Abstract
Background: The combination of all-trans-retinoic acid (ATRA) plus arsenic trioxide (ATO) has revolutionized the treatment of pts with APL. However, despite a remission rate exceeding 96% and 5-year disease-free and overall survival rates reaching 96% and 88%, respectively, death during induction still occur in 4% of the patients. We conducted this study to identify factors that were associated with 30-day mortality, ICU admission, intubation, or differentiation syndrome in patients with APL receiving this combination therapy.
Methods: We retrospectively analyzed the charts of 187 pts with APL who were treated with ATRA and ATO at MD Anderson Cancer Center. Pts with white blood cell count (WBC) > 10x109/L at presentation or during induction received one dose of Gemtuzumab Ozogamicin (GO) (9 mg/m2). Analyzed variables were age, gender, admission (baseline) weight, change in weight (percentage) from baseline to ICU admission or to maximum weight throughout induction cycle, WBC, hemoglobin, platelets, peripheral blasts, lactate dehydrogenase (LDH), creatinine, albumin, and bilirubin at presentation.
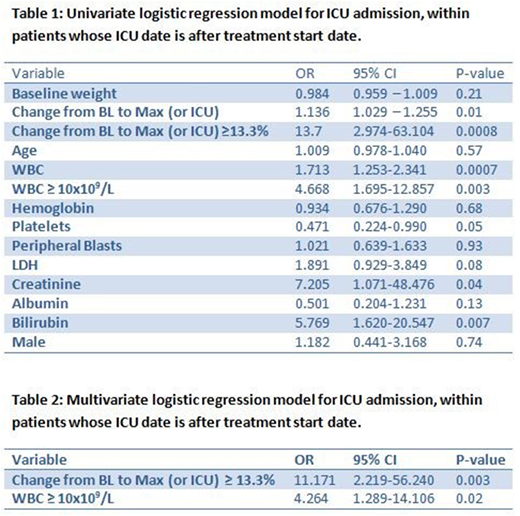
Results: The median age was 50 years (range, 14-84 years), 52% were male, and median WBC count at presentation was 2x109/L (range, 0.3-188x109/L). Median weight at presentation was 91 Kg (range, 49.1-177.6 Kg). During induction, weight increased ≥ 10% from baseline in 26 patients and ≥ 5% in 63 patients. Median increase in weight from baseline to maximum was 3.2% (range, 0-27.5%) and median increase in weight from baseline to ICU admission was 5% (range, 0- 21.6%) after excluding patients who were in ICU before start of treatment (n=27). Differentiation syndrome was reported in 22 (11.8%) patients; four were admitted to ICU and one required intubation. A total of 45 (24.1%) patients were admitted into ICU; 12 of them (26.7%) required intubation. Reasons for ICU admission (one or more reason per patient) included respiratory insufficiency (n=27), intracranial hemorrhage (n=15), leukocytosis (n=7), disseminated intravascular coagulation (n=5), differentiation syndrome (4) and diabetic ketoacidosis (n=1). A total of 7 (3.7%) patients died during induction; 6 of them were in ICU. Causes of death were disseminated intravascular coagulation (DIC) and multi-organ failure in 2 patients, diffuse alveolar hemorrhage (DAH) in 2 patients, intracranial hemorrhage in 1 patient, sepsis in 1 patient, and respiratory arrest in 1 patient. Univariate analysis for 30-day mortality showed that age, high creatinine, low hemoglobin and albumin, were significantly associated with increased risk of death within 30 days. After excluding 27 patients who were already admitted to ICU prior to or at the time of treatment, univariate analysis showed that increase in weight, high WBC, creatinine and bilirubin, and low platelets, were significantly associated with higher risk of ICU admission (Table 1). Based on the fitted multiple logistic regression model, increase in weight of ≥ 13% (p=0.003) and WBC ≥ 10x109/L (p=0.02) were associated with increased risk of ICU admission (Table 2). Increase in weight and low albumin were significantly associated with higher probability of intubation. Additionally, univariate analysis for differentiation syndrome suggested that increase in weight, high WBC and bilirubin were significantly associated with the probability of developing differentiation syndrome. However, based on the fitted multiple logistic regression model, only increase in weight of ≥ 9% (p<0.0001) was associated with an increased risk of differentiation syndrome.
Conclusion: Fluid overload, measured by weight gain, is an unrecognized complication in patients with APL receiving ATRA + ATO +/- GO. In addition to high WBC, fluid overload is associated with an increased risk of ICU admission and intubation but not death during induction.
Jabbour:Pfizer: Consultancy, Research Funding; Novartis: Research Funding; Takeda: Consultancy, Research Funding; Abbvie: Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding. Kadia:Takeda: Consultancy; Celgene: Research Funding; Amgen: Consultancy, Research Funding; Celgene: Research Funding; BMS: Research Funding; Abbvie: Consultancy; Abbvie: Consultancy; Pfizer: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy; Jazz: Consultancy, Research Funding; Jazz: Consultancy, Research Funding; Novartis: Consultancy; Takeda: Consultancy; BMS: Research Funding; Amgen: Consultancy, Research Funding. Daver:ARIAD: Research Funding; Kiromic: Research Funding; ImmunoGen: Consultancy; Pfizer: Research Funding; BMS: Research Funding; Karyopharm: Research Funding; Incyte: Consultancy; Daiichi-Sankyo: Research Funding; Otsuka: Consultancy; Incyte: Research Funding; Novartis: Consultancy; Pfizer: Consultancy; Novartis: Research Funding; Alexion: Consultancy; Sunesis: Research Funding; Sunesis: Consultancy; Karyopharm: Consultancy. DiNardo:Celgene: Honoraria; Abbvie: Honoraria; Karyopharm: Honoraria; Medimmune: Honoraria; Agios: Consultancy; Bayer: Honoraria. Jain:Cellectis: Research Funding; BMS: Research Funding; Pfizer: Research Funding; Verastem: Research Funding; Genentech: Research Funding; Pfizer: Research Funding; Servier: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Celgene: Research Funding; Infinity: Research Funding; Pharmacyclics: Research Funding; Seattle Genetics: Research Funding; Incyte: Research Funding; Adaptive Biotechnologioes: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Astra Zeneca: Research Funding; Genentech: Research Funding; Pharmacyclics: Research Funding; ADC Therapeutics: Research Funding; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Infinity: Research Funding; Abbvie: Research Funding; Seattle Genetics: Research Funding; Incyte: Research Funding; Celgene: Research Funding; Astra Zeneca: Research Funding; Servier: Research Funding; Verastem: Research Funding; Cellectis: Research Funding; Adaptive Biotechnologioes: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Konopleva:Stemline Therapeutics: Research Funding. Cortes:Daiichi Sankyo: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Arog: Research Funding. Ravandi:Macrogenix: Honoraria, Research Funding; Bristol-Myers Squibb: Research Funding; Sunesis: Honoraria; Xencor: Research Funding; Jazz: Honoraria; Sunesis: Honoraria; Orsenix: Honoraria; Macrogenix: Honoraria, Research Funding; Abbvie: Research Funding; Orsenix: Honoraria; Seattle Genetics: Research Funding; Astellas Pharmaceuticals: Consultancy, Honoraria; Abbvie: Research Funding; Seattle Genetics: Research Funding; Amgen: Honoraria, Research Funding, Speakers Bureau; Amgen: Honoraria, Research Funding, Speakers Bureau; Astellas Pharmaceuticals: Consultancy, Honoraria; Bristol-Myers Squibb: Research Funding; Xencor: Research Funding; Jazz: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.