Key Points
HIV-negative UCD and iMCD are heterogeneous at the clinical, immunophenotypic, and pathologic levels.
Complete surgical resection is the primary option of treatment of UCD, while siltuximab is more effective for iMCD than rituximab.
Abstract
Castleman disease (CD) comprises 3 poorly understood lymphoproliferative variants sharing several common histopathological features. Unicentric CD (UCD) is localized to a single region of lymph nodes. Multicentric CD (MCD) manifests with systemic inflammatory symptoms and organ dysfunction due to cytokine dysregulation and involves multiple lymph node regions. Human herpesvirus 8 (HHV-8) causes MCD (HHV-8–associated MCD) in immunocompromised individuals, such as HIV-infected patients. However, >50% of MCD cases are HIV and HHV-8 negative (defined as idiopathic [iMCD]). The clinical and biological behavior of CD remains poorly elucidated. Here, we analyzed the clinicopathologic features of 74 patients (43 with UCD and 31 with iMCD) and therapeutic response of 96 patients (43 with UCD and 53 with iMCD) with HIV-/HHV-8–negative CD compared with 51 HIV-/HHV-8–positive patients. Systemic inflammatory symptoms and elevated inflammatory factors were more common in iMCD patients than UCD patients. Abnormal bone marrow features were more frequent in iMCD (77.0%) than UCD (45%); the most frequent was plasmacytosis, which was seen in 3% to 30.4% of marrow cells. In the lymph nodes, higher numbers of CD3+ lymphocytes (median, 58.88 ± 20.57) and lower frequency of CD19+/CD5+ (median, 5.88 ± 6.52) were observed in iMCD patients compared with UCD patients (median CD3+ cells, 43.19 ± 17.37; median CD19+/CD5+ cells, 17.37 ± 15.80). Complete surgical resection is a better option for patients with UCD. Siltuximab had a greater proportion of complete responses and longer progression-free survival (PFS) for iMCD than rituximab. Centricity, histopathological type, and anemia significantly impacted PFS. This study reveals that CD represents a heterogeneous group of diseases with differential immunophenotypic profiling and treatment response.
Introduction
Castleman disease (CD) represents a group of 3 poorly understood lymphoproliferative disorders that share common histopathological lymph node features but have heterogeneous clinical features, outcomes, and treatment regimens.1 Unicentric CD (UCD) typically involves a slow-growing lymph node at a single anatomical site, which is rarely life-threatening. The cause of UCD is unknown. Multicentric CD (MCD) involves multiple regions of enlarged lymph nodes, systemic inflammatory symptoms, and organ dysfunction due to the dysregulation of cytokines, often including interleukin-6 (IL-6). Human herpes virus-8 (HHV-8) is strongly associated with MCD (HHV-8–associated MCD) and drives cytokine dysregulation in individuals, the vast majority of whom are HIV positive or otherwise immunocompromised.1,2 Additionally, one-third to one-half of MCD cases occur in individuals who are HIV negative and HHV-8 negative; the cause is unknown or “idiopathic” (iMCD).3 Using an insurance claims database, ∼6,500 to 7,700 new cases of CD, including 1,650 cases of MCD, are diagnosed every year in the United States.4
Histopathologically, cases of CD are classified as hyaline vascular (HV) and plasma cell (PC) variants; the PC variant may have HV features.5 In the HV variant, the nodal architecture is altered by increased lymphoid follicles with atrophic or regressed germinal centers, hyalinized vessels, and hypervascularity in the interfollicular space. The PC variant is characterized by hyperplastic germinal centers with sheet-like PCs in the interfollicular space.
The clinical manifestations of CD are heterogeneous. UCD symptoms are often mild and may be related to the enlarged lymph node’s compression of adjacent structures.5 Occasionally, UCD may cause paraneoplastic pemphigus, which is life-threatening.6 HHV-8–associated MCD and iMCD can both present with recurrent episodes of diffuse lymphadenopathy with systemic inflammatory symptoms (fever, weight loss, and/or fatigue), edema, anemia, hypoalbuminemia, and/or multiple organ system dysfunctions, which can be fatal if improperly treated.7,8 Larger cohort studies have described clinical and laboratory presentations associated with HIV-positive and HHV-8–associated MCD,9,10 whereas only small case reports and 1 randomized controlled trial have analyzed the clinical and histopathologic features of CD in HIV-negative and HHV-8–negative individuals.3,6,8
The optimal treatment of CD varies widely across the 3 subtypes, and standard-of-care protocol is lacking in the field. Complete surgical resection is the primary treatment modality for UCD,11,12 but unresectable UCD cases are generally treated like iMCD cases. HHV-8–associated MCD is often well controlled with CD20+-depletion therapy using rituximab9,10 ; antivirals and cytotoxic chemotherapy drugs may be added to the treatment regimen for refractory patients. Tocilizumab, which targets the IL-6 receptor, was approved for iMCD in Japan in 2005, and since then, it has been used as an off-label regimen around the world. Siltuximab, which also targets IL-6, was recently approved for iMCD in countries throughout North America, South America, Europe, and Asia based on the results of a randomized controlled trial where 34% of patients experienced a response to therapy compared with 0% on placebo.7,13,14 Treatment options for iMCD patients who fail anti–IL-6 therapy are more limited and based on experience from small case series. Additional treatment options include radical lymph node resection, glucocorticoids, cytotoxic chemotherapy, immunomodulators, rituximab,15 and anti–IL-1 therapy.16
The lack of longitudinal clinical and immunophenotypic data for CD has made the diagnosis, treatment, and management of the disease challenging. A deeper understanding of the clinical and immunophenotypic features and response to therapy should lead to more accurate diagnoses and more successful treatments. Thus, we performed this study to characterize the diagnostic features, treatments, and prognoses for UCD and iMCD.
Materials and methods
After obtaining approval from The University of Texas MD Anderson Cancer Center’s (MDACC) internal review board, we identified 228 patients with CD who had been diagnosed and treated at the institution between 1 January 1994 and 31 December 2014. Of those, 74 patients had detailed clinical data for analysis. The diagnosis of CD was based on clinical, laboratory, and pathological findings. We excluded patients with concomitant malignancies, HIV infection, and POEMS (polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin pigmentation) syndrome as well as patients without sufficient clinical data. Our study was conducted in accordance with the Declaration of Helsinki. An additional 22 HIV-/HHV-8–negative and 51 HIV-/HHV-8–positive CD patients with treatment data were provided by the Castleman Disease Collaborative Network (CDCN) Research Database and the National Institutes of Health.
Seventy-four patients with clinical and laboratory data were available at diagnosis and throughout treatment. Medical records and diagnostic materials from involved lymph nodes, tissues, or organs, were evaluated in accordance with generally accepted guidelines to confirm the diagnosis of CD.1,17 Twenty-two patients had treatment response and survival data but lacked detailed clinical parameters. MCD was defined by the involvement of ≥2 lymph nodes in at least 2 separate regions, whereas cases with a single focus of disease were classified as UCD. Patient data, including demographics, associated autoimmune disorder, clinical manifestations, laboratory tests, radiological images, bone marrow manifestations, immunophenotypic features of lymph nodes assessed by flow cytometry, treatment, and clinical follow-up, were organized and analyzed. B-symptoms were defined as fevers, night sweats, or weight loss of ≥10% in the previous 6 months. HHV-8 status was determined based on the results of latency-associated nuclear antigen immunocytochemistry (27 cases) and polymerase chain reaction for HHV-8 of peripheral blood (4 cases) in iMCD during active disease; 18 patients with HIV-/HHV-8–positive MCD were previously studied by the National Cancer Institute as described elsewhere (protocol #NCT00099073).9
A dose of 11 mg/kg siltuximab was administered intravenously every 3 weeks per protocol or every 6 weeks at the investigator’s discretion. Patients received 375 mg/m2 rituximab IV weekly for 4 weeks. Chemotherapy included cyclophosphamide, hydroxyldoxorubicin, hydrochloride, vincristine, and prednisone, and the dose, order, and regimen of drugs given were not purely uniform across patients. Follow-up information was generated from a review of each visit record until the time of last follow-up or death. Because no criteria exist to evaluate CD, Cheson criteria18 was used to assess treatment response by us and by the independent radiologists who reviewed our results. We evaluated progression-free survival (PFS) from the date of diagnosis to the date of first progression or recurrence. Follow-up was assessed until 1 April 2016.
Statistical analysis
Patient characteristics and treatment outcomes were summarized using descriptive statistics. The UCD and iMCD groups were compared using χ2, Fisher exact, and the Mann-Whitney tests. We used the Kaplan-Meier method to perform univariate analyses of possible prognostic factors with PFS, and survival curve differences were compared using the log-rank test. A multivariate Cox proportional hazards model was used to identify independent prognostic factors for PFS. P values < .05 were considered statistically significant. All statistical analyses were performed by SPSS software (version 21.0; IBM, Armonk, NY).
Results
Demographic characteristics
A total of 74 patients with CD who were HIV negative were identified from multicenter collaboration. Histopathological and radiological findings were used to classify the cases as UCD (n = 43) and iMCD (n = 31) (Table 1). Of the 31 iMCD cases, results of latency-associated nuclear antigen staining and polymerase chain reaction of peripheral blood for HHV-8 were negative.
The group consisted of 49 white, 12 Hispanic, 8 black, and 3 Asian patients and 2 patients whose race was unknown. There were 30 men and 44 women with a mean age of 46 years (range, 18-78 years) at the time of diagnosis. There were no significant differences in age (>40 years vs ≤40 years) and gender distribution between UCD and iMCD patients.
Clinical manifestations
Patients with iMCD presented with symptomatic complaints more frequently than patients with UCD (80.65% vs 41.86%, P = .007). Patients with iMCD were more likely to have higher rates of B-symptoms (41.93%), history of autoimmune disease (38.71%), hepatomegaly and/or splenomegaly (19.35%), and pleural effusion and/or ascites (12.90%) than those with UCD (P < .05) (Table 1). Nine patients met Iwaki19 criteria for thrombocytopenia, anasarca, fever, reticular fibrosis of bone marrow, and organomegaly (TAFRO) syndrome (1 from MDACC and 8 from the CDCN Research Database), which is a unique subtype of iMCD.
Laboratory and radiological findings
At presentation, patients with iMCD commonly had symptoms of systemic inflammation (Table 2). Of the 62 patients whose platelet counts were measured, a platelet count <150 × 109/L occurred in 5 (16.67%) of the iMCD cases and in no UCD cases (P = .052). Elevation of β2-microglobulin, alkaline phosphatase, and erythrocyte sedimentation rate were significantly more common in iMCD than in UCD (P < .05). Among patients with iMCD, 31.58% and 35.0% had increased immunoglobulin A (IgA) and IgG, respectively, whereas in patients with UCD, these percentages were 0% and 5.88% (P < .05). Patients with iMCD were much more likely to experience anemia than were patients with UCD (P = .002).
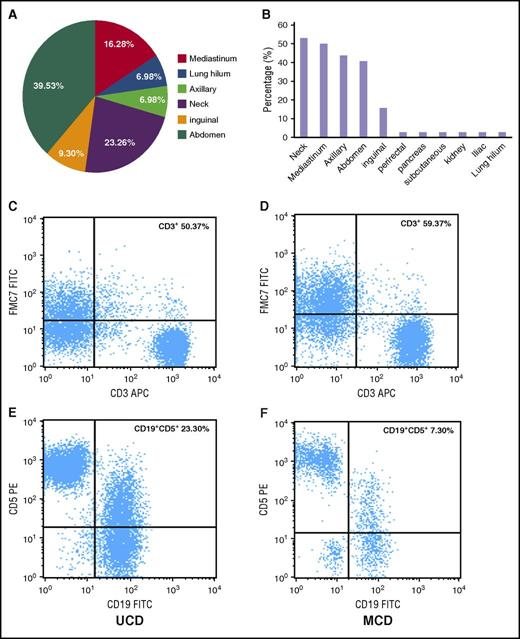
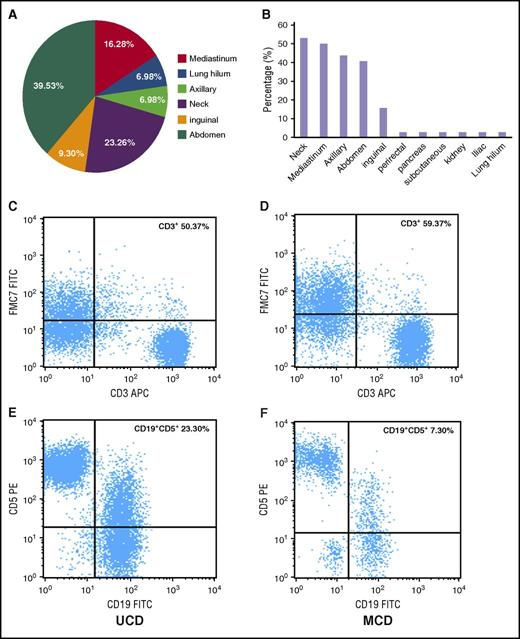
Of the 64 patients who had the record data of computed tomography (CT) or the 23 patients who had positron emission tomography (PET) CT data, all had abnormalities visible on imaging. In patients with UCD, abnormalities were most commonly seen in the abdomen (39.53%), neck (23.26%), and mediastinum (16.28%) (Figure 1A). In patients with iMCD, abnormalities occurred in multiple regions; ∼40% to 55% of patients had abnormalities in the neck, mediastinum, axilla, and/or abdominal regions (Figure 1B). However, there was no significant difference between the 2 groups in terms of the probability of having a large mass (>5 cm) or of having a maximum standardized uptake value in the range of 0.9 to 5.8 during PET-CT (P > .05).
Lymph node involvement by location and immunophenotypic expression (CD3+ and CD5+/CD19+) in patients with UCD and iMCD subtypes of CD. (A) The distribution of lymphadenopathy among patients with HIV-negative UCD. (B) The locations of coexistent lymphadenopathies among patients with iMCD; (C,E) Flow cytometry images of CD3+ and CD5+/CD19+ in UCD; (D,F) flow cytometry images of CD3+ and CD5+/CD19+ in iMCD. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Lymph node involvement by location and immunophenotypic expression (CD3+ and CD5+/CD19+) in patients with UCD and iMCD subtypes of CD. (A) The distribution of lymphadenopathy among patients with HIV-negative UCD. (B) The locations of coexistent lymphadenopathies among patients with iMCD; (C,E) Flow cytometry images of CD3+ and CD5+/CD19+ in UCD; (D,F) flow cytometry images of CD3+ and CD5+/CD19+ in iMCD. FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Bone marrow manifestations
Abnormal bone marrow changes occurred in a significantly higher proportion of patients with iMCD (17 patients [77.28%]) compared with UCD (10 patients [45.45%]) (P < .05) (Table 3). The characteristic morphological appearance of iMCD lymph nodes was not seen in bone marrow specimens. Plasmacytosis is a prominent abnormality in UCD and iMCD compared with normal bone marrow, but there was not a significant difference in plasmacytosis between UCD and iMCD. The percentage of CD45+/CD56+/CD3− cells demonstrating plasmacytosis (26/45) ranged from 3.0% to 30.4% of total nucleated marrow cells, and 57.69% (15/26) had ≥10% plasmacytosis across all cases. Immunophenotyping showed no difference among the expression rates of CD3+, CD3+/CD4+, CD3+/CD8+, and CD19+ (Figure 2).
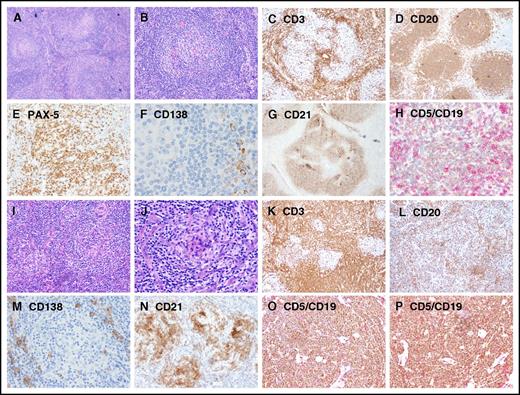
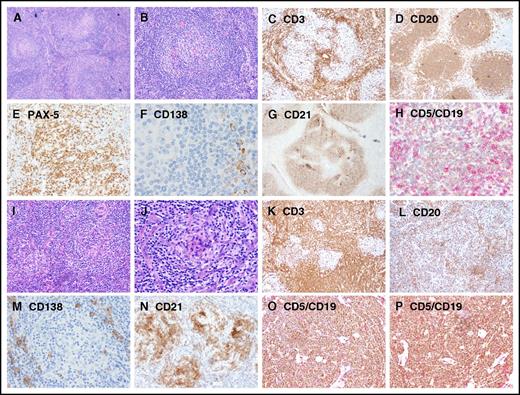
Representative images showing immunohistochemical expression in patients with UCD and iMCD. (A-H) UCD case with B-cell–rich germinal centers and increased CD5+/CD19+ B cells, whereas CD3+ small T cells are relatively sparse. (I-P) iMCD case with dense T cells in the interfollicular regions and decreased CD20/PAX-5 B cells and CD5+/CD19+ B cells. Few polyclonal CD138+ PCs are present around the nodules in both UCD and iMCD. Original magnification ×100 for panels A-D,I-L and ×200 for panels E-H,M-P.
Representative images showing immunohistochemical expression in patients with UCD and iMCD. (A-H) UCD case with B-cell–rich germinal centers and increased CD5+/CD19+ B cells, whereas CD3+ small T cells are relatively sparse. (I-P) iMCD case with dense T cells in the interfollicular regions and decreased CD20/PAX-5 B cells and CD5+/CD19+ B cells. Few polyclonal CD138+ PCs are present around the nodules in both UCD and iMCD. Original magnification ×100 for panels A-D,I-L and ×200 for panels E-H,M-P.
Histopathological and immunophenotypic findings of lymph nodes
The lymph nodes of most patients with UCD (32/43 [74.42%]) were histologically classified as HV subtype. The lymph nodes of patients with iMCD also were most frequently of the HV subtype (16/31 [51.62%]), but this patient group included more lymph nodes of the PC or PC variant subtypes (15/31 [48.38%]) (Table 1).
The immunophenotypic analyses of lymph nodes by flow cytometry are summarized in Table 3 and Figure 1C-F. The number of T cells (CD3+) was higher in iMCD patients than in those with UCD (P = .048), but the ratio of CD3+/CD4+ to CD3+/CD8+ was not significantly different (data not shown). In most cases, the number of B cells was similar between the UCD and iMCD groups, although CD19+/CD5+ lymphocytes were higher in UCD patients than in iMCD patients (P = .032).
Treatment outcomes
Complete surgical resection was performed in 33 out of 43 patients (76.74%) with UCD for diagnosis and as first-line treatment. A total of 30 out of these 33 patients achieved complete remission (CR), but after surgery, 3 patients developed additional adenopathy in a new location. Those 3 patients underwent complete excision again, after which 2 achieved CR and 1 had a recurrence in yet another location. Surgical resection was not possible at the time of diagnosis for 2 patients, because the affected lymph node surrounded the jugular vein. In 1 of these 2 patients, the mass was shrunk >50% with 4000 cGY radiotherapy; in the other, the mass was shrunk >50% with 4 doses of rituximab, which then allowed for complete resection. Similarly, a third patient, who had a mediastinal mass that could not be resected due to cardiac insufficiency, achieved CR after 4500 cGY radiotherapy given in 30 fractions.
Treatments differed considerably among the iMCD cases. Eight patients in MDACC, who had no systemic inflammatory symptoms or complaints at diagnosis, were initially biopsied and observed only. All 8 patients experienced disease progression. Forty-three patients received monoclonal antibody therapy and/or chemotherapy, and >50% of patients received ≥2 agents.
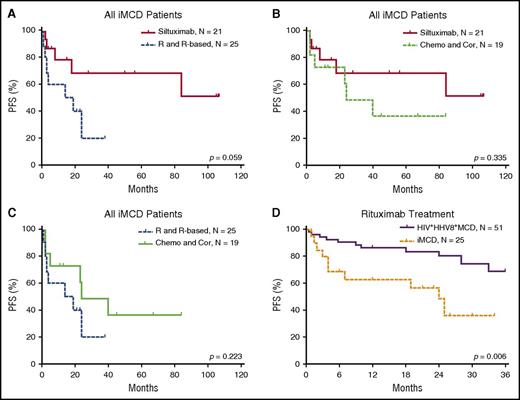
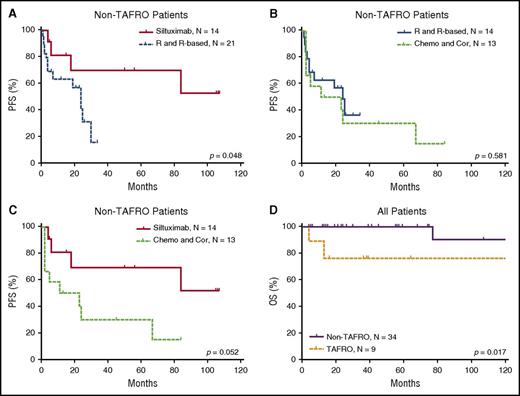
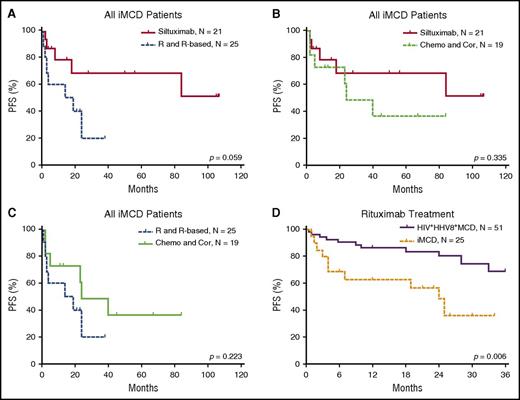
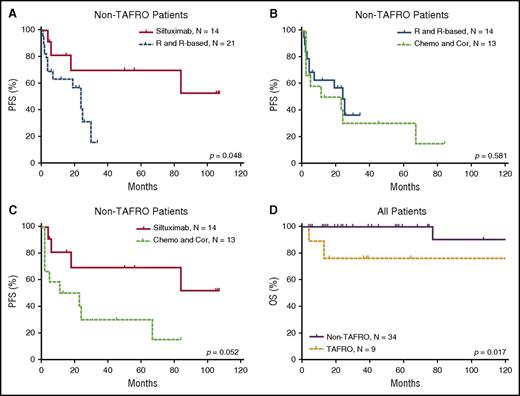
The efficacy results of the iMCD treatment are summarized in Table 4 and Figures 3 and 4. Among the 3 main categories of treatments (siltuximab, rituximab or rituximab-based therapies, and chemotherapy or corticosteroids), siltuximab was associated with a significantly higher rate of CR than rituximab or rituximab-based therapies (P = .034). Of the patients who received siltuximab, 60% received it as a second line therapy. Rituximab or rituximab-based therapies were associated with a significantly poorer PFS rate than siltuximab, and they were no better than chemotherapy or corticosteroids in terms of the CR and PFS rates. However, rituximab was correlated with better PFS among patients with HIV-positive and HHV-8–positive MCD than in those with iMCD (P = .006). Patients with the TAFRO subtype tended to have a poorer overall survival rate than those with the non-TAFRO subtype (P = .017). Among patients with the non-TAFRO subtype, those who received siltuximab had a significantly better PFS rate than those who received rituximab or rituximab-based therapies or chemotherapy or glucocorticoids (P = .048 and P = .052, respectively) (Figure 4A,C).
PFS of patients with iMCD after treatment with different therapies. (A) Among all iMCD patients, siltuximab was not correlated with PFS when compared with rituximab or rituximab-based therapy significantly, although a trend toward better survival is suggested. (B) There was no significant difference in PFS between treatment with siltuximab and treatment with chemotherapy or corticosteroids. (C) There was no significant difference in PFS between treatment with rituximab or rituximab-based therapy and chemotherapy or corticosteroids. (D) Rituximab or rituximab-based therapy was correlated with better PFS in patients with HIV-positive and HHV-8–positive MCD compared with iMCD.9,32,33 Chemo and Cor, chemotherapy or therapy with corticosteroids only; R and R-based, rituximab or rituximab-based therapy.
PFS of patients with iMCD after treatment with different therapies. (A) Among all iMCD patients, siltuximab was not correlated with PFS when compared with rituximab or rituximab-based therapy significantly, although a trend toward better survival is suggested. (B) There was no significant difference in PFS between treatment with siltuximab and treatment with chemotherapy or corticosteroids. (C) There was no significant difference in PFS between treatment with rituximab or rituximab-based therapy and chemotherapy or corticosteroids. (D) Rituximab or rituximab-based therapy was correlated with better PFS in patients with HIV-positive and HHV-8–positive MCD compared with iMCD.9,32,33 Chemo and Cor, chemotherapy or therapy with corticosteroids only; R and R-based, rituximab or rituximab-based therapy.
PFS of patients with non-TAFRO iMCD and overall survival of patients with and without TAFRO. (A) Treatment with siltuximab led to better PFS than rituximab or rituximab-based therapy. (B) Rituximab or rituximab-based therapy and chemotherapy or corticosteroids had similar PFS. (C) Treatment with siltuximab was correlated with better PFS than treatment with chemotherapy or corticosteroids. (D) In all patients, TAFRO syndrome correlated with significantly poorer patient survival. Chemo and Cor, chemotherapy or therapy with corticosteroids only; OS, overall survival; R and R-based, rituximab or rituximab-based therapy.
PFS of patients with non-TAFRO iMCD and overall survival of patients with and without TAFRO. (A) Treatment with siltuximab led to better PFS than rituximab or rituximab-based therapy. (B) Rituximab or rituximab-based therapy and chemotherapy or corticosteroids had similar PFS. (C) Treatment with siltuximab was correlated with better PFS than treatment with chemotherapy or corticosteroids. (D) In all patients, TAFRO syndrome correlated with significantly poorer patient survival. Chemo and Cor, chemotherapy or therapy with corticosteroids only; OS, overall survival; R and R-based, rituximab or rituximab-based therapy.
Univariate survival analysis
Of the 74 patients who were treated at MD Anderson, 29 (16 with UCD and 13 with iMCD) had a relatively short duration of follow-up due to loss of follow-up. Among the remaining 46 cases, the median follow-up duration was 64.66 months (range, 9-275 months). Only 2 patients with iMCD died, 1 of whom met the criteria for TAFRO syndrome.
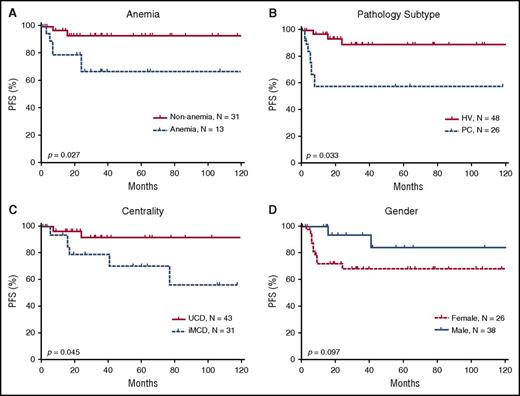
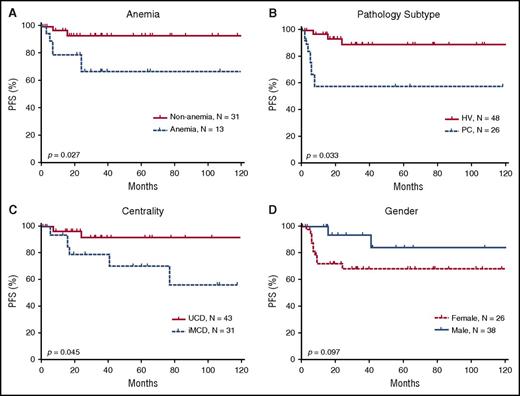
Using the Kaplan-Meier method, we conducted a univariate analysis of PFS with a mean follow-up duration of 64.66 months and identified 3 significant risk factors: multicentricity, PC pathological subtype, and anemia (Figure 5). The univariate analysis did not identify statistically significant differences associated with any other investigated factors, including age, sex, B-symptoms, mass >5 cm, history of autoimmune disease, alkaline phosphatase, lactate dehydrogenase, immunoglobulin level, and bone marrow involvement (data not shown).
Prognostic significance of clinical characteristics in 74 HIV-negative patients with CD. Anemia (A), the pathologic subtypes of PC (B), and multicentricity (C) were correlated with significantly poorer PFS. (D) Sex did not correlate with PFS, although a trend toward better survival is suggested among males. MIX, mixed cellular variant.
Prognostic significance of clinical characteristics in 74 HIV-negative patients with CD. Anemia (A), the pathologic subtypes of PC (B), and multicentricity (C) were correlated with significantly poorer PFS. (D) Sex did not correlate with PFS, although a trend toward better survival is suggested among males. MIX, mixed cellular variant.
Multivariate analysis identified multicentricity as a risk factor
The Cox proportional hazards model was used to perform a multivariate analysis of CD patients’ clinical characteristics, including multicentricity, anemia, and pathological subtype. The results showed that multicentric disease was independently associated with PFS (hazard ratio = 0.236, P = .019). Anemia showed a trend toward being a risk factor for PFS (hazard ratio = 3.075, P = .069 (Table 5).
Discussion
Clinical, laboratory, and treatment data for patients with CD, including UCD and iMCD, are dispersed among case reports, small series, and a single randomized, controlled trial. Using data on clinical, laboratory, and pathologic abnormalities and on treatment outcomes with a median follow-up duration of 6.6 years, we have performed the most comprehensive evaluation of CD in North America to date. In particular, our study provides valuable and compressive information that should advance our understanding of iMCD and its treatment options.
We show the heterogeneity between UCD and iMCD patients as well as within each subtype. The pathogenesis of iMCD is poorly understood at this time. Elevated serum IL-6 levels have been shown to be associated with iMCD, but some patients have normal or only slightly elevated levels of IL-6,20 suggesting that the heterogeneity of this disease may be related to the fact that it is not driven entirely by IL-6 in all patients. In fact, serum IL-6 levels were normal in all 3 patients in whom it was measured. Some case reports suggested that other cytokines may be driving the disease in such patients, including IL-2, VEGF, and IL-1.16,20,21 It is possible that the heterogeneous clinical, histological, and laboratory abnormalities may be explained by different molecular mechanisms. We observed that ∼39% of iMCD patients had a history of autoimmune diseases, which were typically stable at the time of diagnosis. Furthermore, we observed that treatment resulted in improvement or resolution of both CD and signs and symptoms of autoimmune connective tissue disease. Considering the overlap between CD and autoimmune diseases, autoimmunity may be responsible for initiating or perpetuating the cytokine storm in iMCD via autoantibody antigenic stimulation. Alternatively, iMCD may be secondary to the cytokine storm from these autoimmune conditions, or iMCD may have been incorrectly diagnosed. Other possible etiologies for UCD and iMCD include somatic mutations in a small population of clonal cells and a virus other than HHV-8. Further investigation is crucial to better understand the cytokine cascade, specific markers responsible for disease progression, intracellular pathway activation, and pathological microenvironment components mediating HIV-negative CD, especially iMCD.
Our investigation of bone marrow histopathology and immunophenotyping is the largest series reported in patients with HIV-negative CD. Reactive plasmacytosis was the most frequent finding, which has been reported before in case reports.22,23 Ibrahim et al found a similar result in HIV-positive and HHV-8–associated MCD patients.24 PCs originate from B cells and produce antibodies to mediate the humoral immune response.25 In CD, plasmacytosis is often found in the lymph node and is believed to be caused by excess IL-6, but the source and etiology of the IL-6 is unknown. In multiple myeloma, IL-6 can be secreted by both neoplastic PCs and stromal cells.26 Bone marrow PCs are typically long-lived, produce IgG and IgA, and secrete high levels of antibodies without switching antibody classes.
Our study also includes the largest series in which lymph node cells of patients with CD were immunophenotyped. We observed higher numbers of CD3+ T cells and lower numbers of CD19+/CD5+ B cells in iMCD patients than in UCD patients. B cells are known to play an essential role in HHV-8–positive MCD, in which B-cell depletion with rituximab is highly effective.27 Furthermore, T-cell levels are correlated with HHV-8 viral loads in peripheral blood,28 and polyfunctional effector memory HHV-8–specific CD8+ T cells are associated with the pathophysiology of HHV-8–associated MCD.29 However, the roles of B cells, T cells, and other immune cells in UCD and iMCD are unknown.30,31 These cell types may be responsible for the cytokine dysregulation or may be present as a reaction to cytokines. The cytokine-producing cells also may differ between pathologic types. Elucidation of the roles of the various immune cells in CD will be essential in the field.
Our study evaluates the effectiveness of a variety of different treatment regimens.9,11,32,33 In UCD, surgical complete resection is found to be the best first-line treatment.11 In UCD patients refractory to surgical resection or inoperable, rituximab or radiotherapy can be effective. However, standard protocols have not been established for the treatment of iMCD. Our data and the reported literature suggest that iMCD patients treated with glucocorticoids or chemotherapy are less likely to achieve CR (both 10% to 20%), and those patients who do achieve CR often experience recurrence of disease within 1 to 2 years.34 Rituximab is active as monotherapy in HHV-8–associated MCD with or without HIV9,32,33,35 However, although over half of our patients who were treated with rituximab or rituximab-based therapy had a response, only 20% achieved a CR; this percentage is much lower than that reported in the literature for patients with HHV-8–associated MCD (84%).9
Siltuximab effectively controlled and improved the clinical manifestations and PFS in iMCD patients, even among those for whom rituximab failed, and the patients’ mean response rate to siltuximab was significantly higher than that for rituximab or rituximab-based therapy and chemotherapy. Of note, the ∼75% response rate in our series is much higher than the 34% response rate for siltuximab that was observed in the only randomized controlled trial of iMCD.7 The difference in response may be related to improved patient selection, longer follow-up time to achieve a response, and the fact that the threshold for a partial response was less stringent in our series. Although the side effects profile of anti–IL-6 therapy with siltuximab are better tolerated than those of most cytotoxic chemotherapeutic regimens, patients might need lifelong administration of siltuximab, as relapse has been reported on cessation of IL-6 receptor therapy with tocilizumab.36
Approximately one-quarter of patients treated with siltuximab in our series had no response, suggesting that proinflammatory cytokines besides IL-6 may be driving the underlying pathogenesis in some patients. Anakinra, which is an IL-1 receptor antagonist, has been reported to be effective in several CD patients, including 2 patients in the literature who did not respond to anti–IL-6 therapy16,37 and 1 case in our own study. Additional agents that have been tried in CD patients in the literature include bortezomib, cyclosporine, intravenous immunoglobulin, methotrexate, and thalidomide, but there are limited data and prognostic guidance or biomarkers that are available to indicate which patients will respond to these treatments.
Our study also contributes survival data on the largest series of HIV-/HHV-8–negative CD in North America since 2012. In light of the 2014 approval of siltuximab by the US Food and Drug Administration and the increased use of agents other than cytotoxic chemotherapy to treat CD, our data contribute valuable results related to patient outcomes with newer treatment options. Previous series have reported 5-year survival rates ranging from 55% to 77% for HIV-negative MCD.38-40 Only 2 of 31 iMCD patients in our cohort died within the median 6.6-year follow-up period. This could be explained by one of the following: (1) iMCD cases that co-occurred with malignancy were excluded; (2) MD Anderson is a referral center, so acute patients that may die on presentation would not be as well represented, as would be the case in a different setting; (3) the 18% of patients with iMCD patients who were asymptomatic would have been excluded from other series; and (4) newer treatment options may be improving outcomes. Overall survival analysis shows that the TAFRO syndrome is a distinct subtype of iMCD with inferior survival, which is consonant with other reports.19 We found, based on univariate analysis, that PC-type lymph nodes and anemia significantly influenced the PFS in patients with iMCD. In contrast, Talat and Schulte8 reported in a systematic meta-analysis of 416 patients from the literature that PC-type lymph nodes, male sex, and age >37 years appeared to be unfavorable factors influencing the rate of 3-year disease-free survival.
Based on these data, siltuximab appears to be an effective first treatment option for patients with iMCD, whereas rituximab and rituximab-based therapy have relatively inferior efficacy. Given the potential for a delayed response to siltuximab or rituximab monotherapy, corticosteroids may be helpful as an initial adjunct for the improvement of acute symptoms in some patients.
There are a few weaknesses to this study. First, clinical data were missing for some of the enrolled patients, which may have underpowered the true differences in outcomes or affected the relative effectiveness of different treatment categories. Second, we recognize there may be bias due to the types of patients seen at MD Anderson, which is a tertiary referral center. Third, physicians may select more intensive treatments, such as chemotherapy, for patients with more severe disease, thereby possibly achieving better treatment responses. Despite these limitations, we have presented the largest series of CD patients with important observations related to CD’s clinical features, associations with autoimmune disorders, and improved responses to siltuximab treatment.
In summary, the iMCD subtype of CD is a heterogeneous disorder, and little is known about its clinical abnormalities, disease associations, treatments, and outcomes. No standard-of-care regimen has been well developed. We identified significantly elevated CD3+ T cells and decreased CD19+/CD5+ cell populations in lymph nodes of patients with iMCD. We also found that multicentricity, histopathological type, and anemia are significant risk factors for shortened PFS. The use of siltuximab is associated with a greater proportion of complete responses among iMCD treatment options, whereas complete surgical resection remains the optimal approach for patients with UCD. Further investigation is essential to elucidate the roles of CD3+ T cells in iMCD, the etiology of iMCD, and the subgroups of patients that may help predict outcomes or optimal therapies. We anticipate that the International Castleman Disease Consortium program organized by The University of Texas MD Anderson Cancer Center and the global ACCELERATE patient registry and natural history study, currently being organized by the CDCN at the University of Pennsylvania, will generate important information related to clinical abnormalities, treatment options, and outcomes.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study is supported by the National Institutes of Health National Cancer Institute (grants R01CA138688, R01CA187415, and 1RC1CA146299) (Y.L. and K.H.Y.) and in part by the Intramural Research Program of the National Institutes of Health National Cancer Institute (T.S.U. and R.Y.). L.Y. and M.T. are the recipients of a Hematology/Oncology Scholarship Award. K.H.Y. is supported by a The University of Texas MD Anderson Cancer Center Institutional Research Grant Award, an Anderson Lymphoma Specialized Programs of Research Excellence (SPORE) Research Development Program Award, an Anderson Myeloma SPORE Research Developmental Program Award, and the University Cancer Foundation via the Sister Institution Network Fund at The University of Texas MD Anderson Cancer Center. The study is also partially supported by the National Institutes of Health, National Cancer Institute grants P50CA136411 and P50CA142509 and an Anderson Cancer Center support grant (CA016672).
Authorship
Contribution: L.Y. and K.H.Y. conceived and designed the study; L.Y., M.T., Z.Y.X.-M., P.C.B., M.A.A., Y.L., D.C.F., and K.H.Y. performed research performance and wrote the manuscript; L.Y., M.T., J.C., Z.Y.X.-M., R.N.M., J.Z., R.Z.O., S.N., P.C.B., M.A.A., T.S.U., R.Y., L.J.M., Y.L., D.C.F., and K.H.Y. provided ideas, materials, key reagents, and technology; L.Y., M.T., J.C., Z.Y.X.-M., R.N.M., J.Z., R.Z.O., S.N., P.C.B., M.A.A., T.S.U., R.Y., L.J.M., Y.L., D.C.F., and K.H.Y. collected and assembled data under the approved institutional review board and material transfer agreement; L.Y., M.T., J.C., D.C.F., and K.H.Y. analyzed and interpreted data; L.Y., D.C.F., and K.H.Y. edited the manuscript; and all authors gave final approval of the manuscript.
Conflict-of-interest disclosure: D.C.F. has received research funding from Janssen Pharmaceuticals. The research of T.S.U. and R.Y. is partially supported by a CRADA between the National Cancer Institute and Celgene Corp, and the spouse of R.Y. has a patent, assigned to the US Government, on HHV-8 vIL-6. K.H.Y. receives research support from Roche Molecular Systems, Gilead Sciences Pharmaceutical, Seattle Genetics, Dai Sanyo Pharmaceutical, Adaptive Biotechnology, Incyte Pharmaceutical, and HTG Molecular Diagnostics. The remaining authors declare no competing financial interests.
Correspondence: Ken H. Young, Department of Hematopathology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030-4009; e-mail: khyoung@mdanderson.org.
References
Author notes
L.Y. and M.T. contributed equally to this study.