Key Points
Prolonged inhibition of CXCR4/CXCL12 signaling results in exceptional mobilization along with an expansion of the BM HSPC pool.
Reversible inhibition of the CXCR4/CXCL12 axis may represent a novel strategy to restore damaged BM.
Abstract
Interaction between the chemokine receptor CXCR4 and its chief ligand CXCL12 plays a critical role in the retention and migration of hematopoietic stem and progenitor cells (HSPCs) in the bone marrow (BM) microenvironment. In this study, qualitative and quantitative effects of long-term pharmacologic inhibition of the CXCR4/CXCL12 axis on the HSPC compartment were investigated by using 3 structurally unrelated small molecule CXCR4 antagonists. A >10-fold increase in mobilization efficiency was achieved by administering the antagonists as a subcutaneous continuous infusion for 2 weeks compared to a single bolus injection. A concurrent increase in self-renewing proliferation leading to a twofold to fourfold expansion of the HSPC pool in the BM was observed. The expanded BM showed a distinct repopulating advantage when tested in serial competitive transplantation experiments. Furthermore, major changes within the HSPC niche associated with previously described HSPC expansion strategies were not detected in bones treated with a CXCR4 antagonist infusion. Our data suggest that prolonged but reversible pharmacologic blockade of the CXCR4/CXCL12 axis represents an approach that releases HSPC with efficiency superior to any other known mobilization strategy and may also serve as an effective method to expand the BM HSPC pool.
Introduction
Hematopoietic stem cells (HSCs) are characterized by their ability to self-renew and to give rise to all types of mature blood cells.1,2 These unique properties not only allow this rare bone marrow (BM) cell subset to maintain life-long hematopoiesis but also become critically important in the course of hematopoietic stem cell transplantation, the only curative therapy available for many hematologic malignancies as well as some nonmalignant diseases. During the past 2 decades, mobilized peripheral blood stem cells have become the favored graft source for hematopoietic stem cell transplantation.3 Mobilization failure and subsequent low apheresis yields of hematopoietic stem and progenitor cells (HSPCs) that result in delayed or impaired multilineage engraftment can occur in patients undergoing autologous stem cell transplantation and correlates with BM hypoplasia due to prior exposure to cytotoxic therapy.4 Approaches that potently regenerate the BM HSPC pool and release large numbers of HSPCs may provide a novel approach to optimize HSC mobilization and reduce mobilization failures as well as allow for dose-dense or continuation of chemotherapy.
The interaction between the chemokine receptor CXCR4 and its chief ligand CXCL12 plays a major role for HSPC migration as well as their retention in the BM microenvironment.5 Hence, interference with the CXCR4/CXCL12 pathway as a strategy to enforce the release of HSPCs into the circulation is currently being exploited indirectly by the most clinically relevant mobilizing agent to date, granulocyte colony-stimulating factor (G-CSF),6 as well as directly by using the small molecule bicyclam CXCR4 antagonist plerixafor (AMD3100 [Mozobil]).7-9 In addition, CXCR4/CXCL12 signaling has been reported to promote survival of HSPCs10,11 while negatively regulating their proliferation.12-14
In this study, qualitative and quantitative effects of long-term inhibition of the CXCR4/CXCL12 axis, particularly within the HSPC compartment, were investigated. Three different small molecule CXCR4 antagonists were tested: the US Food and Drug Administration–approved bicyclam AMD3100,15 tetrahydroquinoline-derived inhibitor ALT1188,16 and the recently characterized peptidic antagonist POL5551.17 The pharmacologic blockade was compared with the phenotype associated with genetic (irreversible) CXCR4 ablation. Moreover prolonged CXCR4 inhibition was evaluated in a model of chemotherapy-induced BM damage.
Methods
Mice
C57BL/6J (CD45.2) and syngeneic B6.SJL-PtprcaPep3b/BoyJ (CD45.1) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). F1 hybrid mice (CD45.1/2) were obtained through breeding CD45.2 and CD45.1 mice. CXCR4del/del mice were generated by crossing B6.129-Gt(ROSA)26Sortm1(cre/ERT2)Tyj/J (bearing tamoxifen-inducible Cre recombinase [ERT2Cre+]; The Jackson Laboratory) and B6.129P2-Cxcr4tm2Yzo/J (CXCR4flox/flox; The Jackson Laboratory). Subsequent CXCR4 ablation was achieved by using 2 sets of 3 consecutive administrations of tamoxifen (Sigma-Aldrich, St. Louis, MO) in corn oil at 2.5 to 5 mg per mouse intraperitoneally) delivered 4 days apart. Animals were housed at the Washington University Medical School vivarium under specific-pathogen-free conditions or at the Johann Wolfgang Goethe-University Medical School (Frankfurt, Germany) vivarium under non–specific-pathogen-free conditions with autoclaved chow and water ad libitum. All experiments were performed in accordance with the guidelines of the Washington University Animal Studies Committee or approved by the municipal government (Darmstadt, Germany) and the Institutional Animal Care and Use Committee, in agreement with Association for Assessment and Accreditation of Laboratory Animal Care guidelines. After lethal irradiation (9.5 or 11.0 Gy, using a 137Cs source) and transplantation, mice were maintained on antibiotic medication, sulfamethoxazole (0.5 mg/mL) and trimethoprim (0.1 mg/mL) (Hi-Tech Pharmacal, Amityville, NY), given orally in drinking water.
The supplemental Data, available on the Blood Web site, contains descriptions of how cells and tissues were extracted and prepared, lists of reagents used along with details about the treatments, and descriptions of how transplantation assays were performed and analyzed.
Fluorescence-activated cell analysis and sorting
Phosphate-buffered saline and bovine serum albumin (0.5%) buffer were used for all staining and wash steps. Cell labeling was performed according to standard protocols using established marker panels for identification of different subsets in mouse hematopoietic tissues (see supplemental Data for details).
Colony-forming unit assay
Cells were incubated in duplicate in commercially available growth factor–supplemented methylcellulose medium for mouse colony-forming units in culture (CFU-C) (Stem Cell Technologies, Vancouver, BC, Canada, or R&D Systems, Minneapolis, MN) as described.17,18 CFU-C (burst forming unit-erythroid, CFU granulocytle macrophage, and CFU-granulocyte, erythrocyte, monocyte, megakaryocyte) were enumerated after 6 to 8 days of culture.
Quantitative real-time polymerase chain reaction
For analysis of transplanted BM cells (total BM and purified HSCs), RNA was isolated by using an RNA XS column kit (Macherey-Nagel, Bethlehem, PA). Subsequently, an Ambion Turbo DNA-Free Kit (Thermo Fisher Scientific, Waltham, MA) was used to remove genomic DNA contamination, and RNA was reverse transcribed by using the QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA). Quantitative real-time polymerase chain reaction (CXCR4 and glyceraldehyde-3-phosphate dehydrogenase) was performed by using TaqMan Master Mix, probes, and primers (Applied Biosystems, Foster City, CA) listed in supplemental Table 2.
Microarray analysis
RNA from sorted BM Lin–Sca-1+c-kit+ (LSK) cells and 1.5 × 103 LSK CD150+CD48– (LSK SLAM) cells of differently treated (POL5551 infusion, G-CSF, or sham-operated control) mice was prepared using the RNA XS column kit (Macherey-Nagel) and hybridized to the Mouse Gene Expression v2 8 × 60 array (Agilent Technologies, Santa Clara, CA). Quantile normalization and quality assessment of extracted (Feature Extraction Software, Agilent) expression data were performed by using Partek Genomic Suite (Partek Inc., Chesterfield, MO). Differential expression of log2 scale transformed data were then analyzed using limma (Bioconductor; R). R was also used for principal component analysis and clustering analysis. Data can be found at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE88999.
Immunohistochemistry
Dissected hind limbs were fixed for 60 hours in 4% paraformaldehyde (Sigma, St. Louis, MO) at 4°C and then decalcified for 12 days in 14% EDTA (Sigma) pH 7.2 at 4°C. After processing and paraffin embedding, immunohistochemistry was performed as described elsewhere19 with minor modifications. See supplemental Data for further details. Regarding pharmacokinetics, plasma samples were prepared and analyzed as described previously.17
Statistical analysis
Data are presented as mean ± standard error of the mean unless indicated otherwise. Descriptive statistics and Student t tests were calculated by using Excel (Microsoft, Redmond, WA).
Results
Continuous infusion vs bolus administration of CXCR4 inhibitors
We compared the mobilization efficiency of a bolus injection with treatment by continuous infusion using 3 different small molecule CXCR4 antagonists: AMD3100, ALT1188, and POL5551. When the same dose of the protein-epitope mimetic inhibitor POL5551 was administered daily for a period of 2 weeks instead of using a single injection, up to 12-fold to 15-fold higher mobilization was achieved: 8 to 10 × 104 CFU-C or LSK cells and 1.5 × 103 LSK SLAM cells per mL with continuous infusion vs 4 to 6 × 103 CFU-C or LSK and 1 × 102 LSK SLAM cells per mL after bolus POL5551 (Figure 1A-B). Similarly, continuous infusion of ALT1188 or AMD3100 also significantly increased the magnitude of HSPC mobilization relative to bolus injection of either drug (Figure 1A-B). However, the total counts as well as the dramatic increase in circulating HSPC numbers were most pronounced after administration of POL5551. The total leukocyte count, although variably affected by the different compounds, was higher after bolus injection than after infusion (Figure 1C). Pharmacokinetic studies performed in a different cohort of mice using POL5551 demonstrated that maximum plasma levels were achieved within a single day and sustained throughout the treatment period (Figure 1D).
Efficacy of CXCR4 antagonist–based mobilization regimen. (A-C) Comparison of continuous infusion (cont. inf.) and bolus injection. The CXCR4 antagonists POL5551 (POL), AMD3100 (AMD), and ALT1188 (ALT) were administered to C57BL/6 mice as a single intraperitoneal (IP) injection (POL5551, 100 mg/kg; AMD3100, 20 mg/kg; ALT1188, 33 mg/kg) or as a continuous infusion for 2 weeks via subcutaneously implanted pumps (POL5551, 100 mg/kg per day; AMD3100, 20 mg/kg per day; ALT1188, 33 mg/kg per day). Peripheral blood (PB) was analyzed (at 3 hours after injection of bolus POL5551 and ALT1188, at 1 hour after injection of bolus AMD3100, at day 14 [d14] for all groups treated by continuous infusion) for (A) CFU-C/LSK, (B) LSK SLAM, and (C) white blood cell (WBC) concentration. Corresponding counts from age- and sex-matched healthy male mice injected with vehicle or sham-operated are shown as control (ctr.) mean ± standard error of the mean (SEM) (n = 5-10 mice for treated groups; n = 15 for control mice). (D) Pharmacokinetics. C57BL/6 mice were treated with a continuous infusion of POL5551 (50 mg/kg per day for 1 week). Plasma concentration of POL5551 (solid line) was analyzed at the indicated time points. Corresponding CFU-C numbers (dotted line) are shown for comparison. (E-F) Combination of CXCR4 and VLA4 blockade. At the end of treatment with continuous infusion of POL5551 as described in (A-C), mice were randomly assigned to receive either an IP bolus injection of AMD3100 (20 mg/kg) or the VLA4 antagonist CWHM-823 (3 mg/kg). One hour later, (E) CFU-C/LSK and (F) LSK SLAM numbers were quantified. CFU-C, LSK, and LSK SLAM counts obtained before the bolus treatment (ie, from corresponding POL5551 infusion–treated mice [A-C]) are shown for comparison (mean ± SEM; n = 5). ***P < .001; **P < .01; *P < .05. appr., approximately; n.s., not significant.
Efficacy of CXCR4 antagonist–based mobilization regimen. (A-C) Comparison of continuous infusion (cont. inf.) and bolus injection. The CXCR4 antagonists POL5551 (POL), AMD3100 (AMD), and ALT1188 (ALT) were administered to C57BL/6 mice as a single intraperitoneal (IP) injection (POL5551, 100 mg/kg; AMD3100, 20 mg/kg; ALT1188, 33 mg/kg) or as a continuous infusion for 2 weeks via subcutaneously implanted pumps (POL5551, 100 mg/kg per day; AMD3100, 20 mg/kg per day; ALT1188, 33 mg/kg per day). Peripheral blood (PB) was analyzed (at 3 hours after injection of bolus POL5551 and ALT1188, at 1 hour after injection of bolus AMD3100, at day 14 [d14] for all groups treated by continuous infusion) for (A) CFU-C/LSK, (B) LSK SLAM, and (C) white blood cell (WBC) concentration. Corresponding counts from age- and sex-matched healthy male mice injected with vehicle or sham-operated are shown as control (ctr.) mean ± standard error of the mean (SEM) (n = 5-10 mice for treated groups; n = 15 for control mice). (D) Pharmacokinetics. C57BL/6 mice were treated with a continuous infusion of POL5551 (50 mg/kg per day for 1 week). Plasma concentration of POL5551 (solid line) was analyzed at the indicated time points. Corresponding CFU-C numbers (dotted line) are shown for comparison. (E-F) Combination of CXCR4 and VLA4 blockade. At the end of treatment with continuous infusion of POL5551 as described in (A-C), mice were randomly assigned to receive either an IP bolus injection of AMD3100 (20 mg/kg) or the VLA4 antagonist CWHM-823 (3 mg/kg). One hour later, (E) CFU-C/LSK and (F) LSK SLAM numbers were quantified. CFU-C, LSK, and LSK SLAM counts obtained before the bolus treatment (ie, from corresponding POL5551 infusion–treated mice [A-C]) are shown for comparison (mean ± SEM; n = 5). ***P < .001; **P < .01; *P < .05. appr., approximately; n.s., not significant.
When AMD3100 was injected into mice pretreated with POL5551 infusion, as predicted, no further enhancement in mobilization occurred (Figure 1E-F). This is in line with the specificity of both compounds for CXCR4 and/or with CXCR4 mediating the pharmacologic effects of both compounds. Additive to synergistic comobilization by CXCR4 and VLA4 inhibition was previously demonstrated,20,21 albeit not in the context of CXCR4 antagonist infusion. As expected, when VLA4 blockade (injection of the small molecule VLA4 antagonist CWHM-82322 ) was combined with POL5551 infusion, significantly enhanced mobilization was observed (Figure 1E-F).
Effects of long-term inhibition of CXCR4 on hematopoiesis
With up to 8 to 10 × 104 CFU-C and LSK cells per mL found in the circulation (Figure 1A), the question arose of whether such potent mobilization would result in a simultaneous reduction of the BM-resident HSPC pool. Therefore, we concurrently analyzed peripheral blood and BM after continuous infusion of CXCR4 inhibitors. Sham-operated controls as well as mice treated with G-CSF for 3 days (4 doses every 12 hours; maximum proliferation23-25 ) or 5 days (9 doses every 12 hours; maximum mobilization23-25 ) were analyzed for comparison. Continuous infusion of the CXCR4 inhibitors was associated with a significant increase in circulating white blood cell counts and a concomitant decrease in the number of BM leukocytes (Figure 2A). Higher numbers of HSPCs in circulation (Figure 2B,G) did not correspond to decreased numbers of HSPCs in the femur. In fact, depending on the CXCR4 antagonist used, immunophenotypical (LSK as well as LSK SLAM) and short-term functional (CFU-C) analyses showed that compared with steady state, BM-resident HSPCs were increased twofold to fourfold (Figure 2C-F,H). Similar increase was seen in mice treated with G-CSF for 2 days (4 doses).
Mobilization and expansion. C57BL/6 mice were treated with the CXCR4 antagonists POL5551 (100 mg/kg per day for 1 or 2 weeks [wks]), AMD3100 (20 mg/kg per day for 2 weeks,), and ALT1188 (ALT; 33 mg/kg per day for 2 weeks) via subcutaneous infusion pumps. Control groups received G-CSF (100 μg/kg per dose at 4 or 9 doses every 12 hours) or phosphate-buffered saline (PBS). Concurrent analysis of hematopoiesis in PB and BM was performed. (A) WBC concentration in PB and BM. Corresponding CFU-C/LSK numbers are presented in (B) PB and (C) BM. (D) CFU-C/LSK frequency in BM, (E) LSK SLAM count, and (F) LSK SLAM frequency are shown. Representative flow cytometry analyses of LSK and LSK SLAM fraction in (G) PB and (H) BM of differentially treated mice are shown (mean ± SEM; n = 3). ***P < .001; **P < .01; *P < .05.
Mobilization and expansion. C57BL/6 mice were treated with the CXCR4 antagonists POL5551 (100 mg/kg per day for 1 or 2 weeks [wks]), AMD3100 (20 mg/kg per day for 2 weeks,), and ALT1188 (ALT; 33 mg/kg per day for 2 weeks) via subcutaneous infusion pumps. Control groups received G-CSF (100 μg/kg per dose at 4 or 9 doses every 12 hours) or phosphate-buffered saline (PBS). Concurrent analysis of hematopoiesis in PB and BM was performed. (A) WBC concentration in PB and BM. Corresponding CFU-C/LSK numbers are presented in (B) PB and (C) BM. (D) CFU-C/LSK frequency in BM, (E) LSK SLAM count, and (F) LSK SLAM frequency are shown. Representative flow cytometry analyses of LSK and LSK SLAM fraction in (G) PB and (H) BM of differentially treated mice are shown (mean ± SEM; n = 3). ***P < .001; **P < .01; *P < .05.
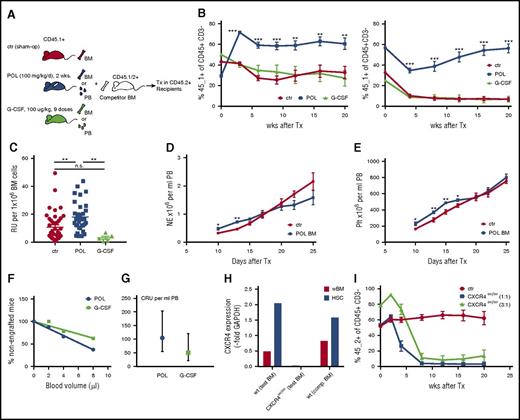
Long-term functional properties of BM HSPCs obtained after 2 weeks of continuous POL5551 infusion were assessed in transplantation assays. In a serial competitive transplantation setting (Figure 3A), POL5551-expanded HSPCs engrafted significantly better than BM cells from untreated or G-CSF–treated mice (Figure 3B). On the basis of the contribution of test BM-derived hematopoiesis in primary recipients, an almost twofold higher frequency of repopulating units was calculated for POL5551 BM (mean ± standard error of the mean: 17.9 ± 1.8 vs 10.8 ± 1.6 relative units per 106 cells in POL5551 BM vs control BM, respectively) (Figure 3C). Furthermore no apparent decline in self-renewal capacity of POL5551-expanded HSPCs was observed, since the POL5551 BM graft also maintained its repopulating advantage in secondary recipients (Figure 3B). Likewise, the more potent mobilization with POL5551 infusion compared with a 5-day course of G-CSF was reflected in a higher competitive repopulating unit frequency as determined by a limiting dilution transplantation assay (Figure 3F-G). In sharp contrast, when genetically CXCR4-ablated BM cells (conditional CXCR4 knockout mice) were injected competitively, a severe repopulating defect was observed beginning at 4 weeks after transplantation, both at 1:1 and 3:1 ratio (Figure 3H-I). Taken together, these data suggest that upon transplantation or soon thereafter, CXCR4 inhibitor-mobilized blood cells or expanded BM regains CXCR4 competency, which then contributes to engraftment in the expected fashion.
Properties of expanded HSPCs. (A) Schematic presentation of competitive transplantation analysis of HSPCs from POL5551 in comparison with G-CSF treated or sham-operated (sham-op) mice. (B-C) Competitive repopulation with differentially treated BM cells. (B) Test BM (n = 3 donor mice; 5 × 105 cells per recipient; CD45.1+) from control mice (sham-op) or mice treated with G-CSF (100 μg/kg per dose, 9 doses every 12 hours) or POL5551 (100 mg/kg per day for 2 weeks via pump) was mixed with competitor BM (n = 4 donor mice; 5 × 105 cells per recipient; CD45.1/2+) and transplanted into lethally irradiated recipients (CD45.2+). PB chimerism analysis of the primary recipients (left) was performed at the indicated time points after transplantation. The percentage of CD45.1+ cells within the graft-derived (ie, CD45.1+ or CD45.1/2+) CD45+CD3– population is shown (data are from 1 of 7 independently performed experiments and represent the mean ± SEM of 5 mice per cohort). At 20 weeks after primary transplantation, BM of the recipients was harvested, pooled, and injected into lethally irradiated secondary (CD45.2+) recipients (2.5 × 106 cells per recipient). Right panel shows PB chimerism measurement in secondary recipients (data represent the mean ± SEM of 5 mice per cohort). (C) The frequency of repopulating units in different test BM suspensions was calculated on the basis of the contribution within the B-cell lymphoid and myeloid (non–T-cell) fraction 16-20 weeks after primary transplant. Data from 7 independently performed transplant experiments (including those presented in Figure 5H) are shown for the control and POL5551-treated BM recipients (mean ± SEM of 34-41 mice per cohort for control and POL treated BM recipients; mean ± SEM of 5 mice in G-CSF BM recipient group). (D-E) Kinetics of noncompetitive engraftment. BM from POL5551 (100 mg/kg per day for 2 weeks of continuous infusion) or sham-op–treated C57BL/6 mice (CD45.2+; 4 donor mice per group) was transplanted noncompetitively into lethally irradiated recipients (CD45.1+; 1 × 106 cells per recipient). Hematopoietic reconstitution was assessed by serial blood count analysis. (D) Neutrophil (NE) and (E) platelet (Plt) counts are shown (data represent the mean ± SEM of 5 recipients per group). (F-G) Competitive repopulating unit (CRU) assay: Small volumes (2, 4, or 8 μL) of POL5551 (100 mg/kg per day for 2 weeks of continuous infusion; pooled from 4 donor mice) or G-CSF (100 μg/kg per dose, 9 doses every 12 hours; pooled from 4 donor mice) mobilized blood (CD45.1+) were cotransplanted with 2.5 × 105 competitor BM cells (CD45.1/2+) into lethally irradiated recipients (CD45.2+; 8-9 mice per dose per mobilizing agent). At 12 weeks after transplantation, CRU engraftment (≥0.5% engraftment in all lineages) was quantified. (F) Percentages of nonengrafted mice were plotted against blood graft volume. Data are represented by f(x) = –5.13+ 103.06 (R2 = 0.91) for G-CSF and f(x) = –7.97x+100.83 for POL5551 (R2 = 0.99). (G) Competitive repopulating unit frequency was determined by using Poisson’s statistic (LCALC software, Stem Cell Technologies) (mean ± upper or lower frequency). (H-I) Genetic blockade of CXCR4 signaling. CXCR4del/del and wild-type (wt) control mice (test BM donors) were generated by injecting tamoxifen (2 sets of 3 consecutive doses) into CXCR4flox/floxERT2Cre+ and CXCR4wt/wtERT2Cre+ mice (both CD45.2+), respectively. (H) CXCR4 expression was quantified via real-time polymerase chain reaction in unfractionated BM (wBM) and sorted HSCs (LSKCD135–CD34–) from test as well as CD45.1/2+ competitor (comp.) BM donors. (I) Two groups of recipients (CD45.1+) received a graft consisting of 5 × 105 test (control or CXCR4del/del; pooled from 3 donor mice each) and competitor BM cells. The 3:1 group (CD45.1+) received a graft composed of 7.5 × 105 CXCR4del/del and 2.5 × 105 competitor cells. Shown is the CD45.2+ percentage within the graft-derived (ie, CD45.2+ or CD45.1/2+) CD45+CD3– population (data represent the mean ± SEM of 3-4 mice per cohort). ***P < .001; **P < .01; *P < .05. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RU, relative unit; Tx, treatment.
Properties of expanded HSPCs. (A) Schematic presentation of competitive transplantation analysis of HSPCs from POL5551 in comparison with G-CSF treated or sham-operated (sham-op) mice. (B-C) Competitive repopulation with differentially treated BM cells. (B) Test BM (n = 3 donor mice; 5 × 105 cells per recipient; CD45.1+) from control mice (sham-op) or mice treated with G-CSF (100 μg/kg per dose, 9 doses every 12 hours) or POL5551 (100 mg/kg per day for 2 weeks via pump) was mixed with competitor BM (n = 4 donor mice; 5 × 105 cells per recipient; CD45.1/2+) and transplanted into lethally irradiated recipients (CD45.2+). PB chimerism analysis of the primary recipients (left) was performed at the indicated time points after transplantation. The percentage of CD45.1+ cells within the graft-derived (ie, CD45.1+ or CD45.1/2+) CD45+CD3– population is shown (data are from 1 of 7 independently performed experiments and represent the mean ± SEM of 5 mice per cohort). At 20 weeks after primary transplantation, BM of the recipients was harvested, pooled, and injected into lethally irradiated secondary (CD45.2+) recipients (2.5 × 106 cells per recipient). Right panel shows PB chimerism measurement in secondary recipients (data represent the mean ± SEM of 5 mice per cohort). (C) The frequency of repopulating units in different test BM suspensions was calculated on the basis of the contribution within the B-cell lymphoid and myeloid (non–T-cell) fraction 16-20 weeks after primary transplant. Data from 7 independently performed transplant experiments (including those presented in Figure 5H) are shown for the control and POL5551-treated BM recipients (mean ± SEM of 34-41 mice per cohort for control and POL treated BM recipients; mean ± SEM of 5 mice in G-CSF BM recipient group). (D-E) Kinetics of noncompetitive engraftment. BM from POL5551 (100 mg/kg per day for 2 weeks of continuous infusion) or sham-op–treated C57BL/6 mice (CD45.2+; 4 donor mice per group) was transplanted noncompetitively into lethally irradiated recipients (CD45.1+; 1 × 106 cells per recipient). Hematopoietic reconstitution was assessed by serial blood count analysis. (D) Neutrophil (NE) and (E) platelet (Plt) counts are shown (data represent the mean ± SEM of 5 recipients per group). (F-G) Competitive repopulating unit (CRU) assay: Small volumes (2, 4, or 8 μL) of POL5551 (100 mg/kg per day for 2 weeks of continuous infusion; pooled from 4 donor mice) or G-CSF (100 μg/kg per dose, 9 doses every 12 hours; pooled from 4 donor mice) mobilized blood (CD45.1+) were cotransplanted with 2.5 × 105 competitor BM cells (CD45.1/2+) into lethally irradiated recipients (CD45.2+; 8-9 mice per dose per mobilizing agent). At 12 weeks after transplantation, CRU engraftment (≥0.5% engraftment in all lineages) was quantified. (F) Percentages of nonengrafted mice were plotted against blood graft volume. Data are represented by f(x) = –5.13+ 103.06 (R2 = 0.91) for G-CSF and f(x) = –7.97x+100.83 for POL5551 (R2 = 0.99). (G) Competitive repopulating unit frequency was determined by using Poisson’s statistic (LCALC software, Stem Cell Technologies) (mean ± upper or lower frequency). (H-I) Genetic blockade of CXCR4 signaling. CXCR4del/del and wild-type (wt) control mice (test BM donors) were generated by injecting tamoxifen (2 sets of 3 consecutive doses) into CXCR4flox/floxERT2Cre+ and CXCR4wt/wtERT2Cre+ mice (both CD45.2+), respectively. (H) CXCR4 expression was quantified via real-time polymerase chain reaction in unfractionated BM (wBM) and sorted HSCs (LSKCD135–CD34–) from test as well as CD45.1/2+ competitor (comp.) BM donors. (I) Two groups of recipients (CD45.1+) received a graft consisting of 5 × 105 test (control or CXCR4del/del; pooled from 3 donor mice each) and competitor BM cells. The 3:1 group (CD45.1+) received a graft composed of 7.5 × 105 CXCR4del/del and 2.5 × 105 competitor cells. Shown is the CD45.2+ percentage within the graft-derived (ie, CD45.2+ or CD45.1/2+) CD45+CD3– population (data represent the mean ± SEM of 3-4 mice per cohort). ***P < .001; **P < .01; *P < .05. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RU, relative unit; Tx, treatment.
POL5551 infusion treatment resulted in a slightly greater expansion within the hematopoietic progenitor (CFU-C and LSK) compartment compared with the more primitive LSK SLAM stem cell fraction (Figure 2C-F). According to published data, initial engraftment derives not from stem cells, but rather from more committed progenitor subsets (LSK and CFU-C) with limited self-renewal capacity.26-28 We therefore reasoned that engraftment of POL5551-expanded BM might be accelerated. Indeed, kinetics of peripheral neutrophil (Figure 3D) and platelet (Figure 3E) count reconstitution after transplantation of lethally irradiated recipients with POL5551-treated BM were significantly faster compared with control BM.
Given the critical role of CXCR4/CXCL12 signaling in B-cell lymphopoiesis,29,30 we evaluated B-cell subsets in the bone marrow of mice after 2 weeks of continuous infusion of POL5551. A substantial reduction of B-cell progenitors (determined as common lymphoid progenitor, B lymphoid progenitor, pre-pro-B cells, pro-B cells, pre-B and pre-B CFU cells) as well as mature B cells (immunoglobulin M–positive mature B and total B220+ cells) was detected in the BM of POL5551-treated mice (supplemental Figure 1). Interestingly, the detrimental effects of continuous CXCR4 signaling perturbation were apparent at earlier stages (common lymphoid progenitor, B lymphoid progenitor, pre-pro-B) compared with G-CSF and, at the same time, slightly less pronounced within the mature B-cell compartment. Of importance, when recipients of differentially treated BM (competitive transplantation) were analyzed for the lineage contribution, no short- or long-term B-cell lineage deficiency was observed (supplemental Figure 1G). Therefore, POL5551 treatment and mediated expansion had no influence on the lineage reconstitution potential of the HSPCs.
CXCR4 blockade results in increased HSPC proliferation
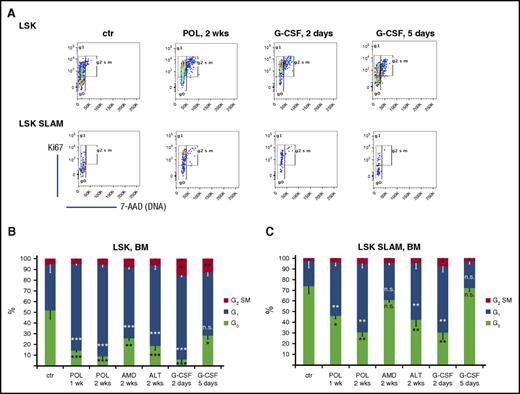
Increased HSPC content after long-term reversible CXCR4/CXCL12 blockade could be a result of better survival (decreased apoptosis and therefore accumulation) and/or decreased differentiation and/or increased as well as skewed toward self-renewal proliferation of the HSPC compartment. We examined the cell cycle status of LSK and LSK SLAM cells on the basis of Ki-67 expression and DNA staining (7-aminoactinomycin D) analysis (Figure 4A). Our analyses revealed that both G-CSF (transiently at days 2 to 3) and CXCR4 inhibitors (at all time points analyzed) induced a twofold to fivefold increase in cycling activity of BM LSK (Figure 4B) and LSK SLAM (Figure 4C) cells. Thus, only 10% to 15% of LSK and 30% to 40% of LSK SLAM cells were found to be quiescent (in G0 phase of the cell cycle) after 1 to 2 weeks of CXCR4 antagonist infusion or 2 days of G-CSF treatment vs 50% to 60% of LSK and 70% of LSK SLAM cells found in G0 under homeostatic conditions (Figure 4). A similar trend in the distribution of the different cell cycle phases was observed in HSPCs isolated from all 3 analyzed hematopoietic tissues (BM, spleen, and peripheral blood), suggesting a lack of preferential mobilization on the basis of the cycling stage of an HSPC (Figure 4; supplemental Figure 2).
Cell cycle analysis. C57BL/6 mice were treated with the CXCR4 antagonists POL5551 (100 mg/kg per day for 1 or 2 weeks), AMD3100 (20 mg/kg per day for 2 weeks), and ALT1188 (33 mg/kg per day for 2 weeks) via subcutaneous infusion. Control groups received G-CSF (100 μg/kg per dose, 4 or 9 doses every 12 hours) or PBS (controls). (A) Representative Ki67 and 7-aminoactinomycin (7-AAD) staining plots gated on LSK (upper panel) or LSK SLAM (lower panel) cells in the BM of differentially treated mice. Distribution of BM (B) LSK and (C) LSK SLAM cells in G0/G1/G2/S/M phases of the cell cycle (data represent the mean ± SEM of 7 mice per cohort). ***P < .001; **P < .01; *P < .05.
Cell cycle analysis. C57BL/6 mice were treated with the CXCR4 antagonists POL5551 (100 mg/kg per day for 1 or 2 weeks), AMD3100 (20 mg/kg per day for 2 weeks), and ALT1188 (33 mg/kg per day for 2 weeks) via subcutaneous infusion. Control groups received G-CSF (100 μg/kg per dose, 4 or 9 doses every 12 hours) or PBS (controls). (A) Representative Ki67 and 7-aminoactinomycin (7-AAD) staining plots gated on LSK (upper panel) or LSK SLAM (lower panel) cells in the BM of differentially treated mice. Distribution of BM (B) LSK and (C) LSK SLAM cells in G0/G1/G2/S/M phases of the cell cycle (data represent the mean ± SEM of 7 mice per cohort). ***P < .001; **P < .01; *P < .05.
Expression profiling of BM LSK SLAM cells from differentially treated mice was assessed by using microarray analyses. Neither principal component (supplemental Figure 3A) nor hierarchical clustering (supplemental Figure 3B) analyses revealed inherent differences between the samples. Of 49 665 detected genes, only 109 were significantly altered (on the basis of the adjusted P value ≤ .05) when comparing POL5551 and untreated HSCs. Within the differentially regulated gene sets, an upregulation of chemokine CXCL12 and cell adhesion molecules VCAM1, ITGA4 (CD49d; α subunit of VLA4), and ITGB1 (CD29; β subunit of VLA4) was observed on POL5551-treated (2 weeks) and G-CSF-treated (5 days) LSK SLAM cells relative to untreated controls (supplemental Figure 3C). Moreover, up- and downregulation of several positive and negative regulators of cycling activity, respectively, were detected (supplemental Figure 3D). Both cyclin D1 and the epigenetic regulator Bmi1 were selectively upregulated in POL5551-treated LSK SLAM cells relative to the untreated and G-CSF controls.
Restoration of steady state hematopoiesis after CXCR4 blockade
We next sought to determine how sustained the effects of POL5551 infusion treatment were. Therefore, the HSPC analyses were performed on days 1, 3, 7, and 14 after pump removal as outlined in Figure 5A. Sham-operated mice as well as a group treated for 2 weeks and analyzed without a subsequent washout period (day 0) were included. HSPCs in circulation dropped to near-homeostatic values within 3 days (80% less within the first 3 days) (Figure 5B) and were indistinguishable from baseline from the next observation (day 7) onward. Similarly, LSK, CFU-C, and LSK-SLAM content in BM was only transiently increased: LSK, CFU-C, and LSK-SLAM levels continued to increase for 3 days, had largely normalized by day 7, and were back to steady state on day 14 after pump removal (Figure 5C-E). A similar trend was observed with regard to the cycling activity of the HSPCs in the BM: twofold to threefold more LSK and LSK SLAM cells were found in G1 phase on days 0, 1, and 3 compared with control mice (Figure 5F). Moreover, significantly fewer HSPCs were actively cycling on day 7, which suggests a compensatory regulation leading to re-establishment of steady state counts along with cycling activity as observed 2 weeks after POL5551 discontinuation. Of note, when assessed in a competitive transplantation setting, only day 0 BM HSPCs displayed a robust repopulating advantage, whereas the contribution of days 1 and 3 BM declined over time (Figure 5G) resulting in lower repopulating unit frequency (Figure 5H). Once again, day 14 BM was indistinguishable from steady state control BM.
Duration of POL5551-induced expansion. (A) Schematic presentation of washout studies. C57BL/6 mice (CD45.2+) were treated with continuous infusion of the CXCR4 antagonist POL5551 (100 mg/kg per day for 2 weeks) and either analyzed directly (day 0 group) or 1, 3, 7, or 14 days after pump removal. Control mice were sham-operated. PB and BM hematopoiesis was analyzed. CFU-C/LSK concentration in (B) blood and (C) BM is shown. (D-E) BM LSK SLAM (D) numbers and (E) frequency are shown. Corresponding cell cycle analysis was performed on BM LSK and LSK SLAM cells and (F) shows G0/G1/G2/S/M phase distribution. (G-H) At the end of the treatment described in (A), BM (test BM) was mixed at a 1:1 ratio with CD45.1/2+ competitor BM (5 × 105 BM cells each per recipient) and transplanted into lethally irradiated CD45.1+ recipients. (G) The CD45.2+ percentage within the graft-derived (ie, CD45.2+ or CD45.1/2+) CD45+CD3– population was analyzed at the indicated time points after transplantation (Tx). (H) At 20 weeks after transplantation, the frequency of repopulating units in test BM suspensions was calculated on the basis of the contribution within the non–T-cell fraction (4-12 mice per treatment [test BM donor] group). The data from control and POL5551-treated BM recipients (Day 0) have also been included in calculations presented in Figure 3C (n = 3 competitor BM donor mice; data represent the mean ± SEM of 5-10 mice per transplant recipient group). ***P < .001; **P < .01; *P < .05.
Duration of POL5551-induced expansion. (A) Schematic presentation of washout studies. C57BL/6 mice (CD45.2+) were treated with continuous infusion of the CXCR4 antagonist POL5551 (100 mg/kg per day for 2 weeks) and either analyzed directly (day 0 group) or 1, 3, 7, or 14 days after pump removal. Control mice were sham-operated. PB and BM hematopoiesis was analyzed. CFU-C/LSK concentration in (B) blood and (C) BM is shown. (D-E) BM LSK SLAM (D) numbers and (E) frequency are shown. Corresponding cell cycle analysis was performed on BM LSK and LSK SLAM cells and (F) shows G0/G1/G2/S/M phase distribution. (G-H) At the end of the treatment described in (A), BM (test BM) was mixed at a 1:1 ratio with CD45.1/2+ competitor BM (5 × 105 BM cells each per recipient) and transplanted into lethally irradiated CD45.1+ recipients. (G) The CD45.2+ percentage within the graft-derived (ie, CD45.2+ or CD45.1/2+) CD45+CD3– population was analyzed at the indicated time points after transplantation (Tx). (H) At 20 weeks after transplantation, the frequency of repopulating units in test BM suspensions was calculated on the basis of the contribution within the non–T-cell fraction (4-12 mice per treatment [test BM donor] group). The data from control and POL5551-treated BM recipients (Day 0) have also been included in calculations presented in Figure 3C (n = 3 competitor BM donor mice; data represent the mean ± SEM of 5-10 mice per transplant recipient group). ***P < .001; **P < .01; *P < .05.
Effects of CXCR4 blockade on BM stroma
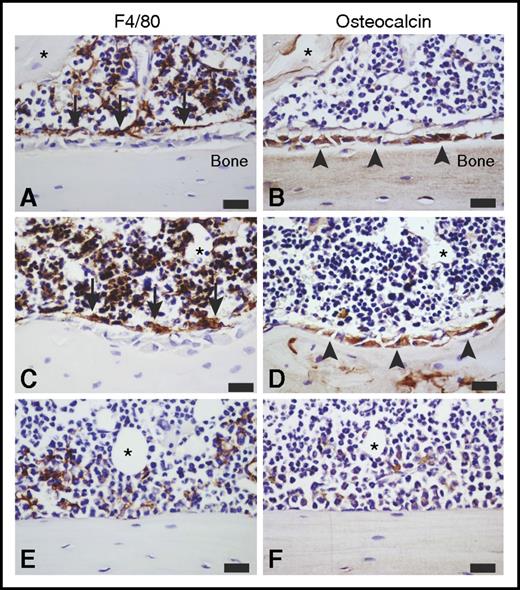
Mobilization with G-CSF has been demonstrated to deplete BM niche components, specifically the bone lining osteoblast lineage cells and macrophages.24,31 We therefore examined the effects of continuous POL5551 infusion (2 weeks) treatment on BM stroma in comparison with saline, a 5-day course of G-CSF, and a bolus POL5551 injection. In contrast to G-CSF, which induced profound depletion of BM osteoblasts and macrophages, no apparent cytological changes were detected in BM exposed to POL5551. The distribution and relative numbers of osteoblast lineage cells and F4/80+ macrophages were largely indistinguishable from those of control mice (Figure 6).
Effects of mobilization and expansion on the BM niche. Representative images of immunohistochemistry staining for F4/80 (brown, left column) and osteocalcin (brown, right column) in serial sagittal cross-sections of the hind limb from C57BL/6 mice treated with (A-B) saline, (C-D) continuous infusion of POL5551 (100 mg/kg per day for 14 days), and (E-F) G-CSF (100 μg/kg per dose, 9 doses every 12 hours) (n = 4). All sections were counterstained with hematoxylin (blue). All images are representative of the endosteal region within the tibial metaphyseal zone. Asterisk in each serial section pair indicates trackable tissue landmarks. (A-D) In saline- and POL5551-treated mice, (B,D, arrowheads) osteocalcin-expressing osteoblasts and (A,C, arrows) F4/80-expressing macrophages can easily be discerned, and they illustrate the F4/80+ osteomac canopy structure associated with the osteoblasts. (E-F) In G-CSF–treated mice, few F4/80+ macrophages are evident, including dramatic loss of (E) osteomac canopy and (F) osteocalcin+ osteoblast-covered bone surface. Original magnification ×60. Scale bars represent 20 μm.
Effects of mobilization and expansion on the BM niche. Representative images of immunohistochemistry staining for F4/80 (brown, left column) and osteocalcin (brown, right column) in serial sagittal cross-sections of the hind limb from C57BL/6 mice treated with (A-B) saline, (C-D) continuous infusion of POL5551 (100 mg/kg per day for 14 days), and (E-F) G-CSF (100 μg/kg per dose, 9 doses every 12 hours) (n = 4). All sections were counterstained with hematoxylin (blue). All images are representative of the endosteal region within the tibial metaphyseal zone. Asterisk in each serial section pair indicates trackable tissue landmarks. (A-D) In saline- and POL5551-treated mice, (B,D, arrowheads) osteocalcin-expressing osteoblasts and (A,C, arrows) F4/80-expressing macrophages can easily be discerned, and they illustrate the F4/80+ osteomac canopy structure associated with the osteoblasts. (E-F) In G-CSF–treated mice, few F4/80+ macrophages are evident, including dramatic loss of (E) osteomac canopy and (F) osteocalcin+ osteoblast-covered bone surface. Original magnification ×60. Scale bars represent 20 μm.
POL5551 treatment improves outcome in a bone marrow failure model
Finally, to test the potential of using continuous CXCR4 antagonist infusion as a strategy to expand HSPC in a clinically relevant setting, we developed a chemotherapy-induced BM damage model. The alkylating agent busulfan (BU) was administered in weekly injections for a period of 4 weeks, whereas control mice received dimethyl sulfoxide (Figure 7A). Eight weeks after the last dose, at the time point when acute response to cytotoxic damage in the form of increased HSPC proliferation ceases (supplemental Figure 4), POL5551 infusion was applied for 2 weeks. Control groups (BU and dimethyl sulfoxide pretreated) were sham-operated. The BM HSPC content was assessed immunophenotypically and showed a restoration of the LSK and LSK SLAM counts upon POL5551 treatment of BU-exposed mice (Figure 7B-C). Furthermore, survival of lethally irradiated transplant recipients was dramatically improved in BU + POL5551 compared with BU only recipients group (Figure 7D). The graph shown in fact overestimates of repopulation capacity of nonexpanded BU-BM, in that 1 of the 2 surviving recipients of BU-BM was almost completely (∼80%) autologously reconstituted (Figure 7E). In contrast, no mixed chimerism (ie, 95% to 100% donor type reconstitution) was found in the recipients of the other 2 groups.
Testing of POL5551-mediated expansion in a disease model. (A) Schematic representation of the BM failure model. Young adult C57BL/6 mice (CD45.2+) were treated with 4 weekly IP doses of the alkylating agent BU (10 mg/kg). Control mice received dimethyl sulfoxide (DMSO). Eight weeks after the last BU/DMSO injection, POL5551 infusion (100 mg/kg per day for 2 weeks) was administered to 1 set of mice from each group and the other set was sham-operated. At the end of the treatment (10 weeks after last BU/DMSO injection), (B) CFU-C/LSK and (C) LSK SLAM numbers in BM were measured. Data represent the mean ± SEM of 5 mice per group. (D) BM from mice (CD45.2+ as in [A]) treated with DMSO (ctr), BU, or BU + POL5551 was transplanted noncompetitively into CD45.1+ or CD45.1/2+ hosts (1 × 106 BM cells per recipient). Survival was monitored for the indicated time period (8 recipients per group at experiment start). Forty weeks later, BM composition of the recipients was analyzed. (E) Measured absolute WBC counts in the BM are shown. ***P < .001; **P < .01; *P < .05.
Testing of POL5551-mediated expansion in a disease model. (A) Schematic representation of the BM failure model. Young adult C57BL/6 mice (CD45.2+) were treated with 4 weekly IP doses of the alkylating agent BU (10 mg/kg). Control mice received dimethyl sulfoxide (DMSO). Eight weeks after the last BU/DMSO injection, POL5551 infusion (100 mg/kg per day for 2 weeks) was administered to 1 set of mice from each group and the other set was sham-operated. At the end of the treatment (10 weeks after last BU/DMSO injection), (B) CFU-C/LSK and (C) LSK SLAM numbers in BM were measured. Data represent the mean ± SEM of 5 mice per group. (D) BM from mice (CD45.2+ as in [A]) treated with DMSO (ctr), BU, or BU + POL5551 was transplanted noncompetitively into CD45.1+ or CD45.1/2+ hosts (1 × 106 BM cells per recipient). Survival was monitored for the indicated time period (8 recipients per group at experiment start). Forty weeks later, BM composition of the recipients was analyzed. (E) Measured absolute WBC counts in the BM are shown. ***P < .001; **P < .01; *P < .05.
Discussion
In this study, the effects of continuous pharmacologic inhibition of CXCR4/CXCL12 signaling were investigated. Similar to what has been previously reported when comparing 1 week of continuous infusion of AMD3100 to a bolus injection of the inhibitor,32 we observed a sustained increase in mobilization efficiency when the infusion period was extended to 2 weeks. This was apparent with all 3 CXCR4 antagonists tested. Improved pharmacokinetics as a result of better protection against proteolytic degradation has been proposed to underlie the significantly amplified mobilization response associated with continuous infusion treatment. While in agreement with this, our findings further suggest a contribution of increased proliferation induced within the BM HSPC pool by prolonged blockade of CXCR4 signaling to the magnitude and maintenance of mobilization response.
The relative independence of CXCR4/CXCL12 and VLA4/VCAM1 axes in promoting retention of HSPCs in the BM was previously established.21,33 Our data showing that the dramatic mobilization induced by continuous infusion of a CXCR4 antagonist could be further enhanced through addition of a VLA4 antagonist confirm the lack of redundancy between the 2 pathways. Moreover, we speculate that the upregulation of VCAM1/VLA4 expression (along with CXCR4/CXCL12 itself), as detected here via microarray analyses, may serve to counteract the robust HSPC egress that occurs during the course of continuous treatment with a CXCR4 inhibitor. It may in fact increase the relative contribution of this pathway to HSC anchorage or increase the number of HSCs depending on this pathway for retention and thus being amenable to mobilization after blockade thereof. Indeed, although the VLA4 antagonist by itself mobilized 1 to 2 × 103 CFU-C cells per mL (data not shown), its combination with POL5551 infusion mobilized more than 4 × 104 CFU-C cells per mL (as the difference between 11 × 104 with combination POL5551 infusion plus CWHM-823 compared with 7 × 104 with POL5551 infusion alone).
Although controversial with regard to the postulated role of CXCR4/CXCL12 signaling for HSPC survival (and homing), thus far all studies performed with genetically Cxcr4-deleted HSPCs, including the one described here, demonstrated a requirement of this axis for efficient engraftment and subsequent repopulation of lethally irradiated recipients.10,13,34 In contrast to this, yet in line with immunophenotypic analyses, transiently pharmacologically CXCR4-inhibited BM as generated here via continuous infusion of POL5551, displayed an approximately twofold higher repopulating capacity, indicating no adverse effects of the treatment on the engraftment capacity of the HSPCs.
Given the unique ability of HSPCs to provide a life-long supply of hematopoietic cells, their cycling activity needs to be subject to tight but adjustable regulation.35,36 Several pieces of evidence from studies with human and murine cells, as well as mouse models, had implied that the CXCR4/CXCL12 axis as a critical mediator of HSPC quiescence.12-14,37,38 In fact, AMD3100 has been shown to facilitate hematopoietic reconstitution after myeloablation and transplantation in part through induction of greater HSPC division.39,40 Our data showing dramatically increased cycling as a result of prolonged pharmacologic blockade of CXCR4 therefore confirm a role for this pathway as a brake for HSPC proliferation. Moreover, in direct comparison with G-CSF–induced proliferation, treatment with a CXCR4 inhibitor seems to favor self-renewing as opposed to differentiating cell division, despite showing a very similar cell cycle distribution. Thus both increased proliferation and relative skewing toward self-renewal may have contributed to the HSPC expansion observed with continuous CXCR4 inhibition and not with G-CSF. An alternative explanation is that continuous CXCR4 inhibition resulted in decreased apoptosis. However, this scenario seems not physiological since it would require substantially faster turnover kinetics of HSPCs at steady state than has been appreciated so far.41-43
Considering the distinct repopulating advantage of CXCR4 antagonist–expanded HSPCs, they may represent an example of HSPC proliferation not accompanied by exhaustion. A similar phenotype was reported for cyclin-dependent kinase inhibitor (CKI) p18-deficient HSPCs.44 p18Inc4c inhibits G1 phase entry, which has also been proposed for CXCR4/CXCL12 signaling.13 Consistent with less restricted G0→G1 transition, we detected increased levels of cyclin D1 (as also reported for genetic CXCR4 ablation13 ) and CDK4/6 as well as Bmi1 in POL5551-treated HSCs, whereas the CKIs p15, p16, and p18 seemed downregulated.
In the course of washout experiments, increased HSC (LSK SLAM) and hematopoietic progenitor cell (LSK and CFU-C) numbers in BM were detected for up to 3 and 7 days following discontinuation of the POL5551 infusion, respectively. However the competitive advantage of BM harvested 3 days after removal of the inhibitor (similar to the day 7 graft) was lost rather early (4-6 weeks; Figure 5G-H) after transplantation. This suggests that despite the identical immunophenotypes of day 0 vs day 3 HSCs, the latter may have been temporarily functionally compromised. One plausible explanation is an induction of transient changes in expanding HSCs and the BM niche, initiated as negative feedback mechanisms to counteract excessive HSC dislodgement as well as proliferation and/or set in motion to restore the niche size after termination of CXCR4 inhibitor treatment. As an example, the responsiveness of HSCs toward proliferative stimuli required for efficient engraftment might have been diminished. Furthermore, expression of known mediators of stemness was possibly altered. Our data thus underscore the importance of the CXCR4/CXCL12 axis in maintaining this stable and seemingly optimal number of HSCs in a homeostatic BM because disruption of this pathway facilitates enlargement of the HSC pool.
Because of the expression of CXCR4 on the surface of the HSPCs, their direct targeting in the course of CXCR4 antagonist administration seems plausible. However, because the receptor is also expressed on different stromal cell populations,45,46 hematopoietic cell extrinsic effects and/or contributions thereof to the observed phenotype cannot be excluded. That said, prolonged blockade of CXCR4 is, to the best of our knowledge, the first pharmacologic approach for in vivo expansion of HSPCs through their direct targeting described to date. For example, treatment with parathyroid hormone47,48 and insulin-like growth factor 149 was associated with (and likely mechanistically caused by) major morphologic changes within BM, including increased (ectopic) formation of trabecular bone, whereas no apparent changes in BM stroma composition and/or structure were found after POL5551 infusion. Moreover, cytologic changes typically associated with G-CSF–induced mobilization (eg, depletion of bone lining osteolineage cells and macrophages)24 were not detected in BM exposed to continuous infusion, which suggests limited effects within the BM niche compartment. Rather, like G-CSF,29,30 continuous CXCR4 blockade resulted in a reduction of BM-associated B-cell progenitors, further illustrating the importance of the CXCR4/CXCL12 axis for B-cell lymphopoiesis.
The re-establishment of steady state HSPC numbers as well as cycling activity was observed 2 weeks after discontinuing infusion. This rapidly reversible nature of induced HSPC expansion made it convenient to evaluate the efficacy of continuous CXCR4 blockade in a model of chemotherapy-damaged BM. The alkylating agent BU was chosen for this study because of its long-term detrimental effects on the murine HSPC compartment while causing limited damage to the BM stroma.50 Thus, we established a BU-based cytotoxic regimen that was associated with severe HSC impairment. The number of phenotypic HSPCs, the number of clonogenic cells and, most pronounced, the radioprotective/repopulating capacity of the marrow from BU-treated mice was irreversibly dramatically reduced. In this model, a 2-week infusion of POL5551 restored the number of immunophenotypically defined HSCs to normal. Moreover, beneficial effects of POL5551 treatment were reflected in significantly improved survival and reconstitution of BU plus POL5551 compared with BU alone BM transplant recipients. This observation, along with increasing evidence of direct and leukemia cell–specific cytotoxic effects of CXCR4 inhibition,51-53 suggests that prolonged CXCR4 blockade via infusion or, given sufficiently stable pharmacokinetics, multiple injections, could be developed as a novel therapeutic avenue alone or in conjunction with chemotherapy for treatment of hematologic malignancies.
In summary, we show that prolonged pharmacologic blockade of CXCR4/CXCL12 signaling leads to superior mobilization of HSPCs, including true stem cells, along with their dramatic expansion in the bone marrow. The latter is likely a result of the increased proliferation and was not associated with an exhaustion of the stem cell pool, because POL5551-expanded HSPCs engrafted efficiently in primary and secondary transplant recipients. Continuous infusion of CXCR4 inhibitors therefore represents a novel pharmacologic model of reversible mobilization and in vivo expansion of HSPCs and may serve as a strategy to restore the HSPC pool after cytotoxic damage or as a method of chemotherapy sensitization of hematologic malignancies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine for help with genomic analysis and the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO, for the use of the Siteman Flow Cytometry Core; Michel Schmitt, Marie-Anne Westwood, and Caroline Kolopp for the pharmacokinetic data provided by Polyphor; and Jean-Pierre Levesque and Daniel Link for revising the manuscript and providing helpful comments and discussion.
This work was supported by Grants No. R01CA83845, R01CA152329, R21CA110489, R21CA132269, R21CA141523, P01CA101937, and P50CA94056 from the National Institutes of Health (NIH), National Cancer Institute (NCI) (J.F.D.). D.K. is a past scholar of the German Academic Exchange Service (postdoctoral fellowship 57054578 [2014-2016]). H.B. is a member of LOEWE Cell and Gene Therapy Frankfurt faculty, funded by Hessian Ministry of Higher Education, Research and the Arts (III L 4-518/17.004 [2013]) and was also supported by Deutsche Forschungsgemeinschaft grant BO3553/1-1. The Siteman Cancer Center is partially supported by NCI Cancer Center Support Grant No. P30CA91842 and by Institute of Clinical and Translational Sciences/Clinical and Translational Science Award Grant No. UL1RR024992 from the NIH, National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research.
This publication is solely the responsibility of the authors and does not necessarily represent the official view of NCRR or NIH.
Authorship
Contribution: D.K., J.K.R., M.S.H., G.A.-E., D.M., L.B., S.M., G.S., E.W., E.C., S.C., and L.G. performed experiments; D.K. analyzed data; W.Y. performed microarray data processing and analysis; D.K., L.G.S., K.D., A.R.P., M.P.R., H.B., and J.F.D. designed the research; D.K., M.P.R., H.B., and J.F.D. wrote the manuscript; and all authors discussed, commented on, and approved the final version of the manuscript.
Conflict-of-interest disclosure: K.D. is an employee of Polyphor, which provided POL5551. The remaining authors declare no competing financial interests.
Correspondence: John F. DiPersio, Division of Oncology, Department of Medicine, Washington University School of Medicine, 660 S. Euclid Ave, Campus Box 8007, St. Louis, MO 63110; e-mail: jdipersi@wustl.edu.
References
Author notes
H.B. and J.F.D. contributed equally to this study.

![Figure 1. Efficacy of CXCR4 antagonist–based mobilization regimen. (A-C) Comparison of continuous infusion (cont. inf.) and bolus injection. The CXCR4 antagonists POL5551 (POL), AMD3100 (AMD), and ALT1188 (ALT) were administered to C57BL/6 mice as a single intraperitoneal (IP) injection (POL5551, 100 mg/kg; AMD3100, 20 mg/kg; ALT1188, 33 mg/kg) or as a continuous infusion for 2 weeks via subcutaneously implanted pumps (POL5551, 100 mg/kg per day; AMD3100, 20 mg/kg per day; ALT1188, 33 mg/kg per day). Peripheral blood (PB) was analyzed (at 3 hours after injection of bolus POL5551 and ALT1188, at 1 hour after injection of bolus AMD3100, at day 14 [d14] for all groups treated by continuous infusion) for (A) CFU-C/LSK, (B) LSK SLAM, and (C) white blood cell (WBC) concentration. Corresponding counts from age- and sex-matched healthy male mice injected with vehicle or sham-operated are shown as control (ctr.) mean ± standard error of the mean (SEM) (n = 5-10 mice for treated groups; n = 15 for control mice). (D) Pharmacokinetics. C57BL/6 mice were treated with a continuous infusion of POL5551 (50 mg/kg per day for 1 week). Plasma concentration of POL5551 (solid line) was analyzed at the indicated time points. Corresponding CFU-C numbers (dotted line) are shown for comparison. (E-F) Combination of CXCR4 and VLA4 blockade. At the end of treatment with continuous infusion of POL5551 as described in (A-C), mice were randomly assigned to receive either an IP bolus injection of AMD3100 (20 mg/kg) or the VLA4 antagonist CWHM-823 (3 mg/kg). One hour later, (E) CFU-C/LSK and (F) LSK SLAM numbers were quantified. CFU-C, LSK, and LSK SLAM counts obtained before the bolus treatment (ie, from corresponding POL5551 infusion–treated mice [A-C]) are shown for comparison (mean ± SEM; n = 5). ***P < .001; **P < .01; *P < .05. appr., approximately; n.s., not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/21/10.1182_blood-2016-10-746909/4/m_blood746909f1.jpeg?Expires=1767731496&Signature=nL7KrNlVGJNz67s4iSkNtWBqiAH3IFquCHtesVygLTwRjwm~V4taHxOljCNgxwf1LouR6B3fwm0Zk0Bd4Ztv1tGXsTAf0u9sT5OXvBy-dnikzAg~tyQks~BXyjmGPnilSMBltJSq3r6gM18ahWCILJ83RMNaT59n3NvJ3eXc9gp6pbFcIUwFL4lUp-3~XOMZWHpPyIiuCS1Zo~PUSYB4tpcBagKEozEf2J~7CBMHBbUMpQj0Zeo5ZqKRqwdk1j~2O79PzH72fSUH3saHURSN1Y1pOUDCLk~RmYdiyZTPuMgf2jgWtnBPisNLow8YdKSZIhxdYDKW6jCo96Y~698E9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Mobilization and expansion. C57BL/6 mice were treated with the CXCR4 antagonists POL5551 (100 mg/kg per day for 1 or 2 weeks [wks]), AMD3100 (20 mg/kg per day for 2 weeks,), and ALT1188 (ALT; 33 mg/kg per day for 2 weeks) via subcutaneous infusion pumps. Control groups received G-CSF (100 μg/kg per dose at 4 or 9 doses every 12 hours) or phosphate-buffered saline (PBS). Concurrent analysis of hematopoiesis in PB and BM was performed. (A) WBC concentration in PB and BM. Corresponding CFU-C/LSK numbers are presented in (B) PB and (C) BM. (D) CFU-C/LSK frequency in BM, (E) LSK SLAM count, and (F) LSK SLAM frequency are shown. Representative flow cytometry analyses of LSK and LSK SLAM fraction in (G) PB and (H) BM of differentially treated mice are shown (mean ± SEM; n = 3). ***P < .001; **P < .01; *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/21/10.1182_blood-2016-10-746909/4/m_blood746909f2.jpeg?Expires=1767731496&Signature=2THeBrdifhfQMj17a0-vWjtQh4uCOK2ZCycTkBrEQ26bS5aQ0QL2WtAjh~78z7UfDCQmD9JqBeL21bFwvMjjkA~CuXKfJb29LR8cU6h8AmOK2tUq3c8cBngnDUF0bPtPxSZYasQ0ZwLjNPyc837WTqCvLyS7GEYxUGtBYIcn3xWP6wdPxILEGIiDybgvhcM9cfm7R9~EK5NkxXxb5Bu7Egz1GewtERKIBug3UChgN-NMNo2RL4ZrirIEz4MXC0JlU1csAgLAUl34pkyXw2ioICfcSiUyGtaZglhrSm30BRnNxVjTQ-Soh-r4ncQ-B-cFCrdOoiluhKaG5oHXBLR8mg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Duration of POL5551-induced expansion. (A) Schematic presentation of washout studies. C57BL/6 mice (CD45.2+) were treated with continuous infusion of the CXCR4 antagonist POL5551 (100 mg/kg per day for 2 weeks) and either analyzed directly (day 0 group) or 1, 3, 7, or 14 days after pump removal. Control mice were sham-operated. PB and BM hematopoiesis was analyzed. CFU-C/LSK concentration in (B) blood and (C) BM is shown. (D-E) BM LSK SLAM (D) numbers and (E) frequency are shown. Corresponding cell cycle analysis was performed on BM LSK and LSK SLAM cells and (F) shows G0/G1/G2/S/M phase distribution. (G-H) At the end of the treatment described in (A), BM (test BM) was mixed at a 1:1 ratio with CD45.1/2+ competitor BM (5 × 105 BM cells each per recipient) and transplanted into lethally irradiated CD45.1+ recipients. (G) The CD45.2+ percentage within the graft-derived (ie, CD45.2+ or CD45.1/2+) CD45+CD3– population was analyzed at the indicated time points after transplantation (Tx). (H) At 20 weeks after transplantation, the frequency of repopulating units in test BM suspensions was calculated on the basis of the contribution within the non–T-cell fraction (4-12 mice per treatment [test BM donor] group). The data from control and POL5551-treated BM recipients (Day 0) have also been included in calculations presented in Figure 3C (n = 3 competitor BM donor mice; data represent the mean ± SEM of 5-10 mice per transplant recipient group). ***P < .001; **P < .01; *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/21/10.1182_blood-2016-10-746909/4/m_blood746909f5.jpeg?Expires=1767731496&Signature=sQgNjJHkzblDq3J8D1VYkDdnxebOmj17v4A6nc3UUtWWFSsqiekYg97swd-VEu8v3qxQlWCYNV4RT-HNLMTVVHWUjbPkky1tAF1M5Xm71P-mwJysTkFsrT0i~b9vOhhnObDJbdb0uvl4OHhwcxiMrK4noUtQsMaFYZRs4XbcMCiaUrBTQ6PkBmZAfLATCJKNw2F9R4Cnbq6qrBmeRCPvzVREEbP~KEBBbA09jeubqX0k0nTNa5lY8VWAGghx~DjRVbKpLW6dI1jZuTQrnhWCIGeLd-6dYAwm5SsMN6ZVFf7lZfxjk7MSLx2V0bG2FcurjPQ~GnLtgkYT0hH4GZfCSw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. Testing of POL5551-mediated expansion in a disease model. (A) Schematic representation of the BM failure model. Young adult C57BL/6 mice (CD45.2+) were treated with 4 weekly IP doses of the alkylating agent BU (10 mg/kg). Control mice received dimethyl sulfoxide (DMSO). Eight weeks after the last BU/DMSO injection, POL5551 infusion (100 mg/kg per day for 2 weeks) was administered to 1 set of mice from each group and the other set was sham-operated. At the end of the treatment (10 weeks after last BU/DMSO injection), (B) CFU-C/LSK and (C) LSK SLAM numbers in BM were measured. Data represent the mean ± SEM of 5 mice per group. (D) BM from mice (CD45.2+ as in [A]) treated with DMSO (ctr), BU, or BU + POL5551 was transplanted noncompetitively into CD45.1+ or CD45.1/2+ hosts (1 × 106 BM cells per recipient). Survival was monitored for the indicated time period (8 recipients per group at experiment start). Forty weeks later, BM composition of the recipients was analyzed. (E) Measured absolute WBC counts in the BM are shown. ***P < .001; **P < .01; *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/21/10.1182_blood-2016-10-746909/4/m_blood746909f7.jpeg?Expires=1767731496&Signature=Olof-JG1cUxWCWJ1Xv-GQWv0KG0xEWvF1KmBu1S-wG~BWf0BjyY6FM9RvezGFFTUQbOCEOL94DS5K8GO8wHfqp~7cOToggDaInIfE2cVqdu-PbTrUGp~sU6LH3ueIwvU-De2uyKvyojmH5fi1veT2HMJ5B1OFJACiU-sry8cX53Oz1YfQk5OAz8wZoOhF724RZ9jrjtmh~EISL6z~eOREW0j6LItWRsX6nHo~PvB58sT6KYNYZac4oUbhVgYiJIrhyunU1N~In2ecAiU7WUO~KZOaUo8dnushNWCGpYszu~bdY9w7m0FRlqwE8ui3C3CZM~VyjO0Kjjbd4DhCPUZww__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 1. Efficacy of CXCR4 antagonist–based mobilization regimen. (A-C) Comparison of continuous infusion (cont. inf.) and bolus injection. The CXCR4 antagonists POL5551 (POL), AMD3100 (AMD), and ALT1188 (ALT) were administered to C57BL/6 mice as a single intraperitoneal (IP) injection (POL5551, 100 mg/kg; AMD3100, 20 mg/kg; ALT1188, 33 mg/kg) or as a continuous infusion for 2 weeks via subcutaneously implanted pumps (POL5551, 100 mg/kg per day; AMD3100, 20 mg/kg per day; ALT1188, 33 mg/kg per day). Peripheral blood (PB) was analyzed (at 3 hours after injection of bolus POL5551 and ALT1188, at 1 hour after injection of bolus AMD3100, at day 14 [d14] for all groups treated by continuous infusion) for (A) CFU-C/LSK, (B) LSK SLAM, and (C) white blood cell (WBC) concentration. Corresponding counts from age- and sex-matched healthy male mice injected with vehicle or sham-operated are shown as control (ctr.) mean ± standard error of the mean (SEM) (n = 5-10 mice for treated groups; n = 15 for control mice). (D) Pharmacokinetics. C57BL/6 mice were treated with a continuous infusion of POL5551 (50 mg/kg per day for 1 week). Plasma concentration of POL5551 (solid line) was analyzed at the indicated time points. Corresponding CFU-C numbers (dotted line) are shown for comparison. (E-F) Combination of CXCR4 and VLA4 blockade. At the end of treatment with continuous infusion of POL5551 as described in (A-C), mice were randomly assigned to receive either an IP bolus injection of AMD3100 (20 mg/kg) or the VLA4 antagonist CWHM-823 (3 mg/kg). One hour later, (E) CFU-C/LSK and (F) LSK SLAM numbers were quantified. CFU-C, LSK, and LSK SLAM counts obtained before the bolus treatment (ie, from corresponding POL5551 infusion–treated mice [A-C]) are shown for comparison (mean ± SEM; n = 5). ***P < .001; **P < .01; *P < .05. appr., approximately; n.s., not significant.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/21/10.1182_blood-2016-10-746909/4/m_blood746909f1.jpeg?Expires=1767731497&Signature=d9GTfhidChLmUscZKzunTW7Lnw8Tinjgn-nz251VssT8cQx3QTYUM6Pdrrb2VvEUCxqAsiJFy5ACgDlyh5WNqMgETDyOJzwThqv2CDNox1-~LihFOisxA5ZaFtr5cNI-iEI55WxX~aEpW5~fktd657fiOGKQ8XkGDPZ-Amb8H9cNhimXOO0SHCcdH7YLb4MheSQwXzodLOg8pra8K5zk~ihTmPIpGdzjNP1jcfDU~Jfxvla0Dq8DQsyPjpjY9M5JX0689LGK0ECaffIUo6b-rv-rUURipFMnN1beci42rDEsD6yh48K-xaq~jzSvEgtp3bA9SUppQrXissxDCjFCUA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Mobilization and expansion. C57BL/6 mice were treated with the CXCR4 antagonists POL5551 (100 mg/kg per day for 1 or 2 weeks [wks]), AMD3100 (20 mg/kg per day for 2 weeks,), and ALT1188 (ALT; 33 mg/kg per day for 2 weeks) via subcutaneous infusion pumps. Control groups received G-CSF (100 μg/kg per dose at 4 or 9 doses every 12 hours) or phosphate-buffered saline (PBS). Concurrent analysis of hematopoiesis in PB and BM was performed. (A) WBC concentration in PB and BM. Corresponding CFU-C/LSK numbers are presented in (B) PB and (C) BM. (D) CFU-C/LSK frequency in BM, (E) LSK SLAM count, and (F) LSK SLAM frequency are shown. Representative flow cytometry analyses of LSK and LSK SLAM fraction in (G) PB and (H) BM of differentially treated mice are shown (mean ± SEM; n = 3). ***P < .001; **P < .01; *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/21/10.1182_blood-2016-10-746909/4/m_blood746909f2.jpeg?Expires=1767731497&Signature=d4-3F7EbqVtpb5~QGi5DqFP~PAA8pcdO5ty~JvHNNjIYGhA0xinwRtrbRoNqJdnZgZnFEBTWYrxdrd-hbgGp0ZDSDM~437fJ2BwSkC4fsqCNvd-Dd8wPgIrWJ2YAhOcxaE~uhTIFjZjcF1So7RYBKM0wm0O1KXqkjHfHovkQ9VZISUTpnIGE4jNqUe0bjoRQU72OUDpAmr-ilw0usUSS15SEPRTdo9FgIkLmNK6I2SDVJFQhG57mExifmt0Vhx3f6wksmyVfLOe4mz7f1TJsvlQytFAa6YUMYjyCvLdPxG7-9NMUezhMqvDEPvIleQ5EOF0pPDh8l3Adc77wKFFcSA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 5. Duration of POL5551-induced expansion. (A) Schematic presentation of washout studies. C57BL/6 mice (CD45.2+) were treated with continuous infusion of the CXCR4 antagonist POL5551 (100 mg/kg per day for 2 weeks) and either analyzed directly (day 0 group) or 1, 3, 7, or 14 days after pump removal. Control mice were sham-operated. PB and BM hematopoiesis was analyzed. CFU-C/LSK concentration in (B) blood and (C) BM is shown. (D-E) BM LSK SLAM (D) numbers and (E) frequency are shown. Corresponding cell cycle analysis was performed on BM LSK and LSK SLAM cells and (F) shows G0/G1/G2/S/M phase distribution. (G-H) At the end of the treatment described in (A), BM (test BM) was mixed at a 1:1 ratio with CD45.1/2+ competitor BM (5 × 105 BM cells each per recipient) and transplanted into lethally irradiated CD45.1+ recipients. (G) The CD45.2+ percentage within the graft-derived (ie, CD45.2+ or CD45.1/2+) CD45+CD3– population was analyzed at the indicated time points after transplantation (Tx). (H) At 20 weeks after transplantation, the frequency of repopulating units in test BM suspensions was calculated on the basis of the contribution within the non–T-cell fraction (4-12 mice per treatment [test BM donor] group). The data from control and POL5551-treated BM recipients (Day 0) have also been included in calculations presented in Figure 3C (n = 3 competitor BM donor mice; data represent the mean ± SEM of 5-10 mice per transplant recipient group). ***P < .001; **P < .01; *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/21/10.1182_blood-2016-10-746909/4/m_blood746909f5.jpeg?Expires=1767731497&Signature=NPfhcckQAElPr7coaxgrtnXRW7bI9aQxJu1irFY8fPdtcykwceL6QOxcPIlOClv-F7ktKnI-XEKcJpYDQxOewd9jXr-07DDVnCzdk4KSr6PEspymM2RIJTAaF4K6yxsOMMYS7wzVw4KhK~4~cQ7k6r1XxBv6NTTZD3-5yCITojW5qhHBUOo6P4UOz8KZDpOeBygbHSjCJYPp-UKaRitkJ4BhgtV3YeOROyVidQ4h6ElrwJRamFpJZlzfPGeJhqKUQNYu2N7hOABE9uTErbeIwz7TeVezVPhHdE42E1H43E8uk2-q6HTvXWRNkFAM7LHydCJUCzzM01hdlHT0skyR-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 7. Testing of POL5551-mediated expansion in a disease model. (A) Schematic representation of the BM failure model. Young adult C57BL/6 mice (CD45.2+) were treated with 4 weekly IP doses of the alkylating agent BU (10 mg/kg). Control mice received dimethyl sulfoxide (DMSO). Eight weeks after the last BU/DMSO injection, POL5551 infusion (100 mg/kg per day for 2 weeks) was administered to 1 set of mice from each group and the other set was sham-operated. At the end of the treatment (10 weeks after last BU/DMSO injection), (B) CFU-C/LSK and (C) LSK SLAM numbers in BM were measured. Data represent the mean ± SEM of 5 mice per group. (D) BM from mice (CD45.2+ as in [A]) treated with DMSO (ctr), BU, or BU + POL5551 was transplanted noncompetitively into CD45.1+ or CD45.1/2+ hosts (1 × 106 BM cells per recipient). Survival was monitored for the indicated time period (8 recipients per group at experiment start). Forty weeks later, BM composition of the recipients was analyzed. (E) Measured absolute WBC counts in the BM are shown. ***P < .001; **P < .01; *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/21/10.1182_blood-2016-10-746909/4/m_blood746909f7.jpeg?Expires=1767731497&Signature=AEkBrcxED4p~8ecUvPJIbgeqUsJ26eZkEnTNiNUhEd5oyPjwf4rOD0nb63Ov1AtSwOT1HkrveZogiMju~VWyKti-sqwetxD6Datyc95Wp-q~6yDFAHFq9RfTNUo6Bezk-ywTP6yrfk47b3gAhXBJR0g5Hde9Wz4H1KwQa3R6AyHS8c02szy5HADBDzRMQ4Iy08toJ4yigaqNG1fMnMZH7D~n7tw7mTOYGnIlxdnGk7213ONkR6DOMcMEIeAa~77ju6EpColCOTdSEKcqAgvO-F0Ue5PwSljFVDuNSfR0ziz~eD~cGxB4ljXlO8fBRHOp45fjywqTtxpXgiJr14J9yQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)