FLT3/ITD mutations are among the most common in AML and are associated with adverse outcome. The addition of FLT3 inhibitors (FLT3-I) to cytotoxic therapy has provided improvements in outcome. We have previously shown that co-occurring mutations impact the clinical implications of FLT3/ITD, where those with NUP98-NSD1 fusions and/or WT1 mutations do particularly poorly (Figure 1A), regardless of ITD allelic ratio (AR). FLT3-I act on mutated and aberrantly activated FLT3, thus may be most effective in AML that is primarily driven by overactive FLT3 signaling. Given the significant overlap between FLT3/ITD and WT1 and NUP98-NSD1, we questioned whether response to FLT3-I may be modulated by these co-occurring variants.
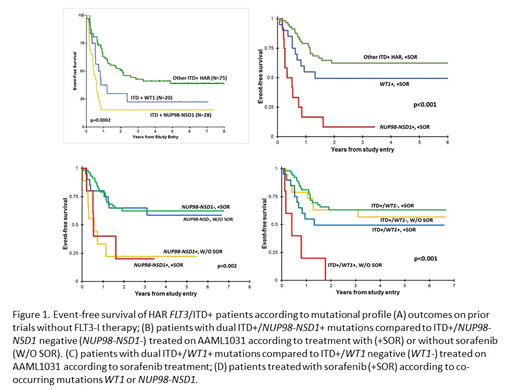
The Children's Oncology Group phase III trial AAML1031 treatment arm for patients with high AR (HAR; >0.4) FLT3/ITD mutations evaluated the safety and efficacy of the FLT3-I sorafenib in combination with chemotherapy and hematopoietic stem cell transplant (HSCT). A total of 136 patients with HAR ITD were enrolled, 92 patients treated on the sorafenib arm (+SOR) with complete clinical and biologic (n=88) data available. An additional 42 patients were included in the primary randomization of +/- bortezomib and consolidation HSCT without sorafenib (w/o SOR). As equivalent outcomes were observed in the primary randomization, this cohort was combined for further analysis to serve as a control to evaluate outcomes of patients not receiving sorafenib. In addition to conventional karyotyping and mutational profiling (determination of the FLT3/ITD and respective AR by fragment length analysis), all patients were evaluated for NUP98-NDS1 by RNAseq and WT1 profiling by NGS or, hotspot sequencing of exons 7 and 9. Response to sorafenib was measured by 3-year overall survival (OS), event-free survival (EFS), and relapse risk (RR).
We compared outcomes for patients +SOR to w/o-SOR to according to co-occurring mutations, specifically NUP98-NSD1, WT1, and triple positive (TP; ITD/NUP98-NSD1/WT1). Patients treated +SOR experienced a combined OS of 62% vs. 68% w/o-SOR (p=0.562). Patients with ITD+/NUP98-NSD1+ disease (WT1+ patients excluded) treated +SOR and w/o-SOR had similar poor outcomes with 3-year EFS of 22% and 20% respectively (p=0.912; Figure 1B). Among patients treated w/o SOR, NUP98-NSD1+ had significantly inferior outcomes compared to NUP98-NSD1-negative (neg) patients (EFS: 20% vs. 65%, p=0.02). NUP98-NSD1+ patients had similar dismal outcomes +SOR treatment, and inferior to NUP98-NSD1-neg patients treated +SOR (p=0.015; Figure 1B). Thus, sorafenib treatment did not improve outcomes of HAR ITD+/NUP98-NSD1+ patients. Analysis of the impact of co-occurring WT1 mutations (NUP98-NSD1+ patients excluded) demonstrated that ITD+/WT1+ patients had an adverse outcome w/o-SOR (EFS 0% and RR 100%), which was significantly inferior to WT1-neg patients treated w/o-SOR (p=0.002 and p=0.003 respectively; Figure 1C). However, WT1+ patients treated +SOR had much improved outcomes with an EFS of 50% and corresponding RR of 27%, which was comparable to WT1-neg patients treated +SOR (Figure 1C). Thus, sorafenib treatment resulted improved outcomes for ITD+/WT1+ patients. Combining all NUP98-NSD1+ patients, TP patients experienced poor EFS of 23%, similar to 21% for ITD+/NUP98-NSD1+/WT1-neg. Thus, we show that NU98-NSD1 fusions drive the dismal prognosis, as ITD+/NUP98-NSD1+ patients, regardless of additional mutations, experienced significantly inferior outcomes compared to other ITD+ patients, including WT1+, that was not improved by treatment with sorafenib in combination with chemotherapy and HSCT (Figure 1D).
We show that sorafenib improved outcomes among the HAR ITD+/WT1+ patients, as this group of patients who did not receive sorafenib had very inferior outcomes, in line with previous findings for this poor risk cohort, while patients treated with sorafenib did much better with outcomes similar to other ITD+ patients. However, sorafenib failed to improve outcomes for NUP98-NSD1+ patients, further highlighting that novel therapeutic strategies will be needed for this group of patients. As the addition of FLT3-I is investigated in HAR FLT3/ITD+ patients, evaluation of its impact across patients with additional oncogenic mutations will be critical to determine which patients will derive the most benefit from this strategy.
No relevant conflicts of interest to declare.
Sorafenib in FLT3/ITD-positive AML
Author notes
Asterisk with author names denotes non-ASH members.