Key Points
Zeb2 controls stem cell pool size and lineage fidelity.
Zeb2 deletion promotes a myeloproliferative phenotype resembling the early stage of primary myelofibrosis.
Abstract
Epithelial-to-mesenchymal-transition (EMT) is critical for normal embryogenesis and effective postnatal wound healing, but is also associated with cancer metastasis. SNAIL, ZEB, and TWIST families of transcription factors are key modulators of the EMT process, but their precise roles in adult hematopoietic development and homeostasis remain unclear. Here we report that genetic inactivation of Zeb2 results in increased frequency of stem and progenitor subpopulations within the bone marrow (BM) and spleen and that these changes accompany differentiation defects in multiple hematopoietic cell lineages. We found no evidence that Zeb2 is critical for hematopoietic stem cell self-renewal capacity. However, knocking out Zeb2 in the BM promoted a phenotype with several features that resemble human myeloproliferative disorders, such as BM fibrosis, splenomegaly, and extramedullary hematopoiesis. Global gene expression and intracellular signal transduction analysis revealed perturbations in specific cytokine and cytokine receptor–related signaling pathways following Zeb2 loss, especially the JAK-STAT and extracellular signal-regulated kinase pathways. Moreover, we detected some previously unknown mutations within the human Zeb2 gene (ZFX1B locus) from patients with myeloid disease. Collectively, our results demonstrate that Zeb2 controls adult hematopoietic differentiation and lineage fidelity through widespread modulation of dominant signaling pathways that may contribute to blood disorders.
Introduction
Hematopoietic stem cells (HSCs) replenish the adult animal with highly specialized blood cells across their lifespan through a process that requires both strict regulation of self-renewal vs differentiation in the progenitor compartment of the bone marrow (BM) as well as flexibility to modulate blood production to meet short-term needs. A variety of factors regulates identity, fate, and activities of HSCs, ranging from cell-intrinsic HSC proteins to extrinsic signals provided by the microenvironment of the stem cell niche. The precise balance between such activating and repressive factors guide HSC responses to these 2 types of cues. A complex network of transcription factors and cooperating chromatin modulators,1-4 which together regulate target gene expression, modulates this balance.
Zeb2, a member of the zinc-finger E-box–binding family of transcription factors,5,6 plays important roles during embryonic development as an epithelial-to-mesenchymal-transition (EMT) modulator,7,8 but also melanocyte,9 neuronal, and oligodendrocyte10-12 cell fate. Zeb2 also influences tumor progression and metastasis because high levels of Zeb2 messenger RNA (mRNA) correlate with poor prognosis and patient outcome.6,13-15 Upregulated expression of EMT transcription factors, including Zeb2, stimulate the acquisition of cancer stem cell properties.16-18 We hypothesized that EMT transcription factors are also essential for adult stem cell function under physiological conditions, such as homeostatic regulation in various tissues, including the adult hematopoietic system.
Previously, we reported that selective inactivation of the Zeb2 allele in the murine hematopoietic compartment resulted in early embryonic lethality around E12.5 (using the Tie2-Cre approach) or neonatal lethality (using Vav-iCre) resulting from intracephalic hemorrhages.19 After colonizing the fetal liver, Zeb2-deficient hematopoietic stem/progenitor cells (HSPCs) exhibit altered adhesion and homing properties, and fail to reenter the blood circulation to colonize the BM cavity.19
Here, we report that Zeb2 inactivation in the adult hematopoietic system leads to severe differentiation defects of hematopoietic cells in a stage-dependent manner, accompanied by HSPCs accumulation and pronounced extramedullary hematopoiesis. The combination of maturation defects and increased myeloproliferation in the absence of Zeb2 resembles human myeloproliferative disorders. Moreover, Zeb2 inactivation within the HSPCs correlates with changes in expression of genes involved in cytokine receptor signaling. These changes modulate signal transduction and affect the differentiation of multiple hematopoietic lineages. Finally, mutation analyses performed on BM samples from patients with myeloid malignancies revealed several unknown point mutations within the Zeb2 gene, which might affect Zeb2 function in humans.
Methods
Mice and genetic mouse intercrosses
The generation of the conditional Zeb2-knockout allele and Mx1-Cre transgenic mice were described previously.20,21 For this study both strains were backcrossed to a C57Bl/6 genetic background for at least 6 generations. Zeb2fl/fl mice were intercrossed with Mx1Cre transgenic mice and bred in-house in a pathogen-free facility. All animal experiments were approved by animal care committees (Landesamt für Natur, Umwelt and Verbraucherschutz, approval no. 8.87-50.10.37.09.157). Deletion of the Zeb2 gene was achieved following exposure of Zeb2fl/fl Mx1-Cre or control (Zeb2fl/fl Mx1-Cre−) mice to polyinosinic:polycytidylic (Poly[I:C]) (GE Healthcare).
Flow cytometric analysis
Fluorescence-activated cell sorting (FACS) analyses were performed on the FACS-Canto cytometer (BD Biosciences) after blood, BM, or spleen cells were stained with antibodies listed in supplemental Table 1, available on the Blood Web site. For cell-cycle assessment, we used the APC-BrdU Flow Kit and for the apoptosis assay the FITC Annexin V Apoptosis Detection Kit I (both from BD Biosciences) according to the manufacturer’s instructions.
Cell sorting and molecular analyses
For molecular analysis BM subpopulations were sorted on a FACS Aria II (Becton Dickinson). For the microarray, RNA isolation, complementary DNA synthesis, and generation of the raw data were performed by MFT Services (Tübingen, Germany). Detailed description of the experimental procedure is described in the supplemental Methods.
Statistical Analysis
Statistical analysis was performed using the unpaired 2-tailed Student t test; significance was set at P values < .05.
Additional experimental procedures are described in the supplemental Methods.
Results
Loss of Zeb2 impairs differentiation in most hematopoietic lineages
Previously, we reported that Zeb2 is abundantly present in hematopoietic cells with high levels of mRNA in the HSPCs,19 which is in high concordance with the Zeb2 expression profiles on the publically available Immgen database (www.immgen.org; supplemental Figure 1A). This prompted us to test whether the main role of Zeb2 is to balance and regulate HSC/HPC function in the adult.
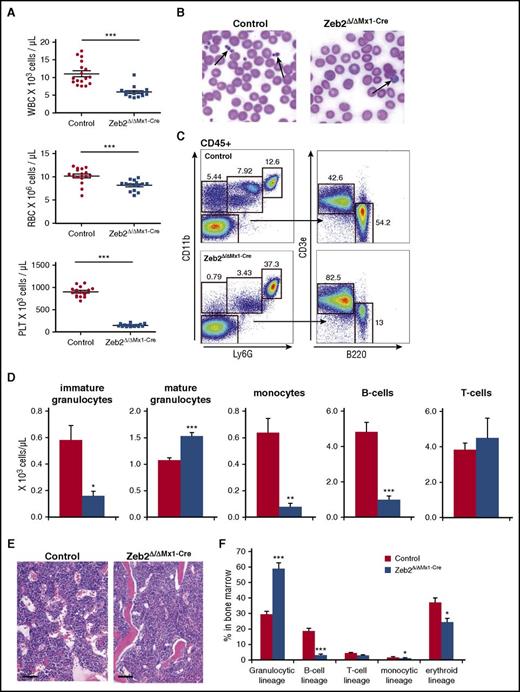
For this, we used drug-inducible, conditional Zeb2 inactivation in adult hematopoietic cells. Eight weeks after Zeb2 inactivation by Poly(I:C) administration, mice exhibited significantly lower number of leukocytes, erythrocytes, and platelets (Figure 1A). Notably, the platelets of Zeb2 mutant mice were increased in size, appearing as giant platelets (Figure 1B), as was the median corpuscular volume of thrombocytes (supplemental Figure 1B). Likewise, the erythrocytes of Zeb2Δ/ΔMx1-Cre mice were significantly larger compared with controls (supplemental Figure 1B). The analyses of leukocyte subpopulation revealed a near-complete loss of B lymphocytes and monocytes, together with an increase in mature granulocytes in Zeb2-deficient mice, whereas T-cell numbers were comparable between Zeb2Δ/ΔMx1-Cre and controls (Figure 1C-D). Blood analysis at subsequent time points (eg, at 12 months) revealed little change in the cell content over time, but a further increase in the number of granulocytes (supplemental Figure 1C).
Zeb2 deletion causes cytopenia in multiple hematopoietic lineages. (A) Absolute numbers of mature blood cells in mice at 8 weeks after Poly(I:C) administration significantly reduces leukocytes, erythrocytes, and thrombocytes in Zeb2Δ/ΔMx1-Cre mice. PLT, platelet; RBC, red blood cell; WBC, white blood cell. (B) Representative images of peripheral blood smears from control and Zeb2Δ/ΔMx1-Cre mice. Smears demonstrate absence of regular thrombocytes but sporadic giant platelets in absence of Zeb2 (arrows). (C) Representative FACS plots and gating strategy of peripheral blood analyses at 8 weeks after Poly(I:C) administration to define monocyte (Ly6G−CD11b+) frequency, immature and mature granulocytes (Ly6GdimCD11b+ and Ly6GhighCD11b+, respectively), B lymphocytes (B220+), and T lymphocytes (CD3e+). (D) Quantified absolute cell numbers of the leukocyte subsets per microliter of blood of control and Zeb2Δ/ΔMx1-Cre mice revealed significant reduction in B cells, monocytes, and immature granulocytes, but comparable counts of mature granulocytes and T cells. (E) Histologic examination of Zeb2Δ/ΔMx1-Cre and control femurs shows comparable BM cellularity. Bar graphs represent 100 µm. (F) Analyses of BM composition of different hematopoietic lineages show a predominant occurrence of granulopoiesis with a relative decrease of all other lineages. Data are from 4 independent Zeb2 inactivation experiments using in total 12 mice (A and D), and 3 experiments with 6 mice per genotype (F). Mean ± SEM is shown. Unpaired, 2-tailed t test was performed to determine significance *P < .05, **P < .01; ***P < .001.
Zeb2 deletion causes cytopenia in multiple hematopoietic lineages. (A) Absolute numbers of mature blood cells in mice at 8 weeks after Poly(I:C) administration significantly reduces leukocytes, erythrocytes, and thrombocytes in Zeb2Δ/ΔMx1-Cre mice. PLT, platelet; RBC, red blood cell; WBC, white blood cell. (B) Representative images of peripheral blood smears from control and Zeb2Δ/ΔMx1-Cre mice. Smears demonstrate absence of regular thrombocytes but sporadic giant platelets in absence of Zeb2 (arrows). (C) Representative FACS plots and gating strategy of peripheral blood analyses at 8 weeks after Poly(I:C) administration to define monocyte (Ly6G−CD11b+) frequency, immature and mature granulocytes (Ly6GdimCD11b+ and Ly6GhighCD11b+, respectively), B lymphocytes (B220+), and T lymphocytes (CD3e+). (D) Quantified absolute cell numbers of the leukocyte subsets per microliter of blood of control and Zeb2Δ/ΔMx1-Cre mice revealed significant reduction in B cells, monocytes, and immature granulocytes, but comparable counts of mature granulocytes and T cells. (E) Histologic examination of Zeb2Δ/ΔMx1-Cre and control femurs shows comparable BM cellularity. Bar graphs represent 100 µm. (F) Analyses of BM composition of different hematopoietic lineages show a predominant occurrence of granulopoiesis with a relative decrease of all other lineages. Data are from 4 independent Zeb2 inactivation experiments using in total 12 mice (A and D), and 3 experiments with 6 mice per genotype (F). Mean ± SEM is shown. Unpaired, 2-tailed t test was performed to determine significance *P < .05, **P < .01; ***P < .001.
The observed cytopenia was not due to BM aplasia, as Zeb2-deficient mice showed similar marrow cellularity (Figure 1E; supplemental Figure 1D). BM composition analyses indicated reduced frequency of B and T cells as well as erythroid lineages in Zeb2Δ/ΔMx1-Cre mice compared with controls, but the granulocytic lineage increased significantly, composing 60% of total BM cells (Figure 1F).
Loss of Zeb2 leads to HSCs accumulation
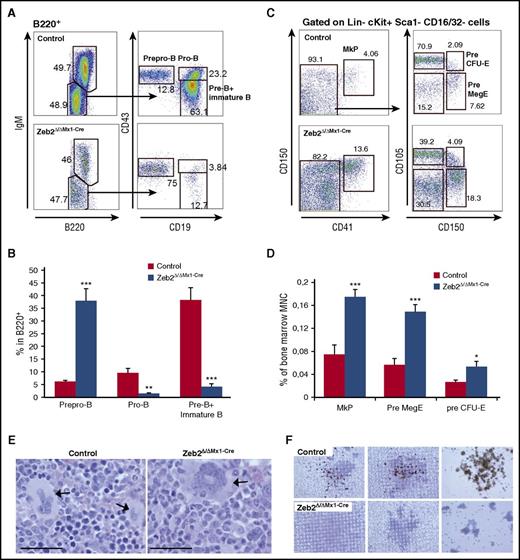
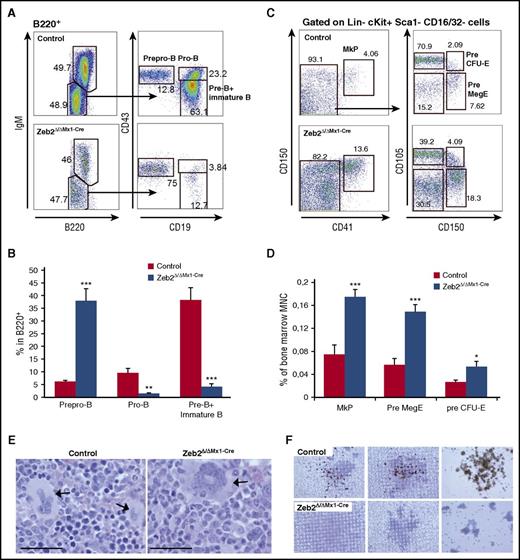
We analyzed the frequency of immature hematopoietic cells within the BM. A significant increase in frequency of immunophenotypically defined HSPCs and of a highly enriched HSC populations were found in the BM of Zeb2Δ/ΔMx1-Cre mice compared with controls (Figure 2A-B). Within myeloid restricted progenitors, we observed a shift toward an increase of megakaryocyte-erythroid progenitors (MEPs) and reduction of granulocyte-monocyte progenitors (Figure 2A,C). A similar pattern was observed when absolute cell numbers were calculated (supplemental Figure 2A).
Zeb2 deficiency alters hematopoietic stem and progenitor compartment. (A) Representative immunophenotypic analysis and gating strategy of control and Zeb2Δ/ΔMx1-Cre hematopoietic stem and progenitor populations at 8 weeks after Poly(I:C) injection. (B) Significant increase of HSPCs (defined as lineage−cKit+Sca1+ [LKS]) and HSC (LKS-CD48−CD150+ [LKS-SLAM]) populations in the absence of Zeb2 compared with control BM. (C) Analyses of lineage restricted progenitor subsets (lineage−cKit+Sca1−) revealed a shift toward more MEP and less granulocyte-monocyte progenitor (GMP) subsets in Zeb2-deficient hematopoietic compartment, whereas no difference in the appearance of CLP and CMP populations was detected. Data are representative of 3 independent experiments using 6 to 7 mice. Percentages refer to all nucleated BM cell and mean ± standard error of the mean (SEM) is shown. CLP, lineage−CD127+cKitdimSca1dim; CMP, lineage−cKit+Sca1−CD34+CD16/32−. Unpaired, 2-tailed t test was performed to determine significance; *P < .05, **P < .01, ***P < .001.
Zeb2 deficiency alters hematopoietic stem and progenitor compartment. (A) Representative immunophenotypic analysis and gating strategy of control and Zeb2Δ/ΔMx1-Cre hematopoietic stem and progenitor populations at 8 weeks after Poly(I:C) injection. (B) Significant increase of HSPCs (defined as lineage−cKit+Sca1+ [LKS]) and HSC (LKS-CD48−CD150+ [LKS-SLAM]) populations in the absence of Zeb2 compared with control BM. (C) Analyses of lineage restricted progenitor subsets (lineage−cKit+Sca1−) revealed a shift toward more MEP and less granulocyte-monocyte progenitor (GMP) subsets in Zeb2-deficient hematopoietic compartment, whereas no difference in the appearance of CLP and CMP populations was detected. Data are representative of 3 independent experiments using 6 to 7 mice. Percentages refer to all nucleated BM cell and mean ± standard error of the mean (SEM) is shown. CLP, lineage−CD127+cKitdimSca1dim; CMP, lineage−cKit+Sca1−CD34+CD16/32−. Unpaired, 2-tailed t test was performed to determine significance; *P < .05, **P < .01, ***P < .001.
To determine whether the changes within the immature hematopoietic compartment were due to changes in apoptosis or proliferation, we performed Annexin V/4′,6-diamidino-2-phenylindole and 5-bromo-2′-deoxyuridine (BrdU) incorporation assays, respectively. No differences in the rate of apoptotic cell death nor in BrdU incorporation over 24 hours were detected between Zeb2-deficient and control HSPCs subpopulations (supplemental Figure 2B-D). Therefore, we surmised that the increased frequencies of HSPCs result from an inability of precursor cells to efficiently engage the differentiation and subsequent maturation program, which causes these precursor cells to accumulate in Zeb2Δ/ΔMx1-Cre mice.
Zeb2 is not essential for HSC self-renewal and acts niche independently
We investigated whether Zeb2-deficient HSCs retain their repopulation capacity after transplantation into lethally irradiated mice. First, we performed BM cell transplants from Zeb2-deficient or control mice into irradiated congenic mice (Figure 3A). Zeb2 mutant BM recipients presented with significantly lower platelets, erythrocytes, and leukocytes, and no donor-derived B cells and monocytes (Figure 3B). However, recipient mice still had an increase in HSC numbers and a shift in myeloid progenitors toward MEPs, as was apparent in donor mice (supplemental Figure 3A,B). To test the self-renewal potential of Zeb2-deficient HSCs further, we transplanted BM from recipients of the first transplant into a second and subsequently third round. Peripheral blood analysis again revealed multilineage reconstitution with the preserved ability for granulocyte and T-lymphocyte differentiation. However, B-cell and monocyte differentiation was nearly absent, as was found in the initial transplant (supplemental Figure 3C). These data exclude the possibility that exhaustion of stem cells caused the observed decreases in peripheral blood cells. Rather, these results suggest that loss of Zeb2 is consistent with a loss of lineage fidelity and decreased differentiation potential.
Zeb2 is dispensable for self-renewal. (A) Schematic of the transplant and analysis setting. (B) Analysis of peripheral blood (PB) cells at 16 weeks posttransplant show significantly fewer WBCs and their subsets, thrombocytes and erythrocytes, in Zeb2Δ/ΔMx1-Cre BM recipients compared with their WT counterpart. (C) Schema of a competitive BM transplant. (D) The relative contribution of donor, competitor, and remaining recipient-derived blood cells in reconstituted recipients at 16 weeks after transplant reveals a significantly reduced contribution of Zeb2Δ/ΔMx1-Cre–derived cells in most lineages.
Zeb2 is dispensable for self-renewal. (A) Schematic of the transplant and analysis setting. (B) Analysis of peripheral blood (PB) cells at 16 weeks posttransplant show significantly fewer WBCs and their subsets, thrombocytes and erythrocytes, in Zeb2Δ/ΔMx1-Cre BM recipients compared with their WT counterpart. (C) Schema of a competitive BM transplant. (D) The relative contribution of donor, competitor, and remaining recipient-derived blood cells in reconstituted recipients at 16 weeks after transplant reveals a significantly reduced contribution of Zeb2Δ/ΔMx1-Cre–derived cells in most lineages.
To determine the engraftment potential of Zeb2-deficient HSPCs in the presence of wild-type (WT) hematopoiesis, we performed a competitive transplant assay. We transplanted a mixture of Zeb2-deficient or control BM cells with WT competitor cells of a congenic mouse strain into lethally irradiated recipient mice (Figure 3C). Peripheral blood analysis revealed a similar decrease of most cell lineages descending from Zeb2-deficient BM (Figure 3D; supplemental Figure 3D).
Given that the Mx1-Cre approach induces gene deletion not only in the hematopoietic but also in the nonhematopoietic BM cells,22,23 we investigated whether the observed phenotype is a consequence of impaired microenvironment changes. We transplanted WT BM cells in previously induced Zeb2Δ/ΔMx1-Cre or control mice (supplemental Figure 3E). Analysis of recipient mice revealed no differences in hematopoietic lineage reconstitution (supplemental Figure 3F). Likewise, a sequential transplant into secondary recipients in a competitive setting revealed no differences in any white blood cell subset, in absolute numbers of test cells, or in contribution ratio (supplemental Figure 3G-H). Therefore, the data suggest that these changes are intrinsic to the HSCs/HPCs rather than to Zeb2-deficient microenvironment alterations.
Zeb2 deficiency impairs differentiation at different stages of hematopoiesis
The discrepancy between the immature cell content in the BM and mature cells in the peripheral blood indicates impaired differentiation. We therefore determined the stage at which the differentiation block occurred within the different lineages. We investigated different stages of B-cell maturation using surface marker expression profiling by flow cytometry. This revealed a differentiation block at the transition from prepro-B to pro-B cells, which caused a substantial reduction in subsequent B-cell lineage subpopulations (Figure 4A-B).
Zeb2 deletion causes impaired differentiation at different stages of maturation. (A) Representative FACS plots demonstrating B lymphopoiesis in control and Zeb2Δ/ΔMx1-Cre BM cells gated on B220-positive population. (B) Frequency calculation of B-cell progenitors within the B220-positive BM subpopulation in 3 repetitive analyses revealed a block at the transition from prepro-B to pro-B cell stage. Data from 3 independent biological replicates are shown as means ± SEM. **P < .01; ***P < .001. (C) Representative FACS plots showing megakaryocytic progenitors in control and Zeb2Δ/ΔMx1-Cre BM cells gated on Lin−cKit+Sca1−CD16/32− population. (D) Analyses of megakaryocytic and erythroid progenitors within total BM cells in 3 repetitive analyses revealed an increase of MkP, PreMegE, and pre CFU-E cells in Zeb2-deficient mice compared with controls. Data from 6 independent biological replicates are shown as means ± SEM. **P < .01; ***P < .001. (E) Representative images of BM sections from femurs from Zeb2Δ/ΔMx1-Cre and control mice showing abnormal morphology in Zeb2-deficient megakaryocytes (arrow) compared with controls. Bar graphs, 50 µm. (F) Representative images of acetylcholinesterase staining of CFU-megakaryocyte colonies demonstrate the absence of mature megakaryocytes in cell cultures derived from Zeb2Δ/ΔMx1-Cre mice.
Zeb2 deletion causes impaired differentiation at different stages of maturation. (A) Representative FACS plots demonstrating B lymphopoiesis in control and Zeb2Δ/ΔMx1-Cre BM cells gated on B220-positive population. (B) Frequency calculation of B-cell progenitors within the B220-positive BM subpopulation in 3 repetitive analyses revealed a block at the transition from prepro-B to pro-B cell stage. Data from 3 independent biological replicates are shown as means ± SEM. **P < .01; ***P < .001. (C) Representative FACS plots showing megakaryocytic progenitors in control and Zeb2Δ/ΔMx1-Cre BM cells gated on Lin−cKit+Sca1−CD16/32− population. (D) Analyses of megakaryocytic and erythroid progenitors within total BM cells in 3 repetitive analyses revealed an increase of MkP, PreMegE, and pre CFU-E cells in Zeb2-deficient mice compared with controls. Data from 6 independent biological replicates are shown as means ± SEM. **P < .01; ***P < .001. (E) Representative images of BM sections from femurs from Zeb2Δ/ΔMx1-Cre and control mice showing abnormal morphology in Zeb2-deficient megakaryocytes (arrow) compared with controls. Bar graphs, 50 µm. (F) Representative images of acetylcholinesterase staining of CFU-megakaryocyte colonies demonstrate the absence of mature megakaryocytes in cell cultures derived from Zeb2Δ/ΔMx1-Cre mice.
Next we analyzed the maturation of megakaryocytic lineage in the BM. Immunophenotypic assessment of progenitor cells according to Pronk et al24 revealed a significant increase in megakaryocytic precursors in Zeb2Δ/ΔMx1-Cre mice compared with controls (Figure 4C-D). Further BM and spleen histological analyses revealed a significant increase of megakaryocytes in Zeb2Δ/ΔMx1-Cre mice (supplemental Figure 4A-B). However, Zeb2-deficient megakaryocytes displayed a rather immature morphology with reduced cytoplasm and less condensed nuclear appearance (Figure 4E). In line with this observation, ex vivo culture of megakaryocytes using colony-forming unit (CFU) megakaryocyte assays revealed a lack of mature, acetylcholinesterase-expressing megakaryocytes in the absence of Zeb2 (Figure 4F). These data indicate that megakaryocytic lineage differentiation is initiated, but show impaired maturation at the terminal differentiation stage in the absence of Zeb2.
Finally, we analyzed the different stages of erythrocytic lineage maturation using flow cytometry according to Socolovsky et al.25 In Zeb2Δ/ΔMx1-Cre BM, we found a significant reduction of mature subsets, such as orthochromatic erythroblasts and a relative accumulation of immature pro-erythroblasts and basophilic erythroblasts (supplemental Figure 4C). In line with these data, colony assays (CFU-erythroid [CFU-E] and burst-forming unit erythroid) revealed no difference in colony-forming potential between Zeb2-deficient and control BM cells (supplemental Figure 4D), indicating impaired differentiation at the transition from pro-erythroblast toward orthochromatic erythroblasts. These data demonstrate that Zeb2 is an important modulator for differentiation processes at various maturation stages in multiple hematopoietic lineages.
Zeb2Δ/Δ Mx1-Cre mice show features of myeloproliferative disease
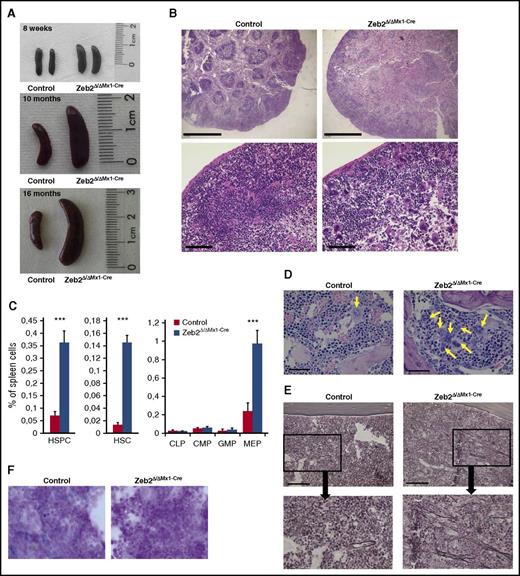
We noticed a significant increase in spleen size in Zeb2Δ/ΔMx1-Cre mice at 8 weeks and a further enlargement at later stages (eg, 10 and 16 months after Poly(I:C) administration) (Figure 5A; supplemental Figure 5A). To determine their cellular content, we performed histological and flow cytometric analyses of the spleens. These analyses revealed destruction of the follicular architecture in Zeb2Δ/ΔMx1-Cre spleens (Figure 5B) and a massive infiltration with HSCs, HSPCs, and MEPs (Figure 5C; supplemental Figure 5B) as well as megakaryocytes, erythropoietic, and granulopoietic cells, corresponding to increased extramedullary hematopoiesis in the absence of Zeb2 (supplemental Figure 5C). This increase in extramedullary hematopoiesis in Zeb2Δ/ΔMx1-Cre mice is reminiscent of myeloproliferative diseases in humans. Furthermore, cell content analyses within the spleens of Zeb2Δ/ΔMx1-Cre BM transplant recipients also revealed significantly increased extramedullary hematopoiesis in recipients of Zeb2-deficient BM compared with spleens of control BM recipients (supplemental Figure 5A). In addition, histology of BM sections revealed an increased frequency of megakaryocytes with an abnormal morphology, as shown in Figure 4E, and these cells also tended to form clusters (Figure 5D). Moreover, similar features were observed in spleens from Zeb2Δ/ΔMx1-Cre mice, with an increase in endomitotic figures and asynchronous maturation (supplemental Figure 5E). Silver staining of the BM cavity revealed a significant increase in the appearance of reticular fibers, which correlated with BM fibrosis grade I (according to Thiele et al26 ) in Zeb2-deficient mice (Figure 5E). Finally, when we examined BM iron content, we found iron depletion in Zeb2Δ/ΔMx1-Cre, but not in control mice (Figure 5E). Together, these features are hallmarks of myeloproliferative diseases, such as primary myelofibrosis in humans. Consistent with this finding, Zeb2Δ/ΔMx1-Cre mice have a significantly shorter life span than controls (supplemental Figure 5F). This phenotype to some extend resembles the GATA1low mouse model of myelofibrosis,27,28 in which increased expression of transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), and other cytokines has been associated with progressive myelofibrosis.28,29 Therefore, we measured the cytokine levels of TGF-β and PDGF in plasma and BM as well as their mRNA levels in whole tibia lysates. In contrast to GATA1low mice, reduced levels of TGF-β and PDGF were detectable in plasma of Zeb2Δ/ΔMx1-Cre mice and showed no difference in BM (supplemental Figures 5G-I), suggesting a different underlying mechanism compared with GATA1low animals.
Zeb2Δ/ΔMx1-Cre mice show characteristic features of myeloproliferative disease. (A) Representative images of spleens from Zeb2Δ/ΔMx1-Cre and control mice at 8 weeks, 10 months, and 16 months after Poly(I:C) administration demonstrating progressive splenomegaly in Zeb2-deficient mice. Data are representative of at least 3 independent experiments. (B) Histological analyses of spleen sections show extensive loss of white pulp compartment in Zeb2-deficient mice. Data are representative of at least 3 independent experiments. Bar graphs, 1000 µm (top); 100 µm (bottom). (C) A significant increase in the appearance of LKS, LKS-SLAM, and MEP populations within the spleen of Zeb2Δ/ΔMx1-Cre mice compared with controls indicate a relocation of hematopoiesis to the spleen. Data are from 6 biological replicates shown as means ± SEM. Unpaired, 2-tailed t test was performed to determine significance; ***P < .01. (D) Hematoxylin and eosin staining of BM sections demonstrate grouping of abnormal megakaryocytes (arrows) in Zeb2Δ/ΔMx1-Cre mice. Bar graphs, 50 µm. (E) Silver staining shows the presence of reticular fibers in the BM space in Zeb2Δ/ΔMx1-Cre mice. Bar graphs, 100 µm. (F) BM smears were subjected to Prussian Blue staining for hemosiderin demonstrating iron depletion in Zeb2Δ/ΔMx1-Cre marrow cells.
Zeb2Δ/ΔMx1-Cre mice show characteristic features of myeloproliferative disease. (A) Representative images of spleens from Zeb2Δ/ΔMx1-Cre and control mice at 8 weeks, 10 months, and 16 months after Poly(I:C) administration demonstrating progressive splenomegaly in Zeb2-deficient mice. Data are representative of at least 3 independent experiments. (B) Histological analyses of spleen sections show extensive loss of white pulp compartment in Zeb2-deficient mice. Data are representative of at least 3 independent experiments. Bar graphs, 1000 µm (top); 100 µm (bottom). (C) A significant increase in the appearance of LKS, LKS-SLAM, and MEP populations within the spleen of Zeb2Δ/ΔMx1-Cre mice compared with controls indicate a relocation of hematopoiesis to the spleen. Data are from 6 biological replicates shown as means ± SEM. Unpaired, 2-tailed t test was performed to determine significance; ***P < .01. (D) Hematoxylin and eosin staining of BM sections demonstrate grouping of abnormal megakaryocytes (arrows) in Zeb2Δ/ΔMx1-Cre mice. Bar graphs, 50 µm. (E) Silver staining shows the presence of reticular fibers in the BM space in Zeb2Δ/ΔMx1-Cre mice. Bar graphs, 100 µm. (F) BM smears were subjected to Prussian Blue staining for hemosiderin demonstrating iron depletion in Zeb2Δ/ΔMx1-Cre marrow cells.
Zeb2 modulates cytokine signaling
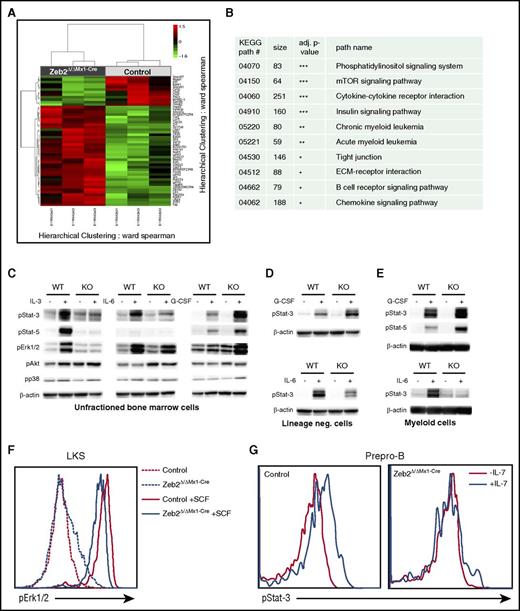
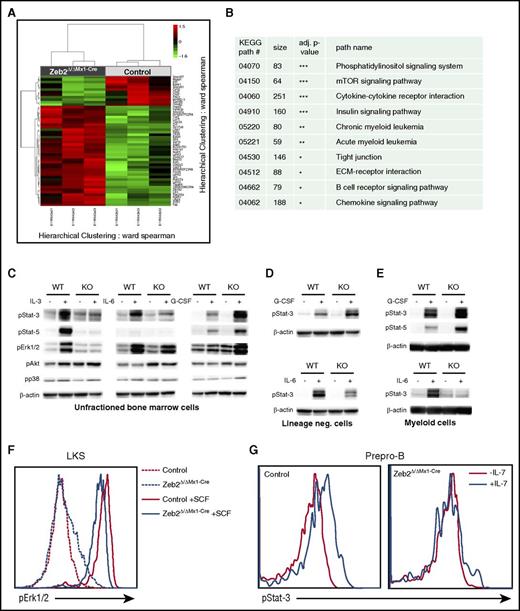
We determined downstream targets responsible for complex hematopoietic differentiation defects associated with Zeb2 loss through a global gene expression analyses focusing on an HSC-enriched BM subset. RNA was extracted from lineage−cKit+Sca1+ (LKS) LKS-CD48−CD150+ (SLAM) cells, which were isolated by flow cytometry 8 weeks after Zeb2 deletion. Figure 6A depicts a heat map of at least twofold up- or downregulated genes, which showed that a vast majority of genes were upregulated in accord with the reported role of Zeb2 as a transcriptional repressor.7,8,10 We performed a statistical association analyses of differentially expressed genes with known KEGG pathways.30 These analyses revealed highly significant alterations in multiple signaling pathways, including cytokine-cytokine receptor interactions, mammalian target of rapamycin, and phosphatidylinositol signaling pathways, respectively (Figure 6B; supplemental Table 5). We then sought to test the functional responsiveness of Zeb2-deficient cells to cytokine stimulation. Stimulation of total BM cells with interleukin-3 (IL-3) or IL-6 resulted in strong activation of JAK/STAT and extracellular signal-regulated kinase (ERK) pathways in WT cells with increased expression of phosphorylated STAT3, STAT5, or ERK. Zeb2-deficient BM cells showed attenuated or nondetectable responses of the JAK/STAT and ERK pathways upon stimulation with IL-6 or IL-3 (Figure 6C). However, stimulation of these cells with granulocyte colony-stimulating factor (G-CSF) resulted in enhanced responsiveness of the JAK/STAT pathway. This finding may explain why granulopoiesis dominates in the mutant BM with no cytopenia in the granulocytic lineage detected. No differences in p38 and AKT phosphorylation levels were observed between WT and Zeb2 mutant cells after different stimulations.
Zeb2 deletion changes the gene expression profile. (A) A heat map of at least 2 fold down- or upregulated genes. Clustered heat map visualizes normalized expression values of genes that showed an at least 2-fold up- or downregulation together with a nominal P value < 1%. The RNA was isolated from 3 pairs of independently sorted control and Zeb2Δ/ΔMx1-Cre HSC (LKS-SLAM) at 8 weeks after Poly(I:C) administration. (B) Selected KEGG pathways with significant alterations after Zeb2 deletion are shown here (the complete list of pathways analyses is included as supplemental Table 5). (C-E) Representative western blots showing phosphorylation of indicated signaling proteins in total lysates of BM cells (C), lineage-negative cells (D), and Mac1+ myeloid cells (E) from Zeb2Δ/ΔMx1-Cre or control mice in the presence of 10 μg/μL G-CSF, IL-3, or IL-6 for 15 minutes. Data shown as a representative plot from at least 3 independently isolated biological replicates. Means ± SEM is shown. (F) Flow cytometric analysis of phosphorylated ERK1/2 in LKS cells treated with the stem cell factor (SCF) of Zeb2Δ/ΔMx1-Cre and control mice revealed reduced signal transduction in absence of Zeb2. (G) Representative FACS plot of phospho-STAT3 expression in prepro-B cells after stimulation with IL-7 shows severely reduced signaling in Zeb2Δ/ΔMx1-Cre prepro-B cells.
Zeb2 deletion changes the gene expression profile. (A) A heat map of at least 2 fold down- or upregulated genes. Clustered heat map visualizes normalized expression values of genes that showed an at least 2-fold up- or downregulation together with a nominal P value < 1%. The RNA was isolated from 3 pairs of independently sorted control and Zeb2Δ/ΔMx1-Cre HSC (LKS-SLAM) at 8 weeks after Poly(I:C) administration. (B) Selected KEGG pathways with significant alterations after Zeb2 deletion are shown here (the complete list of pathways analyses is included as supplemental Table 5). (C-E) Representative western blots showing phosphorylation of indicated signaling proteins in total lysates of BM cells (C), lineage-negative cells (D), and Mac1+ myeloid cells (E) from Zeb2Δ/ΔMx1-Cre or control mice in the presence of 10 μg/μL G-CSF, IL-3, or IL-6 for 15 minutes. Data shown as a representative plot from at least 3 independently isolated biological replicates. Means ± SEM is shown. (F) Flow cytometric analysis of phosphorylated ERK1/2 in LKS cells treated with the stem cell factor (SCF) of Zeb2Δ/ΔMx1-Cre and control mice revealed reduced signal transduction in absence of Zeb2. (G) Representative FACS plot of phospho-STAT3 expression in prepro-B cells after stimulation with IL-7 shows severely reduced signaling in Zeb2Δ/ΔMx1-Cre prepro-B cells.
To exclude the possibility that the differences in signaling activity in response to G-CSF was solely due to the changes in cell composition between the control and Zeb2-deficient BM, we performed the same analyses on isolated myeloid BM cells and lineage-negative cells, mostly stem and progenitor cells. Similar to unfractioned BM, JAK/STAT signaling in both fractions was substantially reduced in Zeb2-deficient cells upon IL-6 treatment but augmented upon G-CSF stimulation in enriched myeloid cells (Figure 6D-E).
To further examine the signaling activity in small BM subsets, like the HSPCs or B-cell precursors, we employed a flow cytometry based approach because the low cell numbers precluded the use of western blots. The treatment of LKS cells with stem cell factor or thrombopoietin (TPO) revealed a reduced ERK, AKT, or STAT3 phosphorylation, respectively (Figure 6F; supplemental Figure 6A-C). Stimulation of the prepro-B cell subset with IL-7 revealed no detectable activation of JAK/STAT signaling pathway in Zeb2-deficient cells (Figure 6G).
Given that different cytokines induce unique responses within the same signaling pathway, we predicted a difference in the expression of the corresponding receptors. We found no changes in the expression of the G-CSF receptor mRNA in Zeb2-deficient myeloid cells. However, IL-7 and IL-6 receptor mRNA were severely reduced in the prepro-B-cells and myeloid progenitors, respectively (supplemental Figure 6D). Whether IL-7 and IL-6 receptor levels are direct transcriptional targets of Zeb218 or whether their expression is altered through epigenetic changes in gene expression remains to be determined. Finally, we sought to exclude that Zeb2-deficient BM cells gained some cytokine-independent signaling activity, as this has been reported for other mouse models of myeloproliferative syndroms.31-34 Using colony-forming assays, we found no evidence for cytokine-independent cell growth in the absence of Zeb2 (supplemental Figure 6E).
Zeb2 mutations found in patients with myeloid malignancy
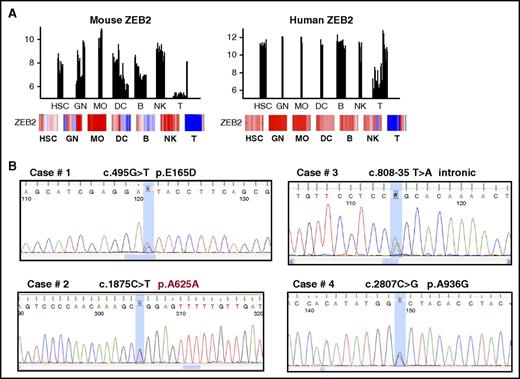
To investigate the potential impact of Zeb2 on human hematopoiesis, we first compared the expression level of Zeb2 in different subsets of hematopoietic cells using the publically available Immgen database (http://www.immgen.org). Similar to the expression pattern in mice, high levels of Zeb2 expression were found in multiple hematopoietic precursor and lineage subsets (Figure 7A). Interestingly, comparison of Zeb2 expression in healthy human hematopoietic cells with different subtypes of acute myelogenous leukemia (AML) using BloodSpot (www.bloodspot.eu)35 revealed high expression of Zeb2 in most AML samples, comparable with the expression levels found in immature hematopoietic cells (supplemental Figure 7A). Given the observed phenotype upon inactivation of Zeb2 in mice and similarly high expression of Zeb2 in hematopoietic cells in humans, we wondered whether genetic alterations within the Zeb2 gene are common within myeloid neoplasias. A screening for mutations and deletions was carried out in a series of 707 cases of myeloid diseases including myeloproliferative neoplasias (MPN), myelodysplastic syndromes (MDS), and AML (Table 1). No deletions were found in any of the 470 cases of myeloid diseases analyzed. Using bidirectional-sequencing analyses, we detected 4 of 237 cases (1.7%) of previously unknown heterozygous variations in 4 patients with myeloid neoplasia, in particular essential thrombocythemia and AML (Table 2; Figure 7B). Three of these variations were not found in 200 samples from healthy donors, indicating the variations could be new putative mutations. In case 2, the variation was germline as confirmed by sequencing of DNA from nails. Similarly, the intronic variation in case 3 with AML could also be germline because it was found in the buccal scrub after successful allogeneic stem cell transplantation. Case 4 had a very complex karyotype and a history of treatment of non-Hodgkin lymphoma.
Zeb2 mutation in human hematopoietic malignancies. (A) Comparison of Zeb2 expression between murine and human hematopoietic cell subsets based on the Immgen database. (B) Sequencing analyses of Zeb2 allele revealed point mutations in 4 of 237 cases.
Zeb2 mutation in human hematopoietic malignancies. (A) Comparison of Zeb2 expression between murine and human hematopoietic cell subsets based on the Immgen database. (B) Sequencing analyses of Zeb2 allele revealed point mutations in 4 of 237 cases.
To investigate a possible impact of these mutations on protein functionality, we performed some computational and initial functional analyses on 2 mutations: p.E165D and p.A936G. Although both amino acids are not part of a previously reported ZEB2 functional domain, both are highly conserved during evolution, suggesting they are of importance for ZEB2 function (supplemental Figure 7B). PSIPRED, a secondary structure prediction method, suggests that E165 is part of α helix, which shows homology with a region in insulinoma-associated protein 1, a Zinc-finger domain-containing DNA-binding transcription factor that plays key roles in neurogenesis36,37 and neuroendocrine cell differentiation.38 A936 is predicted to be part of a β sheet localized between the C-terminal binding protein–interaction domain and the carboxyterminal Zinc-finger cluster of ZEB2. To further investigate the impact of these mutations on Zeb2 protein stability we have cloned aminoterminally 3xFlag/strep-tagged versions of these mutant Zeb2 forms into the ROSA26 locus of ESC and analyzed for protein expression via western blot. No obvious changes in Zeb2 protein levels were observed between WT and mutant forms of Zeb2 (supplemental Figure 7C), suggesting that these amino acids changes are not altering the ZEB2 protein stability or proteolytic degradation.
Altogether, results obtained in Zeb2 mutant mice and in human samples indicate that ZEB2 is a major regulator of hematopoietic differentiation and its alterations may have impact on myeloid malignancies in both mouse models and humans.
Discussion
We show that Zeb2 is a master regulator of differentiation in multiple cell lineages in adult hematopoiesis, in addition to the documented role of Zeb2 in embryonic hematopoiesis.19 In contrast to the embryonic system, adult Zeb2-deficient mice can survive for many months despite severe cytopenia in multiple lineages. There were also distinct differences in cell lineages that depend on intact Zeb2 during embryonic and adult hematopoiesis. Whereas Zeb2 deletion during embryonic stages results in severely reduced myeloid colony formation,19 granulopoiesis is not affected during adult hematopoiesis in Zeb2Δ/ΔMx1-Cre mice. Rather B lymphopoiesis and terminal differentiation of megakaryocytes and erythrocytes are dependent on Zeb2 expression. Together with findings in previous studies, also in other organ systems than hematopoiesis, we suggest that Zeb2 primarily regulates differentiation. The importance of Zeb2 as an intrinsic factor for cellular differentiation and maturation has indeed been reported in other cell types, including neurons and oligodendroglial cells,10-12,39 or in melanocyte maturation,9 and have also been observed in mouse embryonic stem cells (J.J.H., D.H., and S.G., unpublished results). Our observations are also consistent with recent reports that demonstrated Zeb2 is essential for cell fate decision, maturation and function of mast cells,40 natural killer cells,41 and T cells.42 Surprisingly, Zeb2 deficiency did not alter HSC self-renewal. In cancer, increased Zeb2 levels were associated with cancer stem cell acquisition and self-renewal potential,18 so Zeb2 loss should predicate stem cell loss. However, we demonstrate that Zeb2 deletion causes accumulation of immature hematopoietic cells and is not essential for self-renewal of normal adult HSCs.
The differentiation impairment phenotype following Zeb2 inactivation is very complex and appears to involve different hematopoietic stages at the stem and progenitor cell levels and the multiple cell lineage differentiation at various maturation stages. B lymphopoiesis was affected at a relatively early stage at the transition from the prepro-B to pro-B cell stage, whereas megakaryocytes and erythrocytes were affected at the later stages of terminal differentiation. The underlying molecular mechanism(s) of how Zeb2 directs cell differentiation in different cellular subsets through maturation at different stages poses specific challenges. Indeed, the emerging knowledge is that Zeb2 might have multiple interacting partners and modify various transcriptional programs in a cell or signaling receptor type–specific manner. Interestingly, we observed differential signal transduction intensity in response to TPO, IL-6, or G-CSF stimulation within the same signaling pathway. In addition to reduced expression of corresponding receptors, other factors can contribute to the diverse signaling responses. IL-6 signaling is not necessary for HSPCs homeostasis; however, TPO and IL-6 are required for terminal differentiation, particularly in megakaryocytes.43 Similarly, IL-7 signaling is crucial for B-cell differentiation at the transition from prepro-B to the pro-B cell stage.44,45 Thus, impaired JAK-STAT signaling by the IL-7 receptor is sufficient to block B-cell maturation at this corresponding stage of differentiation. Similarly, at early stages of T-cell differentiation and in T--cell acute lymphoblastic leukemia, Zeb2 appears to be an activator of IL-7 receptor expression with corresponding activation of the JAK-STAT signaling18 ; but, Zeb2 may be a repressor of IL-7 receptor at later stages of T-cell maturation.42
Finally, we demonstrated multiple phenotypic and molecular changes in Zeb2-deficient hematopoietic systems that resemble a myeloproliferative disorder seen in patients and some other mouse models of myeloproliferative disorders, particularly GATA1low mice. Screening for genetic alterations in human myeloid neoplasias revealed that ZEB2 mutations are rather rare events in these diseases. Similarly, Zeb2 mutations have been reported in some cases of MDS patients (http://cancer.sanger.ac.uk/cosmic). Of note, because we have reported previously the development of AML in mice that overexpress Zeb2,18 it seems that the overexpression of Zeb2 and not loss-of-function mutations predominantly leads to malignancy. However, we cannot exclude the possibility that ZEB2 loss of function or mutation may contribute to human MDS. Thus, the biological relevance of these mutations remains uncertain and requires additional studies to define their possible impact on ZEB2 function. However, deletion and deleterious mutations are only 2 of the possible mechanisms associated with alteration of gene function; additional transcriptional or posttranscriptional regulations affecting proper Zeb2 expression might be in place. Members of the miR-200 family were found to directly target the mRNA of ZEB2 and thus posttranscriptionally regulate their protein levels.46-49 In fact, in the accompanying manuscript, Pellman et al demonstrated high levels of Zeb2 expression in AML cells that is associated with loss of mir200 family expression. Zeb2 downregulation was associated with reduced proliferation and increased apoptosis of leukemic cells as well as induction of differentiation into granulocytic lineages. Of note, consistent with this finding in human cells, we observed a preferential granulocytic differentiation in Zeb2-deficient murine hematopoiesis. Overexpression of Zeb2 in a context of T-cell differentiation also results in malignant transformation and acute T-lymphoblastic leukemia.18 In addition, Zeb2 has been recently described as a fusion partner of the PDGFRB kinase in acute B-lymphoblastic leukemia,50 resulting in cytokine-dependent activation of proliferation and STAT5 phosphorylation. Thus, our results are in line with increasing evidence that the EMT regulator Zeb2 is essential in the orchestration of differentiation processes and the development of malignancies.
The microarray data set has been submitted to the Gene Expression Omnibus database (accession number GSE87305).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge the assistance of the Flow Cytometry Core Facility at the Institute of Molecular Medicine and Experimental Immunology, University of Bonn, which is supported in part by the Deutsche Forschungsgemeinschaft (grant no. HBFG-109-517).
This work was supported by grants from the German Research Foundation (Deutsche Forschungsgemeinschaft JA1967/3-1 and JA1967/5-1) (V.J.), China Scholarship Council (W11106) (J.L.), National Natural Science Foundation of China (81561138002) (T.C.), Italian Association Against Cancer (IG 15525) (C.M.), Fund for Scientific Research – Flanders (FWO-V; G.0568.13) (J.J.H.) and (G.0782.14) (D.H.), the Belgian Federation for the Study Against Cancer, and Australian National Health and Medical Research Council (grants 1047995 and 1051485). The work is also part of the DevRepair (P7/07) IAP-VII network, which supports J.J.H. and D.H. S.G. is a postdoctoral fellow of the FWO-V. We thank Life Science Editors for editing services (www.lifescienceeditors.com).
Authorship
Contribution: J.L., T.R., S.G., I.G., H.F., C. Mecucci, J.J.H., and V.J. conceived and designed the experiments. J.L., T.R., S.S., C.C.G., M.B., L.D., N.M., R.L.S., C. Matteucci, N.F., E.R., T.P., and R.L.S. performed the experiments. J.L., T.R., S.G., I.G., H.F., C. Mecucci, P.H., and V.J. analyzed the data. V.J., J.J.H., P.B., T.C., D.H., and C. Mecucci contributed reagents/materials/analysis tools. J.L., S.G., J.J.H., D.H., and V.J. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Viktor Janzen, Department of Internal Medicine III, Division of Hematology/Oncology/Rheumatology, University of Bonn, Sigmund-Freud-Str. 25, 53105 Bonn, Germany; e-mail: viktor.janzen@ukb.uni-bonn.de.
References
Author notes
J.L., T.R., and S.G. contributed equally to this manuscript.


![Figure 2. Zeb2 deficiency alters hematopoietic stem and progenitor compartment. (A) Representative immunophenotypic analysis and gating strategy of control and Zeb2Δ/ΔMx1-Cre hematopoietic stem and progenitor populations at 8 weeks after Poly(I:C) injection. (B) Significant increase of HSPCs (defined as lineage−cKit+Sca1+ [LKS]) and HSC (LKS-CD48−CD150+ [LKS-SLAM]) populations in the absence of Zeb2 compared with control BM. (C) Analyses of lineage restricted progenitor subsets (lineage−cKit+Sca1−) revealed a shift toward more MEP and less granulocyte-monocyte progenitor (GMP) subsets in Zeb2-deficient hematopoietic compartment, whereas no difference in the appearance of CLP and CMP populations was detected. Data are representative of 3 independent experiments using 6 to 7 mice. Percentages refer to all nucleated BM cell and mean ± standard error of the mean (SEM) is shown. CLP, lineage−CD127+cKitdimSca1dim; CMP, lineage−cKit+Sca1−CD34+CD16/32−. Unpaired, 2-tailed t test was performed to determine significance; *P < .05, **P < .01, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/4/10.1182_blood-2016-05-714659/4/m_blood714659f2.jpeg?Expires=1768019079&Signature=ms5OfFn2iqzzmFRTWKqSZvr9Xlyvz7~T29mvZUVG7ZqJuL7abOOI3FJU2WFalFYuwfvM15pF07~sipG7FjvkErbsLMpqH5vl8EDIgyKO5y8l8Q1oOwHZwWoNBCL5K2loHaGTzy5DnqWO7hgMXUJ~HPgHC-6d3~vFquSJkHV~VOS7BfsL~OO5vbAtGO2CNdxywIwq-Um9WMTdImufu0iEuGzIAcY7zZv6iKKIWihg2QbWZD5z1huVN345jGLKUnWRZeV~s15NSh3IV4X0mS3QYMeBI9ojiVQWw6704gEx61KO85sM-1TqN40uq74M4qFxXfhP7URo6GflSPtCi6v66w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



![Figure 2. Zeb2 deficiency alters hematopoietic stem and progenitor compartment. (A) Representative immunophenotypic analysis and gating strategy of control and Zeb2Δ/ΔMx1-Cre hematopoietic stem and progenitor populations at 8 weeks after Poly(I:C) injection. (B) Significant increase of HSPCs (defined as lineage−cKit+Sca1+ [LKS]) and HSC (LKS-CD48−CD150+ [LKS-SLAM]) populations in the absence of Zeb2 compared with control BM. (C) Analyses of lineage restricted progenitor subsets (lineage−cKit+Sca1−) revealed a shift toward more MEP and less granulocyte-monocyte progenitor (GMP) subsets in Zeb2-deficient hematopoietic compartment, whereas no difference in the appearance of CLP and CMP populations was detected. Data are representative of 3 independent experiments using 6 to 7 mice. Percentages refer to all nucleated BM cell and mean ± standard error of the mean (SEM) is shown. CLP, lineage−CD127+cKitdimSca1dim; CMP, lineage−cKit+Sca1−CD34+CD16/32−. Unpaired, 2-tailed t test was performed to determine significance; *P < .05, **P < .01, ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/4/10.1182_blood-2016-05-714659/4/m_blood714659f2.jpeg?Expires=1768379782&Signature=mB87soLbqDoWTniMtme38hIF82SHlOE~hzwwE-HbIlb1d1jU1RobuZH6u1Gg5DhJBhnILVBCXnrtCqXbb8qEKaRjLlf4tY2Co58LIKN6PyL9qhZP5Vo5VtVDHgy3PA8N7gggmRkOEpoWVeyRt0a8HImpHvFogMVAUVin3V8ai9B5DcAAzuiO8GKpd0lnf4v3~vAve8~w6fjZsCKE2rYUhbOvald0YuC9p2QKujXqpsAimcAAl4jFByB5nAvWQza8W7d5YR8xKmSuNV0ZSl2qkp5RIX02JbQoHel3d4oFXGif5RN1VI8a1R1lVI1IdJ3OnqR-zzb0Q4Az8VBmX-E9Tw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)