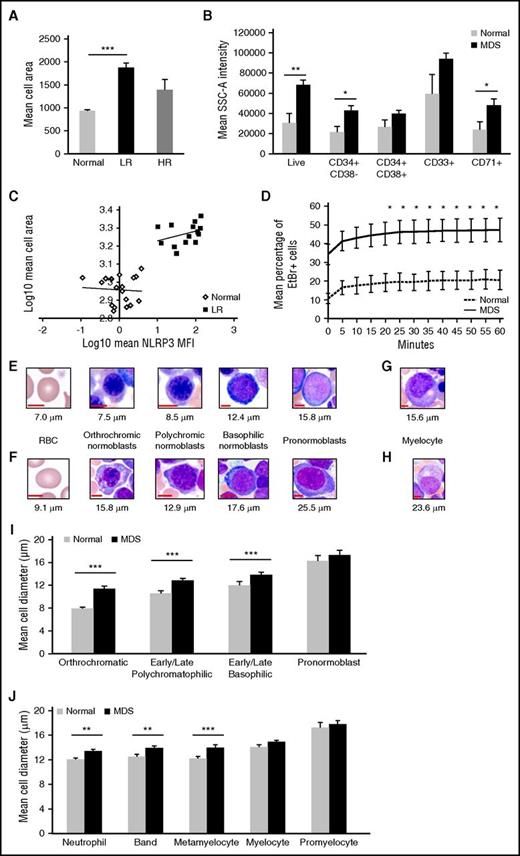
Cell swelling is a hallmark of pyroptosis following activation of plasma membrane cation channels, which compromises membrane integrity and triggers mitochondrial membrane depolarization.28 Confocal image analyses of MDS BM-MNCs revealed a significantly larger mean cell area compared with controls (Figure 3A). Further, this phenotype was accentuated in LR-MDS when compared with normal donors (P = 6.0 × 10−5), whereas there were no significant differences in HR-MDS. These findings were corroborated using side scatter area (SSC-A) flow cytometry measurements, a validated reference for cell size,29 in ungated BM-MNCs from LR-MDS patients (n = 7) and controls (n = 6), as well as in antigenically distinct hematopoietic lineages (Figure 3B).29 Finally, mean NLRP3 MFI correlated with mean cell area in LR-MDS patients, but not HR-MDS patients (r = 0.49) (P = 7.8 × 10−3) (Figure 3C).
MDS precursors evidence cell swelling, a pyroptotic hallmark. (A) Mean cell area was quantified from confocal images of BM-MNCs from normal donors (n = 6) vs MDS patient specimens (n = 7 lower risk, n = 3 higher risk). (B) Flow cytometric analysis of mean SSC-A intensity of BM-MNCs isolated from normal donors (n = 6) or LR-MDS patients (n = 7). MDS BM-MNCs have mean cell area that is 2.0-fold greater than ungated BM-MNCs (P = .017), 2.2-fold greater than stem cells (CD34+CD38−; P = .019), 1.5-fold greater than progenitor cells (CD34+CD38+), 1.6-fold greater than immature myeloid progenitors (CD33+), and 2.0-fold greater than erythroid progenitors (CD71+; P = .038). (C) NLRP3 MFI correlates with BM-MNC area in LR-MDS patients (r = 0.49, n = 7). (D) EtBr dye incorporation in BM-MNCs from normal donors (n = 3) and MDS patients (n = 3) was measured at 5-minute intervals by flow cytometry. (E, left to right) Photomicrograph images from normal donors illustrating normal red blood cell (RBC; 7.0 μm) followed by normal erythroid lineage maturation of nucleated BM precursors with corresponding cell diameter. (F) Corresponding images from MDS BM aspirates, demonstrating an oval macrocyte (RBC, 9.1 μm) followed by dysplastic and megaloblastic erythroid lineage maturation. (G) Normal myelocyte. (H) Enlarged dysplastic myelocyte with mild hypogranulation in MDS. (I-J) Erythroid (I) and myeloid (J) lineage maturation comparison of mean cell diameter in BM of normal donors (n = 4) vs MDS patients (n = 4). Maturation is depicted as most to least mature cell populations from left to right. Error bars represent SE. *P < .05, ** P < .01, and ***P < .001.
MDS precursors evidence cell swelling, a pyroptotic hallmark. (A) Mean cell area was quantified from confocal images of BM-MNCs from normal donors (n = 6) vs MDS patient specimens (n = 7 lower risk, n = 3 higher risk). (B) Flow cytometric analysis of mean SSC-A intensity of BM-MNCs isolated from normal donors (n = 6) or LR-MDS patients (n = 7). MDS BM-MNCs have mean cell area that is 2.0-fold greater than ungated BM-MNCs (P = .017), 2.2-fold greater than stem cells (CD34+CD38−; P = .019), 1.5-fold greater than progenitor cells (CD34+CD38+), 1.6-fold greater than immature myeloid progenitors (CD33+), and 2.0-fold greater than erythroid progenitors (CD71+; P = .038). (C) NLRP3 MFI correlates with BM-MNC area in LR-MDS patients (r = 0.49, n = 7). (D) EtBr dye incorporation in BM-MNCs from normal donors (n = 3) and MDS patients (n = 3) was measured at 5-minute intervals by flow cytometry. (E, left to right) Photomicrograph images from normal donors illustrating normal red blood cell (RBC; 7.0 μm) followed by normal erythroid lineage maturation of nucleated BM precursors with corresponding cell diameter. (F) Corresponding images from MDS BM aspirates, demonstrating an oval macrocyte (RBC, 9.1 μm) followed by dysplastic and megaloblastic erythroid lineage maturation. (G) Normal myelocyte. (H) Enlarged dysplastic myelocyte with mild hypogranulation in MDS. (I-J) Erythroid (I) and myeloid (J) lineage maturation comparison of mean cell diameter in BM of normal donors (n = 4) vs MDS patients (n = 4). Maturation is depicted as most to least mature cell populations from left to right. Error bars represent SE. *P < .05, ** P < .01, and ***P < .001.
To assess pore formation, influx of the membrane-impermeable cationic dye ethidium bromide (EtBr) was assessed by flow cytometry. MDS specimens incubated with autologous BM plasma displayed rapid and sustained elevation of EtBr influx compared with normal BM-MNCs (Figure 3D) that was evident as early as 20 minutes in MDSs (P = .041) and sustained through 1 hour of dye exposure (P = .014). Finally, analysis of normal and MDS BM morphology confirmed the larger cell size in MDSs by maturation stage and lineage (Figure 3E-J.).
Inhibition of pyroptosis promotes effective hematopoiesis in MDSs
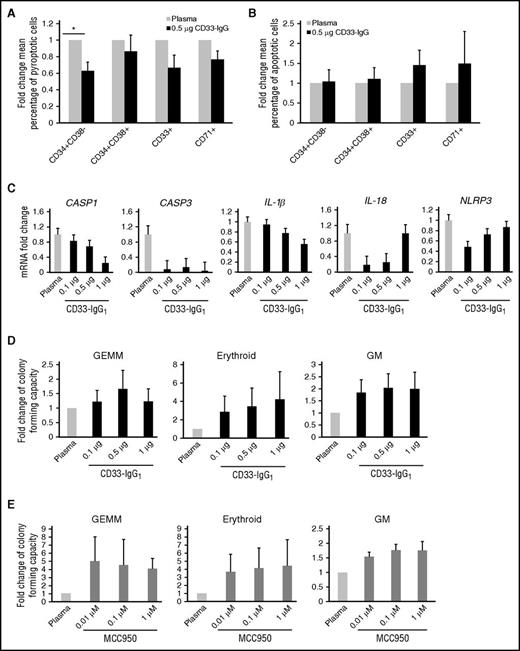
To assess the role of S100A9 in pyroptosis in MDSs, we tested if an S100A9 high-affinity chimeric decoy receptor (CD33-IgG1) could neutralize this alarmin in autologous BM plasma and ameliorate pyroptosis in MDS BM-MNCs. Notably, treatment with the CD33-IgG1 chimera markedly reduced the fraction of pyroptotic cells without altering the fraction of apoptotic cells (Figure 4A-B). Overall, short-term incubation with the chimera was sufficient to reduce the pyroptotic cell fraction across lineages, with an 81% reduction in stem cells, 57% reduction in progenitors, 90% reduction in CD33+, and 42% reduction in CD71+ erythroid progenitors. Short-term CD33-IgG1 incubation also significantly reduced the MDSC fraction, confirming that S100A9 promotes expansion of MDSCs (data not shown). Consistent with this, CD33-IgG1 reduced S100A9-induced transcriptional priming, decreasing levels of CASP1, IL-1β, IL-18, and NLRP3 transcripts in MDS BM-MNCs when compared with treatment with autologous BM plasma alone (n = 5) (Figure 4C). CASP3 expression was also markedly reduced, consistent with late caspase-3 activation after mitochondrial depolarization.30 Note that as a result of the IgG1-Fc conjugation, high concentrations of the chimera led to crosslinking of the Fc domains and aggregation that masked dose-dependent transcriptional effects of S100A9 neutralization.
Inhibition of pyroptosis abrogates MDS HSPC death and augments CFC. (A-B) Fold change in the mean percentage of (A) pyroptotic or (B) apoptotic cells in each respective lineage in LR-MDS BM-MNCs (n = 6) incubated with autologous BM plasma and 0.5 µg CD33-IgG1 chimera for 24 hours. Values are normalized to autologous BM plasma-incubated MDS BM-MNCs. (C) qPCR analysis of BM-MNCs isolated from LR-MDS patients (n = 5) treated for 24 hours with CD33-IgG1. (D-E) CFC was assessed in BM-MNCs from LR-MDS patient specimens (n = 3) treated with increasing concentrations of CD33-IgG1 (E) or the inflammasome inhibitor MCC950 (E). Error bars represent SE.
Inhibition of pyroptosis abrogates MDS HSPC death and augments CFC. (A-B) Fold change in the mean percentage of (A) pyroptotic or (B) apoptotic cells in each respective lineage in LR-MDS BM-MNCs (n = 6) incubated with autologous BM plasma and 0.5 µg CD33-IgG1 chimera for 24 hours. Values are normalized to autologous BM plasma-incubated MDS BM-MNCs. (C) qPCR analysis of BM-MNCs isolated from LR-MDS patients (n = 5) treated for 24 hours with CD33-IgG1. (D-E) CFC was assessed in BM-MNCs from LR-MDS patient specimens (n = 3) treated with increasing concentrations of CD33-IgG1 (E) or the inflammasome inhibitor MCC950 (E). Error bars represent SE.
To test if S100A9 neutralization could improve hematopoiesis in MDSs, colony-forming capacity (CFC) was assessed after plating MDS BM-MNCs in autologous BM plasma and increasing concentrations of CD33-IgG1 (Figure 4D) or of MCC950 (Figure 4E), a small-molecule inhibitor of the NLRP3 inflammasome.31 Notably, neutralization of S100A9 or inhibition of the NLRP3 inflammasome markedly improved the CFC of MDS progenitors (up to 6.6-fold greater than controls). Thus, pyroptotic pathway inhibition abrogates MDS HSPC death and promotes effective hematopoiesis.
S100A9 is sufficient to provoke HSPC pyroptosis in vivo
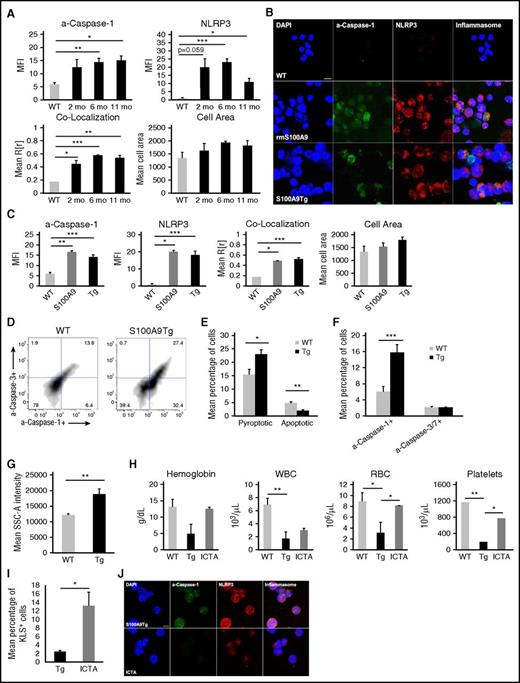
To test if forced expression of S100A9 is sufficient to induce pyroptosis in vivo, we assessed phenotypes manifest in an S100A9-transgenic (S100A9Tg) mouse model that phenocopies human MDSs.11 Confocal fluorescence microscopy analyses of BM cells from the tibia and femurs of S100A9Tg vs wild-type (WT) mice at 2 (n = 4), 6 (n = 4), and 11 (n = 5) months of age showed that caspase-1 activation increased with age in the BM cells of S100A9 transgenics, increasing 2.1-fold at 2 months, 2.4-fold at 6 months (P = 3.3 × 10−3), and 2.5-fold at 11 months (P = .010). Similarly, in S100A9Tg mice, NLRP3 levels were increased 21.1-fold at 2 months (P = .059), 25.6-fold at 6 months (P = 2.2 × 10−4), and 12.1-fold at 11 months (P = .018) (Figure 5A). Accordingly, formation of NLRP3 inflammasome complexes also significantly increased in an age-dependent fashion, with 2.6-fold greater colocalization in the 2-month-old S100A9Tg mice (P = .017), 3.3-fold in 6-month-old mice (P = 1.0 × 10−6), and 3.2-fold in 11-month-old mice (P = 1.2 × 10−3) (Figure 5A).
Pyroptosis is the principal mechanism of HSPC death in S100A9Tg mice. (A) Confocal image analysis of BM cells isolated from WT (n = 2), 2-month-old (n = 4), 6-month-old (n = 5), and 11-month-old (n = 4) S100A9Tg mice. (B) Representative micrograph (original magnification ×2520, 7.5 µm scale) depicting inflammasome formation in BM cells from WT cells, WT cells treated for 24 hours with 5 µg/mL rmS100A9, and BM cells from S100A9Tg mice (DAPI, blue; a–caspase-1, green; and NLRP3, red; merged images show inflammasome formation). (C) Quantitative analysis of confocal images of BM cells isolated from WT mice (n = 2), WT BM cells treated for 24 hours with 5 µg/mL rmS100A9 (n = 2), or BM cells from S100A9Tg mice (n = 13). (D) Representative scatterplots of pyroptotic and apoptotic KLS cells isolated from WT and transgenic mice. (E) Mean percentage of pyroptotic vs apoptotic KLS cells in WT (n = 6) and S100A9Tg mice (n = 6). (F) Mean percentage of total a–caspase-1+ and a–caspase-3/7+ KLS cells isolated from WT (n = 6) and S100A9Tg mice (n = 6). (G) Flow cytometric analysis of mean SSC-A intensity of BM cells isolated from WT (n = 6) and S100A9Tg mice (n = 6) (P = 1.0 × 10−2). (H) At 6 months of age, S100A9Tg mice were treated with 50 mg/kg ICTA. Shown are changes in hemoglobin, white blood cell (WBC), RBC, and platelet counts in WT (n = 4), S100A9Tg (n = 5), and ICTA-treated S100A9Tg mice (n = 5). (I) Mean percentage of KLS+ HSPCs in untreated vs ICTA-treated S100A9Tg mice. (J) Representative micrograph (original magnification ×2520, 7.5 µm scale) depicting inflammasome formation in BM cells harvested from untreated S100A9Tg mice or mice treated with ICTA by oral gavage for a total of 8 weeks (DAPI, blue; a–caspase-1, green; and NLRP3, red; merged images show inflammasome formation). Error bars represent SE. *P < .05, ** P < .01, and ***P < .001. See also supplemental Figure 3.
Pyroptosis is the principal mechanism of HSPC death in S100A9Tg mice. (A) Confocal image analysis of BM cells isolated from WT (n = 2), 2-month-old (n = 4), 6-month-old (n = 5), and 11-month-old (n = 4) S100A9Tg mice. (B) Representative micrograph (original magnification ×2520, 7.5 µm scale) depicting inflammasome formation in BM cells from WT cells, WT cells treated for 24 hours with 5 µg/mL rmS100A9, and BM cells from S100A9Tg mice (DAPI, blue; a–caspase-1, green; and NLRP3, red; merged images show inflammasome formation). (C) Quantitative analysis of confocal images of BM cells isolated from WT mice (n = 2), WT BM cells treated for 24 hours with 5 µg/mL rmS100A9 (n = 2), or BM cells from S100A9Tg mice (n = 13). (D) Representative scatterplots of pyroptotic and apoptotic KLS cells isolated from WT and transgenic mice. (E) Mean percentage of pyroptotic vs apoptotic KLS cells in WT (n = 6) and S100A9Tg mice (n = 6). (F) Mean percentage of total a–caspase-1+ and a–caspase-3/7+ KLS cells isolated from WT (n = 6) and S100A9Tg mice (n = 6). (G) Flow cytometric analysis of mean SSC-A intensity of BM cells isolated from WT (n = 6) and S100A9Tg mice (n = 6) (P = 1.0 × 10−2). (H) At 6 months of age, S100A9Tg mice were treated with 50 mg/kg ICTA. Shown are changes in hemoglobin, white blood cell (WBC), RBC, and platelet counts in WT (n = 4), S100A9Tg (n = 5), and ICTA-treated S100A9Tg mice (n = 5). (I) Mean percentage of KLS+ HSPCs in untreated vs ICTA-treated S100A9Tg mice. (J) Representative micrograph (original magnification ×2520, 7.5 µm scale) depicting inflammasome formation in BM cells harvested from untreated S100A9Tg mice or mice treated with ICTA by oral gavage for a total of 8 weeks (DAPI, blue; a–caspase-1, green; and NLRP3, red; merged images show inflammasome formation). Error bars represent SE. *P < .05, ** P < .01, and ***P < .001. See also supplemental Figure 3.
To determine if S100A9 was sufficient to trigger pyroptosis in mouse HSPCs, BM cells isolated from WT mice were treated with 5 µg/mL rmS100A9, and inflammasome formation was assessed by confocal microscopy (Figure 5B-C). As predicted, MFI of a–caspase-1 and NLRP3 significantly increased after rmS100A9 treatment (n = 2) when compared with controls (n = 2) (P = 7.5 × 10−3 and .017, respectively). Notably, MFI values from rmS100A9-treated cells of WT mice were comparable to those found in the BM cells of S100A9Tg mice (n = 13) (Figure 5C), and rmS100A9 treatment of WT BM cells markedly induced inflammasome complexes (P = .023).
To compare pyroptosis and apoptosis in vivo, BM cells were isolated from 7-month-old WT (n = 6) and 9-month-old S100A9Tg (n = 6) mice, and a–caspase-1 and a–caspases-3/7 were assessed by flow cytometry in the KLS (c-Kit+Lin−Sca-1+) HSPC population. The mean percentage of pyroptotic KLS cells was significantly higher in S100A9Tg mice than in WT mice (P = .052), whereas WT BM cells had a higher apoptotic index (P = 7.1 × 10−3) (Figure 5D-E). Additionally, the percentage of a–caspase-1+ KLS cells was 2.6-fold higher in S100A9Tg mice than in WT mice (P = 4.2 × 10−3), while there were no significant difference in a–caspase-3/7+ between the 2 cohorts (Figure 5F). S100A9Tg BM cells also had a significant increase in mean cell area, as assessed by SSC-A intensity measurements (Figure 5G). Finally, to test if in vivo inflammasome inhibition improves hematopoiesis in S100A9Tg mice, aged mice (n = 5) were treated with ICTA, an icariin derivative that inhibits NLRP3 inflammasome activation, every other day for 8 weeks (supplemental Figure 3). ICTA treatment markedly improved hemoglobin, leukocyte, red blood cell, and platelet counts in transgenic mice (Figure 5H) and was accompanied by a significant increase in the percentage of KLS+ HSPCs (P = .047) (Figure 5I), consistent with restored effective hematopoiesis. Finally, NLRP3 activation was dramatically reduced in BM cells from ICTA-treated S100A9Tg mice (Figure 5J). Thus, pyroptosis is the principal mechanism driving HSPC cell death in MDS and S100A9Tg mice.
S100A9 and MDS SGMs trigger pyroptosis and β-catenin activation via ROS
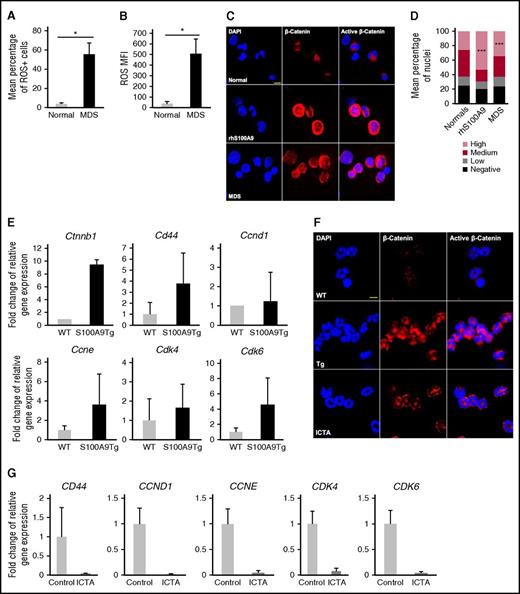
ROS act as DAMP intermediates that activate the Wnt/β-catenin axis, which is activated in MDSs.32-36 Thus, we hypothesized that ROS generated by either S100A9 or somatic gene mutations (SGMs) would direct activation of β-catenin in MDSs. In accordance with this, the mean percentage of ROS positive cells was increased 16.5-fold in MDS BM-MNCs (n = 5) compared with normal donors (n = 2) (P = .011) (Figure 6A), with corresponding significant increases in ROS MFI (P = .028) (Figure 6B). Further, nuclear β-catenin levels were increased in MDS BM-MNCs (n = 6) compared with normal donors (n = 3), as well as in normal BM-MNCs treated with 5 µg/mL rhS100A9 compared with untreated BM-MNCs (P = .043 and P = 6.38 × 10−7, respectively) (Figure 6C-D). Finally, β-catenin mRNA levels were increased 9.5-fold in BM cells of S100A9Tg mice compared with WT BM cells, with corresponding upregulation of Wnt/β-catenin target genes (Figure 6E). As expected, this phenotype was associated with significant increases in nuclear β-catenin in S100A9Tg BM cells compared with WT BM cells, and levels of nuclear β-catenin were reduced following in vivo treatment with ICTA (Figure 6F), which was associated with a reduction in expression of Wnt/β-catenin target genes (Figure 6E). Similarly, treatment of MDS BM-MNCs with ICTA suppressed nuclear β-catenin and Wnt/β-catenin target gene expression (Figure 6G). Thus, the S100A9-to-NLRP3 inflammasome circuit is necessary and sufficient to drive activation of β-catenin in MDS.
S100A9 induces ROS through NADPH oxidase to activate β-catenin. (A-B) The percentage of (A) ROS positive cells and (B) ROS-MFI assessed by flow cytometry in BM-MNCs isolated from MDS patients (n = 5) and normal donors (n = 2). (C) Representative micrograph (original magnification ×2520, 7.5 µm scale) of β-catenin expression in normal BM-MNCs (n = 3), normal BM-MNCs treated with 5 µg/mL rhS100A9 (n = 3), and MDS BM-MNCs (n = 6) (DAPI, blue; and β-catenin, red; merged images show nuclear β-catenin localization). (D) Quantitation and scoring of confocal images based on the presence of no, low, medium, or high nuclear β-catenin. (E) Wnt/β-catenin target gene expression in WT and S100A9Tg BM cells. (F) Representative micrograph (original magnification ×2520, 7.5 μm scale) of β-catenin expression in WT (n = 5), S100A9Tg (n = 5), and S100A9Tg mice that were treated with ICTA (n = 5) by oral gavage for 8 weeks (DAPI, blue; and β-catenin, red; merged images show nuclear β-catenin localization). (G) Wnt/β-catenin target gene expression in MDS BM-MNCs (n = 4) treated for 48 hours with ICTA. Error bars represent SE. *P < .05 and ***P < .001.
S100A9 induces ROS through NADPH oxidase to activate β-catenin. (A-B) The percentage of (A) ROS positive cells and (B) ROS-MFI assessed by flow cytometry in BM-MNCs isolated from MDS patients (n = 5) and normal donors (n = 2). (C) Representative micrograph (original magnification ×2520, 7.5 µm scale) of β-catenin expression in normal BM-MNCs (n = 3), normal BM-MNCs treated with 5 µg/mL rhS100A9 (n = 3), and MDS BM-MNCs (n = 6) (DAPI, blue; and β-catenin, red; merged images show nuclear β-catenin localization). (D) Quantitation and scoring of confocal images based on the presence of no, low, medium, or high nuclear β-catenin. (E) Wnt/β-catenin target gene expression in WT and S100A9Tg BM cells. (F) Representative micrograph (original magnification ×2520, 7.5 μm scale) of β-catenin expression in WT (n = 5), S100A9Tg (n = 5), and S100A9Tg mice that were treated with ICTA (n = 5) by oral gavage for 8 weeks (DAPI, blue; and β-catenin, red; merged images show nuclear β-catenin localization). (G) Wnt/β-catenin target gene expression in MDS BM-MNCs (n = 4) treated for 48 hours with ICTA. Error bars represent SE. *P < .05 and ***P < .001.
To determine if SGMs can trigger NLRP3 inflammasome activation, ROS, pyroptosis, and β-catenin activation, we first investigated these phenotypes in TF-1 myeloid leukemia cells engineered to express GFP-labeled WT or U2AF1 splicing factor gene mutants.5 The percentage of pyroptotic cells was increased 4.6-fold in S34F U2AF1 mutant vs WT U2AF1 cells, which was associated with increased a–caspase-1 (P = .044) and annexin-v (P = .021) (Figure 7A-H). Further, there was increased ASC oligomerization and NLRP3 inflammasome activation in S34F cells, as evidenced by increased binding of NLRP3 and ASC (Figure 7I), maturation of caspase-1 and IL-1β (Figure 7J), and the generation of ASC monomers and higher-order ASC complexes (Figure 7K).
U2AF1 mutations manifest in MDS provoke pyroptosis and induce NOX-dependent activation of β-catenin. (A) Representative density plot of inflammasome formation based on ASC oligomerization. (B) Quantitation of ASC in WT, S34F, and S34F cells treated with DPI for 24 hours. (C) Representative scatter plots of pyroptotic cells by flow cytometry. (D) Mean percentage of pyroptotic cells in mutant and WT cells. (E-H) Mean percentage of total (E) a–caspase-1+ and (F) annexin V+ cells, as well as the MFI of (G) a–caspase-1 and (H) annexin V assessed by flow cytometry. (I) Binding of ASC to NLRP3 (IP of NLRP3, IB of NLRP3 and ASC). (J) Western blot of cleaved caspase-1 and IL-1β maturation. (K) Immunoblot of ASC monomers and higher-order ASC complexes following chemical crosslinking of cell lysates. (L) Mean cell area quantitated from confocal images. (M) Incorporation of EtBr measured by flow cytometry at 5-minute intervals. (N-O) Mean percentage of (N) ROS positive cells and (O) ROS MFI assessed by flow cytometry. (P) Representative micrograph (original magnification ×1890, 10 µm scale) of β-catenin expression in U2AF1 WT, S34F-expressing, or S34F-expressing cells treated with NAC or DPI for 24 hours prior to staining (DAPI, blue; and β-catenin, red; merged images show nuclear β-catenin localization). (Q) Quantitation and scoring of confocal images based on the presence of no, low, medium, or high nuclear β-catenin. (R) Representative density plot of inflammasome formation based on ASC oligomerization in S34F cells treated with 10 µM ICTA. (S) CFC assessed in WT, S34F, and S34F cells treated with increasing concentrations of ICTA (0.01-10 µM). The mean number of colonies is representative of 4 replicates per condition. Error bars represent SE. *P < .05, ** P < .01, and ***P < .001. Data are representative of 3 independent experiments. See also supplemental Figures 4-7.
U2AF1 mutations manifest in MDS provoke pyroptosis and induce NOX-dependent activation of β-catenin. (A) Representative density plot of inflammasome formation based on ASC oligomerization. (B) Quantitation of ASC in WT, S34F, and S34F cells treated with DPI for 24 hours. (C) Representative scatter plots of pyroptotic cells by flow cytometry. (D) Mean percentage of pyroptotic cells in mutant and WT cells. (E-H) Mean percentage of total (E) a–caspase-1+ and (F) annexin V+ cells, as well as the MFI of (G) a–caspase-1 and (H) annexin V assessed by flow cytometry. (I) Binding of ASC to NLRP3 (IP of NLRP3, IB of NLRP3 and ASC). (J) Western blot of cleaved caspase-1 and IL-1β maturation. (K) Immunoblot of ASC monomers and higher-order ASC complexes following chemical crosslinking of cell lysates. (L) Mean cell area quantitated from confocal images. (M) Incorporation of EtBr measured by flow cytometry at 5-minute intervals. (N-O) Mean percentage of (N) ROS positive cells and (O) ROS MFI assessed by flow cytometry. (P) Representative micrograph (original magnification ×1890, 10 µm scale) of β-catenin expression in U2AF1 WT, S34F-expressing, or S34F-expressing cells treated with NAC or DPI for 24 hours prior to staining (DAPI, blue; and β-catenin, red; merged images show nuclear β-catenin localization). (Q) Quantitation and scoring of confocal images based on the presence of no, low, medium, or high nuclear β-catenin. (R) Representative density plot of inflammasome formation based on ASC oligomerization in S34F cells treated with 10 µM ICTA. (S) CFC assessed in WT, S34F, and S34F cells treated with increasing concentrations of ICTA (0.01-10 µM). The mean number of colonies is representative of 4 replicates per condition. Error bars represent SE. *P < .05, ** P < .01, and ***P < .001. Data are representative of 3 independent experiments. See also supplemental Figures 4-7.
ROS generation by NADPH oxidase-1 (NOX1) has been linked to Wnt/β-catenin activation.32,33 Treatment of S34F-expressing cells with the NOX1/4-specific inhibitor GKT137831 or with the pan-NOX inhibitor DPI profoundly reduced ASC oligomerization and thereby NLRP3 inflammasome assembly; thus, NLRP3 activation in response to SGMs is NOX1/4 dependent (Figure 7B). Moreover, elevated levels of ROS in U2AF1-S34F cells were abrogated by GKT137831 or DPI treatment (Figure 7N-O). Treatment with GKT137831 or DPI had no effect on U2AF1-WT cells (supplemental Figure 4). U2AF1-S34F cells also displayed significantly increased mean cell area (P = .035), EtBr influx (Figure 7L-M), mean percentage ROS+ cells (P = 1.5 × 10−3), and ROS MFI (P = .032) (Figure 7N-O), accompanied by nuclear localization of β-catenin (Figure 7P-Q). Notably, treatment of U2AF1-S34F mutant cells with the antioxidant N-acetylcysteine (NAC) or the NOX inhibitor DPI effectively reduced β-catenin activation in U2AF1-S34F cells (P = 3.8 × 10−3 and P = 2.5 × 10−6, respectively) (Figure 7P-Q); thus, β-catenin activation is initiated by NOX-derived ROS generation. Finally, treatment of U2AF1-S34F mutant cells with ICTA suppressed inflammasome activation, as evidenced by a reduction in ASC polymerization, and restored CFC to that of WT cells (Figure 7R and 7S). Thus, reduced survival of cells harboring this MDS splicing mutation is driven by NLRP3 inflammasome-directed pyroptosis, while β-catenin activation may support propagation of the clone.
This NLRP3 inflammasome circuit was also evaluated in BM cells from the conditional knockin Sf3b1-K700E mouse, which expresses an RNA splicing subunit gene mutation found in MDSs and displays an MDS phenotype.37 Sf3b1-K700E BM cells (n = 6) displayed similar increases in the percentage of pyroptotic and apoptotic cells, with a significant increase in total a–caspase-1+ cells (P = .014) compared with WT BM (n = 6) (supplemental Figure 5A-B). Further, a–caspase-1 and a–caspase-3/7 MFIs were both significantly increased in Sf3b1-K700E mutant BM cells (P = .030 and P = 6.92 × 10−3, respectively) (supplemental Figure 5C), and this was accompanied by increased inflammasome assembly (supplemental Figure 5D). Accordingly, NLRP3 protein expression was increased 1.9-fold in Sf3b1-K700E cells (P = .063), which was accompanied by NLRP3 inflammasome assembly (supplemental Figure 5E).
Inflammasome activation in Sf3b1-K700E mutant BM cells was confirmed by marked ASC polymerization compared with WT BM (P = 8.4 × 10−3). Further, this was dependent upon NOX-generated ROS, as (1) ASC oligomerization was significantly reduced in Sf3b1-K700E mutant-expressing BM cells following treatment with NAC (P = 2.68 × 10−3) or DPI (supplemental Figure 5F-G) and (2) the mean percentage of ROS+ cells and ROS MFI were markedly increased in Sf3b1-K700E mutant BM cells, which was extinguished by treatment with NAC or DPI (supplemental Figure 5H-I). Finally, nuclear localization of β-catenin was also significantly increased in Sf3b1-K700E mutant BM compared with WT BM (P = .04), which was also reduced by treatment with NAC (P = 2.0 × 10−3) or DPI (P = 1.8 × 10−2) (supplemental Figure 5J-K).
Notably, pharmacologic inhibition of the NLRP3 inflammasome in Sf3b1-K700E BM cells restored CFC, illustrating the importance of inflammasome activation in the attrition of mutant cells (supplemental Figure 5L). Similar findings were also manifest in mutant vs WT SRSF2-expressing HEK293T cells (supplemental Figure 6), as well as in BM-MNCs obtained from MDS murine models driven by epigenetic regulatory gene mutations (ASXL1 and TET2)38,39 (supplemental Figure 7). To confirm that somatic gene mutations prime HSPCs for pyroptosis in MDSs, we performed comparative analyses of published gene expression profiles from human and murine SRSF2 (GSE65349) and U2AF1 mutants (GSE30195 and GSE66793) vs WT, TET2 knockout (GSE27816), and primary MDS (GSE19429) vs normal HSPCs and found uniform upregulation of pyroptosis effectors, consistent with transcriptional priming.39-43 Importantly, in MDS BM specimens, BM plasma concentration of S100A9 positively correlated with NLRP3 MFI, percentage and MFI of plasma ASC specks, and the presence of SGMs and variant allele frequency (supplemental Figure 8). Furthermore, both the percentage and MFI of plasma ASC specks were significantly increased in MDS patients harboring SGMs (supplemental Figure 8D-E). Finally, percentage of pyroptotic erythroid precursors significantly increased in parallel with splicing gene mutation variant allele frequency and the number of SGMs. Thus, MDS SGMs prime cells to undergo pyroptosis, which promotes self-renewal and contributes to an inflammatory microenvironment that is driven by NOX-derived ROS.
Discussion
Heretofore, ineffective hematopoiesis in MDSs has been attributed to high fractions of proliferating BM progenitors undergoing apoptosis within an unexplained inflammatory microenvironment.2,6 Nearly 2 decades ago, it was reported that MDS HSPCs generate IL-1β in short-term cultures, which directly correlated with the extent of cell death as measured by DNA fragmentation.10 We present evidence that these and other biological features of MDSs are explained by activation of the NLRP3 pattern recognition receptor by S100A9 and ROS DAMP intermediates that induce inflammasome assembly, β-catenin nuclear translocation and pyroptotic cell death. Notably, pyroptosis-associated gene transcripts and inflammasome assembly are profoundly upregulated in MDSs independent of genotype. Pyroptotic, but not apoptotic, cells are markedly increased in MDS stem cells, progenitors, and erythroid precursors. Accordingly, knockdown of NLRP3 and caspase-1, but not caspase-3, significantly reduced the pyroptotic fraction in MDS BM-MNCs. Moreover, MDS HSPCs are selectively primed to undergo pyroptosis, due in part to upregulation of pattern recognition receptors directing pyroptosis, the expansion of MDSCs,11 and transcriptional priming of inflammasome components. Importantly, neutralization of S100A9 in MDS BM plasma or pharmacologic inhibition of inflammasome assembly suppressed pyroptosis and restored effective hematopoiesis in vitro and in the S100A9Tg mouse model of MDSs. Thus, pyroptosis, a caspase-1–dependent inflammatory cell death, impairs HSPC survival in MDSs.
S100A8/S100A9 heterodimers activate both NF-κB and NLRP3 inflammasome assembly via a NOX/ROS–dependent mechanism.23,44-46 Intracellularly, S100A8/9 heterodimers serve as a scaffold for the membrane assembly and activation of the NOX complex,47,48 which generates ROS via transfer of electrons across membranes to generate superoxide.49 As also shown here, NOX regulates both priming and activation of NLRP3 inflammasomes, including the activation of caspase-1 and IL-1β secretion.44 Moreover, transcription and nuclear localization of β-catenin are redox and NOX1 dependent.50,51 Although MDSCs are a key paracrine source of S100A9 in the MDS BM microenvironment,11 we show here that MDS HSPCs also express high intracellular S100A9 across lineages, suggesting that inflammasome activation may be sustained by intracrine DAMP stimulation and, upon cytolysis, reinforce paracrine TLR4 activation and BM MDSC expansion. Indeed, Schneider et al recently reported that Rps14 haplodeficiency induces S100A8/9 expression to direct TLR4-dependent cell-intrinsic death of polychromatic erythroblasts.52 Comparative transcriptome analysis also shows that S100A8/9 is highly upregulated in Srsf2 P95H mutant as well as Ezh2-deleted mouse models.53,54 Additionally, our findings that catalytically active pyroptotic ASC specks are released from the cytosol into the extracellular space suggests that specks may further reinforce bystander inflammation in the microenvironment in a non–cell-autonomous fashion.25,55 Importantly, NOX1/4 inhibition suppressed activation of the inflammasome and β-catenin both in MDS patient–derived BM-MNCs and in cells harboring varied classes and types of MDS founder gene mutations. Thus, S100A9 induces NOX1/4 to drive ROS-dependent inflammasome assembly, pyroptosis, and β-catenin activation, explaining the proliferation and inflammatory cell death manifest in MDSs.
Another hallmark of MDSs is the larger cell size of BM precursors and macrocytosis. A characteristic feature of NLRP3 inflammasome activation is cell swelling due to the activation of cation channels.28 We show that activation of pattern recognition receptors triggers expansion in size and volume of MDS progenitors via influx of cations by membrane channels that are activated by NOX-derived ROS.56-59 Our findings show that MDS BM-MNCs display increased influx of the membrane-impermeable cationic dye EtBr, confirming pore formation. Additionally, flow cytometry assessment of BM cell size according to lineage and stage of maturation confirmed significantly larger BM precursors in MDS patients than in normal controls that increased directly with NLRP3 mean fluorescence intensity. Thus, S100A9-mediated NOX activation and inflammasome-initiated pyroptosis explain the characteristic larger cell size, proliferation, and inflammatory cell death manifest in MDSs.
Somatic gene mutations in MDSs trigger Rac1/NOX–dependent ROS generation,60,61 which we show activates both inflammasome assembly and Wnt/β-catenin signaling, a pathway known to promote leukemia stem cell self-renewal. NOX-derived ROS activates β-catenin by oxidation and dissociation of nucleoredoxin (NRX) from disheveled (Dvl), which in turn inactivates the β-catenin destruction complex.62 We show that ROS and nuclear β-catenin are profoundly increased in MDS HSPCs and that S100A9 treatment of normal BM-MNCs is sufficient to trigger NOX/ROS–dependent nuclear translocation of β-catenin and the activation of Wnt/β-catenin target genes. Similarly, BM-MNCs from S100A9Tg mice and cells expressing varied RNA splicing gene (U2AF1, SF3B1, and SRSF2) and epigenetic regulatory gene (ASXL1 and TET2) mutations found in MDSs similarly undergo pyroptosis, pore formation, and cell volume expansion and express high levels of nuclear β-catenin and Wnt/β-catenin target genes that are suppressed by inhibition of the NLRP3 inflammasome or NOX1/4. Finally, the proportion of pyroptotic erythroid progenitors in primary MDSs increased with somatic gene mutation allele burden and mutation complexity. Thus, both S100A9-induced NOX activation and MDS gene mutations initiate pyroptosis through superoxide generation to drive β-catenin activation and enable a proliferative advantage to the MDS clone.
In conclusion, despite genetic heterogeneity, inflammasome activation underlies the biological phenotype in LR-MDS, whereby DAMP signals and MDS gene mutations license a common redox-sensitive inflammasome platform to drive pyroptotic death, elaborate inflammatory cytokines, activate cation influx, and support propagation of the MDS clone through β-catenin activation (supplemental Figure 9). These findings provide a common platform that accounts for the biological features of MDS and suggest that strategies targeting S100A9 neutralization or inhibition of pyroptosis signaling offer therapeutic promise in LR-MDS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank to Seishi Ogawa and Masashi Sanda of Kyoto University (Kyoto, Japan) for kindly providing U2AF1 WT and U2AF1-S34F-expressing cell lines. They also thank Benjamin Ebert for providing BM cells from Sf3b1 K700E conditional knockin mice and corresponding WT littermates and Omar Abdel-Wahab for providing BM cells from Asxl1 knockout, Tet2 knockout, DKO, and corresponding control BM.
This work was supported by National Cancer Institute, National Institutes of Health T32 training grant 5T32 CA115308-08 (A.A.B.) and National Institutes of Health K01 career award CA187020 (E.A.E.). This work was supported in part by the Edward P. Evans Foundation, The Taub Foundation Grants Program for S, the Flow Cytometry and Microscopy Core Facilities at the H. Lee Moffitt Cancer Center and Research Institute (a National Cancer Institute–designated Comprehensive Cancer Center [P30-CA076292]), the Foundation Nuovo Soldati, the Phillippe Foundation, and “Les amis de la faculte de medecine de Nice.”
Authorship
Contribution: A.A.B. designed and performed research, collected, interpreted, and analyzed data, and wrote the manuscript; K.L.M., E.A.E., X.C., J.J., L.Z., Q.Z., B.A.I., and T.C. performed research and collected data; D.A.S., E.P., R.K., L.S., J.L.C., S.W., and A.F.L. analyzed and interpreted data; A.A.B.R. and M.A.C. synthesized MCC950 and performed formulation and ADME/pharmacokinetic work; R.C.C. and L.A.O. performed MCC950 cell-based screening. A.F.L. designed research and analyzed and interpreted data; and A.F.L. and J.L.C. authored revisions to the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alan F. List, 12902 Magnolia Dr, SRB-CEO, Tampa, FL 33612; e-mail: alan.list@moffitt.org.

![Figure 1. Fulminant pyroptosis is manifest in HSPCs and progeny in MDSs. (A) Quantitative polymerase chain reaction (qPCR) analyses of expression of pyroptosis-associated genes in BM-MNCs isolated from MDS patient specimens (n = 10 total, n = 5 LR-MDS, and n = 5 HR-MDS) compared with normal BM-MNCs (n = 5). (B) Representative confocal fluorescence micrograph (original magnification ×2520, 7.5 µm scale) of a–caspase-1 and NLRP3 expression in MDS vs normal BM-MNCs (DAPI, blue; a–caspase-1, green; and NLRP3, red; merged images show inflammasome formation). (C) Quantitative analysis of confocal images of BM-MNCs isolated from MDS patients (n = 7 LR-MDS, n = 3 HR-MDS) and normal donors (n = 6). (D) Binding of ASC to NLRP3 in LR-MDS BM-MNCs compared with normal donors (immunoprecipitation [IP], NLRP3; immunoblot [IB], NLRP3, ASC). Data are representative of 3 independent experiments. (E) Immunoblot following chemical crosslinking of BM-MNC lysates derived from normal donors (n = 3) and LR-MDS patients (n = 3). (F) Quantitation of inflammasome activation based on ASC oligomerization in BM-MNCs from LR-MDS (n = 5) vs normal BM-MNCs (n = 3). (G) Mean percentage of ASC specks and speck MFI in the BM plasma of LR-MDS specimens (n = 6) compared with normal BM plasma (n = 3). (H) The mean percentage of pyroptotic cells by hematopoietic lineage in LR-MDS (n = 8) vs normal donors (n = 8). (I-J) Mean percentage of (I) total a–caspase-1+ and (J) a–caspase-3/7+ cells assessed by hematopoietic lineage in LR-MDS (n = 8) and normal donors (n = 5). (K) Comparison of the mean percentage of pyroptotic vs apoptotic cells by hematopoietic lineage in LR-MDS specimens (n = 8). (L) Comparison of the mean percentage of a–caspase-1+ vs a–caspase-3/7+ cells in the same LR-MDS patients (n = 8). (M) Mean percentage of pyroptotic cells following knockdown of NLRP3, CASP1, and CASP3 by short hairpin RNA–directed silencing of LR-MDS BM-MNCs (n = 4 NLRP3, n = 3 CASP1 and CASP3). Error bars represent standard error (SE). *P < .05, ** P < .01, and ***P < .001. See also supplemental Figures 1 and 2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/128/25/10.1182_blood-2016-07-730556/4/m_blood730556f1.jpeg?Expires=1768439406&Signature=LxjWPn5r2Z0Js8qjlcrKB2ex7Sy4LUeAbhqJpvlRzpcQuKrVoJyJZgXt0xWVONkZGrTDhd~rAASySYJEjhqIcSd3SBk-bSxHKOpBIV0X~JP9Nrc~BFKL1VvTr4PhalWOEJoQZgyrWrFoXPLDiYX5HP14ICsUo1sZrrIbZHA9r13AGAJLkm2VTklUByuEN8AmYgHuVvMCrWe4bKtdNS-4y0pJZwWhJfwGVLfnybFf25vyXfc5kITCbS7wi098ExLyEvRD9HmjOfGVw2tZIl2op3EWgFAo6RGfnxL5hD1AluHIVc1-JyJDdVWcyMIE396pEui9XcD5PWhPD~idFbE9KQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)