Key Points
RP2D of PEV 20 mg/m2 in PEV/AZA combo did not alter toxicity profile of AZA; dose-limiting toxicities were transiently elevated AST/ALT.
In treatment-naive older AML patients, the intent-to-treat ORR was 50%.
Abstract
Pevonedistat (TAK-924/MLN4924) is a novel inhibitor of NEDD8-activating enzyme (NAE) with single-agent activity in relapsed/refractory acute myeloid leukemia (AML). We performed a phase 1b study of pevonedistat (PEV) with azacitidine (AZA) based on synergistic activity seen preclinically. Primary objectives included safety and tolerability, and secondary objectives included pharmacokinetics (PK) and disease response. Patients ≥60 years with treatment-naive AML (unfit for standard induction therapy) received PEV 20 or 30 mg/m2 IV on days 1, 3, and 5 combined with fixed-dose AZA (75 mg/m2 IV/subcutaneously) on days 1 to 5, 8, and 9, every 28 days. The most common treatment-emergent adverse events were constipation (48%), nausea (42%), fatigue (42%), and anemia (39%). In total, 11 deaths were observed and considered unrelated to study therapy by the investigators. Transient elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were dose limiting. The recommended phase 2 dose (RP2D) of PEV in this combination is 20 mg/m2. PEV PK was not altered by the addition of AZA. Overall response rate (ORR) based on an intent-to-treat analysis was 50% (20 complete remissions [CRs], 5 complete remission with incomplete peripheral count recovery, 7 partial remissions [PRs]), with an 8.3-month median duration of remission. In patients receiving ≥6 cycles of therapy (n = 23, 44%), ORR was 83%. In patients with TP53 mutations, the composite CR/PR rate was 80% (4/5). Two of these patients stayed on study for >10 cycles. Baseline bone marrow blast percentage or cytogenetic/molecular risk did not influence ORR. This study was registered at www.clinicaltrials.gov as #NCT01814826.
Introduction
Current therapy in acute myeloid leukemia (AML) is inadequate.1-5 Although some progress has been made in this disease, the prognosis for older patients (deemed unfit to receive intensive chemotherapy) remains very poor.4,5 The use of hypomethylating agents as alternative induction therapies for these patients has become commonplace. Two large randomized studies reported higher rates of remission for older patients treated with 5-azacitidine (AZA) compared with conventional care approaches, which included supportive care.6,7 Considering the widespread use of AZA in older patients who are not candidates for chemotherapy, combination studies with promising new agents are actively enrolling.8
We previously reported the therapeutic potential of single-agent pevonedistat (PEV) (previously TAK-924/MLN4924) in patients with AML.9 PEV is a small-molecule inhibitor of the NEDD8-activating enzyme (NAE), which processes NEDD8 (neural cell developmentally downregulated 8) for binding to target substrates.10-12 The best-characterized NAE targets in cells are the cullin-RING E3 ubiquitin ligases, which direct the degradation of specific substrates (eg, p27, CDT1, and Nrf-2) through the proteasome.13-17 In response to PEV treatment, impaired NAE activity leads to Cullin-RING E3 ubiquitin ligase substrate accumulation, causing antiproliferative effects in AML.18 A variety of mechanisms are implicated in driving these effects, including disruption of cellular redox via stabilization of pIKB (a critical mediator of cell killing),18 DNA replication, and cell cycle arrest.19 In a phase 1b study of patients with relapsed/refractory AML and myelodysplastic syndrome (MDS), PEV was administered as a 1-hour IV infusion on days 1, 3, and 5 (schedule A, n = 27) or days 1, 4, 8, and 11 (schedule B, n = 26) every 21 days.9 The maximum tolerated doses (MTDs) for schedules A and B were 59 and 83 mg/m2, respectively. On schedule A, elevation of alanine aminotransferase (ALT)/aspartate aminotransferase (AST) was dose limiting. Multiorgan failure was dose limiting on schedule B. Overall response rate (ORR) in patients treated at or below the MTD was 17% (4/23; 2 complete remissions [CRs] and 2 partial remissions [PRs]) for schedule A and 10% (2/19; 2 PRs) for schedule B.9
To identify clinically effective PEV combinations, a high-throughput viability screen in AML cells confirmed that combined treatment using PEV with either decitabine or AZA was synergistically lethal by combination index and blending synergy analysis.19 In the case of AZA, combined treatment with PEV significantly increased DNA damage and cell death when compared with either agent alone, as measured by immunoblotting and flow cytometry analysis of cell cycle distributions. In vivo studies were performed in AZA-resistant HL-60 and THP-1 xenografts. Although the doses of PEV and AZA would be subtherapeutic if used as single-agent treatment, the combination led to complete and sustained tumor regression in these models.19 The mechanisms underlying the observed synergistic effects are currently under investigation.19 Considering the promising clinical data for PEV as a single agent and its enhanced antitumor activity when combined with AZA in laboratory models, we conducted a phase 1b trial of PEV combined with AZA for older patients with AML deemed unfit to receive intensive chemotherapy.
Materials and methods
Patients
Eligible patients were ≥60 years old with untreated AML who were considered unlikely to benefit from standard induction defined by ≥1 of the following: age ≥75 years, presence of antecedent MDS, adverse cytogenetic risk, and Eastern Cooperative Oncology Group performance status (ECOG PS) of 2. Other inclusion criteria included ECOG PS of 0 to 2, adequate renal function (calculated creatinine clearance >50 mL/min), adequate hepatic function (bilirubin within normal range, AST and ALT ≤2.5 × upper limit of normal [ULN]), and adequate cardiac function (B-type natriuretic peptide ≤1.5 × ULN, left ventricular ejection fraction ≥50%, and pulmonary artery systolic pressure ≤1.5 × ULN). Exclusion criteria included treatment with an investigational antileukemic agent within 14 days prior to entering study, uncontrolled intercurrent illness, and known infection with HIV and/or viral hepatitis B or C. Moderate and strong CYP3A inhibitors or chronic continuous use of CYP3A inducers were not permitted. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. All patients provided written informed consent, and the trial was approved by the local institutional review board for each enrolling site.
Study design
This open-label, phase 1b study was conducted at 10 sites across the United States. The primary objectives were to determine the dose-limiting toxicities (DLTs) and MTDs of PEV when combined with fixed doses of AZA. Secondary objectives included descriptions of PEV pharmacokinetics (PK) in whole blood and a preliminary assessment of antitumor activity. PEV was administered via a 60-minute IV infusion on days 1, 3, and 5 in escalating doses beginning at 20 mg/m2. AZA was administered IV only during dose escalation and IV or subcutaneously (SC) during dose expansion, in standard doses (75 mg/m2), on days 1 to 5, 8, and 9. Cycles were repeated every 28 days, and treatment continued until disease progression or unacceptable toxicity. Dose escalation was performed using continual reassessment method (CRM), which used noninformative β priors with a target DLT rate of 25%. The MTD was determined to be the highest dose level at which at least 6 patients were dosed (at any dose level) and the CRM algorithm did not recommend escalation or de-escalation. DLTs were defined in cycle 1 only as grade ≥3 toxicity related to study drug (exceptions were arthralgia/myalgia despite optimal use of analgesia, fatigue <1 week, hypophosphatemia, and prolonged partial thromboplastin time /activated partial thromboplastin time without clinical bleeding). Upon determining the MTD, there was a preplanned expansion of 55 patients at the MTD to better define the safety profile and gather a preliminary assessment of efficacy at the recommended phase 2 dose (see supplemental Figure 1 [available on the Blood Web site] for the Consolidated Standards of Reporting Trials diagram describing patient disposition in the study).
Safety and efficacy assessments
Patient demographics and medical history were recorded at baseline. Adverse event (AE) assessments, physical examination, vital signs, and ECOG PS were documented at baseline and on day 1 of subsequent cycles for the duration of the study. Safety was assessed from informed consent to 30 days after final doses of study therapy. Treatment-emergent (all-cause) AEs were graded according to the National Cancer Institute’s Common Terminology Criteria for AEs, version 4.03.20 Patients with AML were assessed for efficacy according to published International Working Group criteria.21
PK analysis
Serial blood samples for the determination of PEV concentrations were obtained during the first cycle of treatment, at prespecified time points before and up to 48 hours after the start of the infusion on days 1 and 5. Noncompartmental analyses (using WinNonlin software, Version 6.2, Pharsight Corporation, Cary, NC) were used to estimate the observed maximum concentration (Cmax; theoretical end-of-infusion concentration), the time at which Cmax occurred (Tmax), the area under the plasma concentration–time curve from time 0 to 24 hours postdose (AUC24), the area under the plasma concentration–time curve from time 0 to the end of the dosing interval (AUC0-τ), and, data permitting, the terminal disposition phase half-life (t1/2).
Statistical analysis
Response rates were reported along with their 95% confidence intervals (CIs) using a 2-sided exact binomial test. Overall survival (OS) and 1-year survival rates along with their 2-sided 95% CIs were estimated using Kaplan-Meier methods.
NGS
High-quality DNA extracted from either bone marrow aspirates and/or blood, together with matched buccal swab samples, was available for 33 of 61 patients from MTD cohort at the time of screening. A targeted next-generation sequencing (NGS) panel consisting of 116 genes, comprising genes implicated in myeloid neoplasms as well as genes involved in pathways modulated by PEV (supplemental Table 1A) was constructed. Samples were sequenced on an Illumina HiSeq with 76-bp paired-end reads to meet a mean target coverage of 500× (±5%) (tumor average coverage = 10 430×, normal average coverage = 10 296×) as measured by the Broad’s Picard bioinformatics pipeline. Demultiplexed, aggregated Picard BAM files were analyzed to identify single-nucleotide variants and insertions/deletions. Single-nucleotide variants and insertions/deletions were identified with VarScan.v2.3.9, and false positives were filtered with the fpfilter function. After false-positive removal, alterations in highly mutated AML genes were kept if they matched the table of known variations (supplemental Table 1B). Genes with low AML mutation frequency were kept if the P value was < .01 and the coverage was 100× or greater. Notably, this targeted NGS methodology did not allow for the identification of mutations in the CEBPα gene and FLT3-ITD mutations.
Results
Sixty-four patients were enrolled into 2 dose levels in this study and included in all assessments of safety, demographics, and baseline disease characteristics. Efficacy assessments were confined to the MTD cohort patients (PEV 20 mg/m2 + AZA [n = 61]) treated at the recommended phase 2 dose for all subsequent phase 2 and 3 studies.
Patient characteristics
Patient demographics are displayed in Table 1. Sixty-four patients with a median age of 75 years (range, 61-89 years) were treated. Of these, 53% were male. Most patients (78%) had an ECOG PS of 0 to 1. Over half of the patients enrolled had de novo AML (56%). Median marrow blast percentage was 38.5% (range, 5-92), 50% had intermediate-risk, 28% had adverse-risk, and 3% had favorable-risk cytogenetics.
DLTs and MTD determination
PEV dosing was started at 20 mg/m2 (n = 6) and increased to 30 mg/m2 (n = 3) in the absence of DLTs. At the 30-mg/m2 dose level, 2 of the three patients experienced a DLT: 1 patient had persistent grade 2 bilirubin elevation and 1 patient had reversible grade 4 AST elevation. Transaminase and bilirubin elevations were transient and clinically inconsequential in both patients (resolving to grade 1 or baseline levels within 1 week of withdrawal from study). The MTD for PEV was declared at 20 mg/m2 when combined with AZA in standard doses, based on the final posterior estimate of probability of toxicity at 20 mg/m2 being 24% using the CRM model. In the MTD expansion cohort (n = 55), 2 additional patients experienced DLTs (grade ≥3 transaminase elevation) and were successfully rechallenged with a reduced dose of PEV. Both patients remained on study without further hepatic toxicity.
Safety
Treatment-emergent AE data for the patients in the intention-to-treat cohort (n = 64) are presented in Table 2. Patients received a median of 4 cycles (range, 1-37), and 23 out of 64 patients (36%) received ≥6 cycles of therapy. The most common AEs were constipation (48%), nausea (42%), fatigue (42%), and anemia (39%). Fifty-three patients (83%) experienced grade ≥3 AEs; the most frequent (≥15%) were anemia and febrile neutropenia (each 30%), thrombocytopenia (23%), neutropenia (20%), and pneumonia (17%). Increased liver enzymes (grade ≥3 increase in either AST or ALT) were reported in 6% of patients. Forty-four patients (69%) experienced serious AEs; the most frequent (≥10%) were febrile neutropenia (25%) and pneumonia (14%). In addition to the 2 patients who withdrew due to DLTs, 2 additional patients withdrew from the study due to febrile neutropenia, which was considered by the investigator to be related to both PEV and AZA. There were 11 on-study deaths due to progression of disease or disease-related events that were unrelated to study therapy.
PK
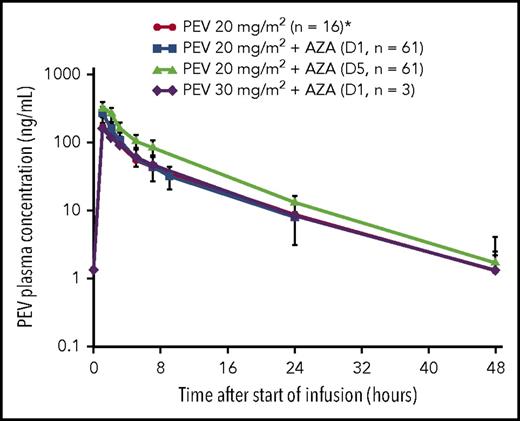
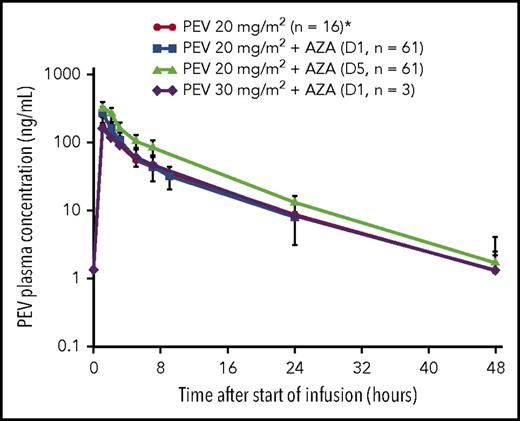
All relevant PK parameters of PEV administered in combination with AZA are summarized in Tables 3 and 4. Mean and individual PK profiles of PEV on cycle 1, day 1, and day 5 exhibited a biphasic disposition phase, whereby PEV plasma concentrations were measurable up to 24 hours postdose in all patients and up to 48 hours postdose in approximately half of the patients (Figure 1). Systemic exposure data indicate that PEV PK was not altered in the presence of AZA when compared with historical single-agent data.9,22 Additionally, when comparing individual PK profiles on day 5 vs day 1, PEV exposures remained unchanged following 5 days of continuous dosing with AZA (observed accumulation ratio ∼1; Figure 1).
Mean (standard deviation) PK profile after 1-hour IV infusion of PEV in combination with AZA in elderly patients with treatment-naive AML. *Derived from single-agent PEV data in patients with AML.9
Mean (standard deviation) PK profile after 1-hour IV infusion of PEV in combination with AZA in elderly patients with treatment-naive AML. *Derived from single-agent PEV data in patients with AML.9
Efficacy
A total of 64 patients were treated (ITT population). Among the 3 patients treated at the 30-mg/m2 PEV dose, 1 patient discontinued following a best response of stable disease, which lasted ∼1 month, and discontinued due to a serious adverse event (SAE) (grade 3 pneumonia); 1 patient achieved a CR (which had a duration of ∼4 months), at which point the patient discontinued treatment due to progressive disease; and 1 patient discontinued the study prior to the first disease assessment because of symptomatic deterioration (supplemental Figure 1). Among the 61 patients in the MTD cohort, 9 had no postbaseline disease assessments (supplemental Figure 1). One patient withdrew consent, 1 was lost to follow-up, and 7 discontinued treatment prior to their first marrow assessment due to experiencing SAEs: 3 patients had pneumonia, and 1 patient each had sepsis, mental health status change, pulmonary edema/congestive heart failure, or multiple organ failure.
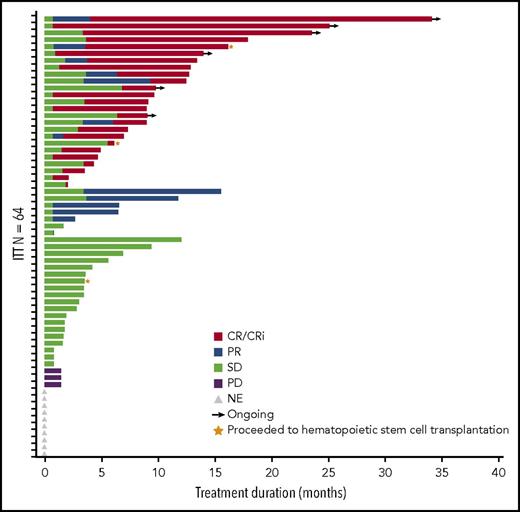
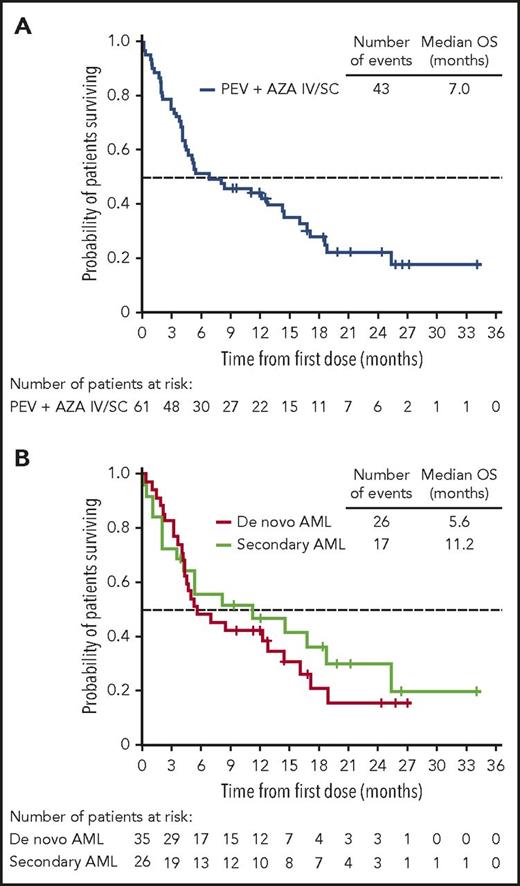
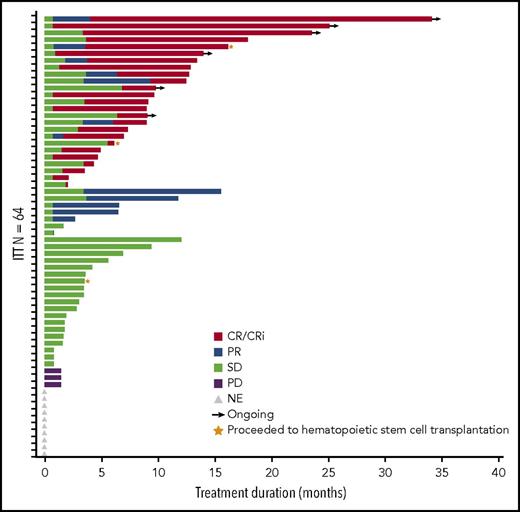
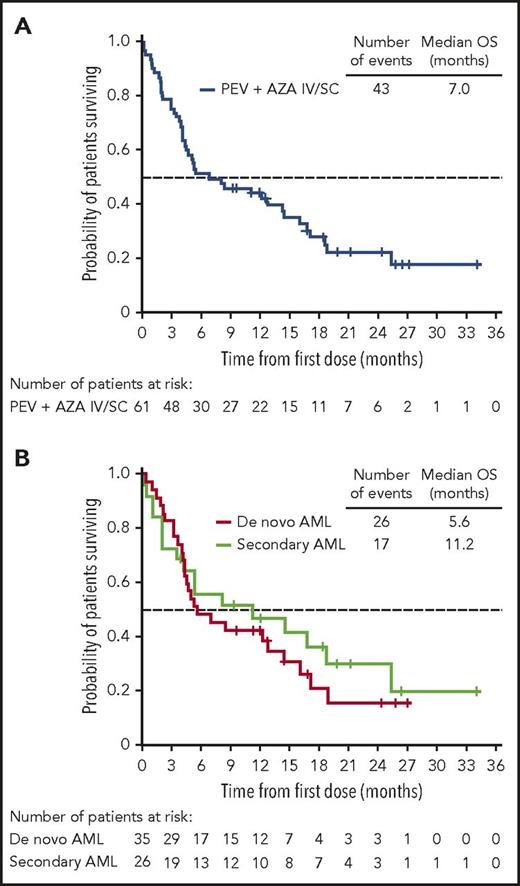
The ORR in the 64-patient ITT cohort was 50% (20 CR, 5 complete remission with incomplete peripheral count recovery (CRi), and 7 PR), with a median duration of remission of 8.3 months (95% CI, 5.52-12.06 months; Figure 2). Of the responding patients, 63% (20/32) responded within the first 2 cycles of treatment (supplemental Table 2), 14 had responses lasting ≥4 cycles, and 2 proceeded to allogeneic stem cell transplantation. In total, 3 patients proceeded to stem cell transplantation, as they met physiologic requirements and agreed to pursue the treatment; details of these patients are provided in supplemental Figure 1. The ORR was respectively 52% (13/25; 7 CR, 3 CRi, and 3 PR) vs 49% (19/39; 13 CR, 2 CRi, and 4 PR) for patients with low (<30%) vs high (≥30%) marrow blast percentage; 53% (19/36; 12 CR, 3 CRi, and 4 PR) vs 46% (13/28; 8 CR, 2 CRi, and 3 PR) for de novo vs secondary AML patients; and 44% (14/32; 9CR, 1 CRi, and 4 PR) vs 44% (8/18; 5 CR, 2 CRi, and 1 PR) for intermediate-risk vs adverse-risk patients. As expected, patients were more likely to respond if they received ≥6 cycles vs <6 cycles of treatment (ORR 83% [19/23]; 14 CR, 2 CRi, and 3 PR) vs 32% (13/41; 6CR, 3 CRi, and 4 PR]) (Table 5). We further scrutinized timing of the responses achieved among the patients and observed that the majority of patients (91%) achieved responses within the first 2 to 4 cycles regardless of how long they have been treated (supplemental Table 2). Fourteen patients (half with adverse cytogenetics) remained on study for more than 1 year (≥13 cycles, maximum ≥48 cycles; 2 were still active on study at the time of submission of the manuscript) in whom the best responses were CR/CRi (n = 11), PR (n = 2), or stable disease (SD; n = 1). Among the entire cohort of 61 patients treated at the MTD (median follow-up of 21.2 months), survival at 6 months was 52% (95% CI, 38%−63%) and 45% at 1 year (95% CI, 32%−57%); median OS was 7 months (95% CI, 4.5−14.5 months) and 11.2 months (95% CI, 3.5−25.3 months) vs 5.6 months (95% CI, 4.3−14.4 months) for secondary AML vs de novo patients (Figure 3; supplemental Table 3). We evaluated the OS differences between patients who had achieved CR, CRi/PR, and no CR/CRi/PR and observed statistically significant differences (log-rank P < .05) between the Kaplan-Meier survival curves (CR vs CRi/PR groups showed median OS of 18.8 months vs 8.3 months, respectively; supplemental Figure 2). In the MTD cohort, the median OS was 11.2 months (95% CI, 4.5 months to NE) vs 5.2 months (95% CI, 3.5-14.4 months) for patients with low (<30%) vs high (≥30%) marrow blasts and 16.1 months (95% CI, 3.6-25.3 months) vs 5.3 months (95% CI, 4.3-12.8 months) for patients aged 65 to 74 vs ≥75 years, respectively.
Duration of response. Bar length reflects duration of response. End of duration is due to progressive disease (PD), last disease assessment, or last follow-up. Gray triangles indicate the 10 patients who did not have postbaseline disease assessment and are listed as not evaluable (NE) but included as “nonresponders” in the ITT analysis. SD, stable disease.
Duration of response. Bar length reflects duration of response. End of duration is due to progressive disease (PD), last disease assessment, or last follow-up. Gray triangles indicate the 10 patients who did not have postbaseline disease assessment and are listed as not evaluable (NE) but included as “nonresponders” in the ITT analysis. SD, stable disease.
Kaplan-Meier survival analyses in the MTD cohort. (A) OS of patients treated at the MTD, including the 9 response-unevaluable patients. (B) OS of de novo AML vs secondary AML patients.
Kaplan-Meier survival analyses in the MTD cohort. (A) OS of patients treated at the MTD, including the 9 response-unevaluable patients. (B) OS of de novo AML vs secondary AML patients.
Molecular analysis results
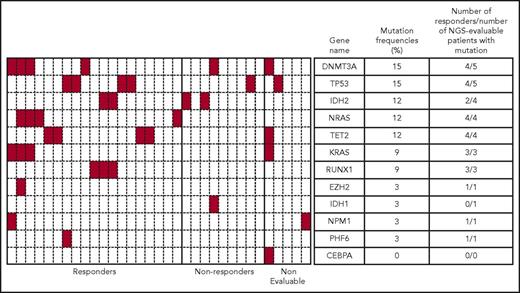
Targeted NGS identified a heterogenous mutation profile for the tumor DNA samples sequenced. Only 46 of the 116 genes sequenced were shown to be mutated in this patient cohort, with 1 to 7 genes mutated per patient. Figure 4 provides a summary of baseline mutations correlated with response for 12 frequently mutated AML genes. In this subset analysis, the mutational frequencies of these genes, except for TP53 (5/33 patients; 15%), were consistent with frequencies previously reported.23 The mutation status and frequencies for the remaining genes are provided in supplemental Figure 3.
Heatmap showing mutational status of 12 frequently mutated genes and response data for patients in the MTD cohort. Genetic mutation data for 33 of 61 patients identified using a targeted NGS panel are shown. Each column represents a single patient, and each row represents a single gene. Presence of a mutation in any gene is denoted in red. Mutation frequency = (number of patients with mutation/number of NGS-evaluable patients) × 100. In this figure, responders = CR + CRi + PR.
Heatmap showing mutational status of 12 frequently mutated genes and response data for patients in the MTD cohort. Genetic mutation data for 33 of 61 patients identified using a targeted NGS panel are shown. Each column represents a single patient, and each row represents a single gene. Presence of a mutation in any gene is denoted in red. Mutation frequency = (number of patients with mutation/number of NGS-evaluable patients) × 100. In this figure, responders = CR + CRi + PR.
Discussion
Optimal management of newly diagnosed AML patients who are unfit for induction therapy is a topic of considerable debate. Guideline recommendations for the treatment of this group include the use of hypomethylating agents (AZA or decitabine).24 AZA was shown to prolong OS compared with conventional care regimens (CCRs) in the subset of older patients with 20%−30% bone marrow blasts on the phase 3 AZA-001 trial.7 Similarly, AZA was associated with a median OS of ∼10 months in patients with AML who participated on the Austrian AZA registry.25 The phase 3 AZA-AML-001 study6 prospectively randomized older unfit patients with increased marrow blasts (> 30%) to receive AZA or CCR (physician’s choice of best supportive care only, low-dose cytarabine, or standard induction chemotherapy). In this trial, response rates for AZA vs CCR were 27.8% vs 25.1%, median OS was 10.4 vs 6.5 months, and 1-year survival was 46.5% vs 34.2%. In an attempt to improve on these data, a number of early-phase clinical trials have tested the potential of newer agents when combined with AZA.8,26-28 Of these, PEV, a novel inhibitor of NAE, potently impairs the viability of cancer cells in laboratory models of AML18 as well as other tumor types.12,29-42 Several preclinical PEV combination approaches have now been published (including PEV/AZA combinations in AML).19,30,43-59 Safety and efficacy data are available on over 300 patients treated with PEV from early-phase studies in both solid and hematologic malignancies, including AML.9 Here, we tested for the first time the potential of PEV to enhance the activity of AZA in patients with AML considered unfit for intensive chemotherapy.
Overall, the combination of PEV and AZA in this older population was well tolerated. The nature and frequency of the toxicities typically observed for AZA monotherapy (fatigue, gastrointestinal toxicity, myelosuppression, and SC injection site pain)6,7 did not change significantly with the addition of PEV in this study. Transient elevation in liver enzymes was dose limiting for 4 patients, none of whom experienced clinical sequelae. Two of these patients were successfully rechallenged with lower doses of PEV and remained on protocol. PEV-related hepatic toxicity has been reported in other studies.36-38,60 In the dose-escalation phase of this study, we used a more conservative lower starting dose of 20 mg/m2 of PEV compared with doses used in previous single-agent phase 1 studies, primarily to ensure safety. Azacitidine is metabolized in the liver; hepatoxicity with single-agent AZA has been noted in both animal studies and in patients,61 and elevated liver function tests had previously been determined to be dose limiting in single-agent PEV phase 1 studies.9 Proposed on-target mechanisms accounting for this toxicity include the disruption of cytoskeletal proteins in hepatic cells as well as sensitization of these cells to toxic cytokines, such as tumor necrosis factor α.60 The main reasons for withdrawal from study were disease progression and AEs (mainly unrelated to study therapy). Four patients (6.2%) came off protocol for therapy-related events (2 of these patients had DLTs; the other 2 had febrile neutropenia considered by investigators to be therapy related). Eleven patients (17.1%) died of disease progression, and no toxic deaths were reported.
The median number of cycles of treatment with AZA plus PEV was 4, and most patients received ≥6 cycles of therapy. This is noteworthy as the addition of new agents to AZA can increase toxicity and potentially compromise total AZA exposure if patients are withdrawn early. In a recent placebo-controlled randomized study (n = 102), the oral histone deacetylase inhibitor pracinostat8 failed to increase the efficacy of AZA alone in patients with high-risk MDS. This was attributed to higher rates of early discontinuation due to AEs (within the first 2 cycles) for patients treated with combined therapy. In the North American Intergroup MDS study62 comparing AZA plus lenalidomide vs AZA plus vorinostat (a histone deacetylase inhibitor) vs AZA alone (n = 277), patients randomized to the combination treatment were more likely to discontinue therapy early, more likely to undergo off-protocol dose modification, and less likely to undergo subsequent bone marrow biopsies to assess response. This study also failed to demonstrate an advantage for AZA combinations over AZA alone in a similar patient population.
In the current phase 1b study, the ORR was 50%, which includes 10 patients in the ITT analysis who either withdrew consent or suffered from clinical deterioration prior to their first disease assessment. The characteristics of responses observed in this trial suggest benefit from the addition of PEV. Most responding patients achieved their responses within 2 cycles of therapy (63%), and almost all the responses reported occurred within 4 cycles of therapy (91%). Notably, bone marrow blast percentage or cytogenetic risk did not appear to influence the likelihood of achieving a response following treatment with PEV and AZA in this study. For patients receiving <6 cycles (n = 41) of therapy, ORR was 32%; for those who received ≥ 6 cycles (n = 23) of therapy, ORR was 83%. These responses are explained, in part, by the favorable nonoverlapping toxicity profile of PEV, which allowed for optimal AZA dosing in this study. In patients with TP53 mutations, the composite CR/PR rate was 4 out of 5 (80%). Two responding patients stayed on study for >10 cycles. Further review of the molecular pathology reports collected from sites identified 3 additional patients with mutations in TP53; 2 achieved a CR. In total, 6 out of 8 TP53-mutated patients achieved CR/CRi/PR, and 4 out of 6 remained on study for >10 cycles. The mutational frequency of TP53 on this study (8/61 [13.1%]) was comparatively higher than that previously reported (13.1% vs 7%),63 perhaps suggesting a more aggressive disease in the patient population enrolled in this study, as TP53 alterations in AML are generally associated with older age, genomic complexity, monosomal karyotype, and shorter OS.63 Nonetheless, responses were seen in patients who often have refractory disease. It is worth noting that in a recent prospective study of patients with AML, those with TP53-mutated AML responded well to extended-dose decitabine, implying a potential advantage to strategies that include azanucleosides in TP53-mutated AML.64 Beyond TP53 mutations, NGS performed on a subset of treated patients (Figure 4) did not reveal a robust biomarker of response to PEV but did confirm responses independent of mutational profile.
The development of PEV as a new therapeutic strategy for patients with myeloid neoplasms continues with rational combination studies informed by preclinical studies planned with both standard (PEV plus decitabine and PEV plus low-dose cytarabine) and investigational agents (eg, Bcl2 inhibitors and others) in AML, MDS, and in “overlap syndromes” (MDS/myeloproliferative neoplasms). Further studies will be guided by a deeper understanding of responsiveness to therapy with PEV. For example, given that PEV can repress nuclear factor κB−dependent gene expression, it has been proposed that PEV could modulate overexpression of the nuclear factor κB−dependent microRNA MIR155HG, which may offer an advantage to patients with normal-karyotype AML, where this microRNA adversely impacts survival.47 In summary, in this phase 1b trial for older patients with AML who were unfit for high-dose induction therapy, combined treatment with PEV and AZA was well tolerated. The timing and frequency of responses suggest potential benefit from the addition of PEV to a standard regimen of single-agent AZA.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank their patients for participating in this study.
This research was supported by Takeda Pharmaceuticals, Inc.
Authorship
Contribution: R.T.S., W.T., S.V., I.P.-D., H.M.F., F.S., B.J.D., and M.R.S. were involved in the conception and design of the study; R.T.S., S.C., M.B.M., J.F.Z., J.M.F., J.C., H.P.E., J.G.B., W.T., S.V., I.P.-D., H.M.F., A.B.D., F.S., B.J.D., and M.R.S. were responsible for the collection and assembly of data; R.T.S., S.C., M.B.M., J.F.Z., J.M.F., J.C., H.P.E., J.G.B., W.T., S.V., I.P.-D., H.M.F., A.B.D., F.S., B.J.D., D.V.F., and M.R.S. were involved in data analysis and interpretation; R.T.S. and M.R.S wrote the manuscript; and all authors were responsible for reviewing and approving the final draft of the manuscript.
Conflict-of-interest disclosure: R.T.S. is a consultant for Novartis and receives research funding from Millennium. S.C. is a consultant for Janssen, Pharmacylics, and AbbVie and receives research funding from AbbVie. J.F.Z. receives honoraria from Agios, Celgene, and Tolero and research funding from Merck and Millennium. J.M.F. receives honoraria from Novartis, Pfizer, Karyopharm, and Medscape and research funding from Millennium. J.C. is on the Millennium speakers bureau and receives honoraria from Millennium. H.P.E. is a consultant for Novartis, Celgene, Seattle Genetics, Amgen, Daiichi Sankyo, Sunesis, Janssen, Ariad, and Pfizer; on the data safety and monitoring board at Incyte and Gylcomimetics; on the speakers bureau at Incyte and Celgene; and receives research funding from Seattle Genetics, Amgen, Daiichi Sankyo, Janssen, Celator, Millennium, Astellas, Agios, and Juno. W.T. is a consultant for Millennium. S.V., I.P.-D., H.M.F., A.B.D., F.S., B.J.D., and D.V.F. are Millennium employees. M.R.S. is a consultant for and receives research funding from Astex, Incyte, Millennium, Sunesis, and TG Therapeutics; is on the data safety and monitoring board at Celgene and Gilead; and is a consultant for and has equity in Karyopharm. J.G.B. and M.B.M. declare no competing financial interests.
Correspondence: Ronan T. Swords, Leukemia Program, 1475 NW 12th Ave, Clinical Research Building (CRB), 610G, Sylvester Comprehensive Cancer Center, Miller School of Medicine, University of Miami, Miami, FL 33136; e-mail: rswords@med.miami.edu; and Michael R. Savona, Division of Hematology/Oncology, 777 Preston Research Building, 2200 Pierce Ave, Vanderbilt-Ingram Cancer Center, Vanderbilt University Medical Center, Nashville, TN 37232; e-mail: michael.savona@vanderbilt.edu.