Background
In patients with Myelodysplastic Syndromes (MDS), TP53 mutations associate with high-risk presentation, complex karyotype, acute myeloid leukemia (AML) progression and poor response to hematopoietic stem cell transplantation. These associations highlight the relevance of TP53 as a prognostic and predictive biomarker. Consistent with its role as a tumor suppressor, bi-allelic targeting of the TP53 locus is a frequent but not an obligatory event. Despite the central role of TP53 in MDS, the clinical implications of TP53 mutations in the context of allelic state have not been extensively studied.
Methods
Under the auspices of the International Working Group for Prognosis in MDS, we sequenced a representative cohort of 3,324 peri-diagnosis MDS patients on a next generation sequencing (NGS) panel optimized for myeloid disease. Conventional G-banding analysis (CBA) was available for 2,931 patients. Focal (~3MB) gains and deletions and regions of NGS-derived copy-neutral loss of heterozygosity (cnLOH) were assessed using an in-house algorithm CNACS. Putative oncogenic mutations in TP53 were characterized by consideration of normal controls and established population databases. A validation cohort of 1,120 samples with independent but comparable molecular and clinical annotation was sourced from a compendium of Japanese MDS data to include JALSG-MDS212, JMDP registry, and regional registries.
Results
NGS-derived ploidy alterations and CBA show a high genome-wide concordance. From NGS profiles, 11% of patients (n=360) are subject to cnLOH, of which 80 target the TP53 locus. We characterize 490 TP53 mutations in 380 patients, representing 11% of the cohort. Amongst those patients, 22% (n=85) and 21% (n=78) have a deletion or a cnLOH involving the TP53 locus, respectively. Taken together, these segregate patients into two TP53 states: a mono-allelic state where one wild type allele remains (33% of TP53 mutated patients, n=126); and a multi-hit state where TP53 is altered multiple times by either mutations, deletions or cnLOH (67% of TP53 mutated patients, n=254).
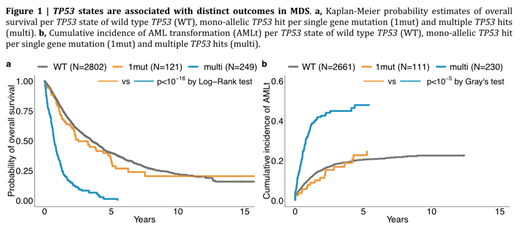
We find that TP53 state shapes clinical presentation and outcomes. Mono-allelic TP53 patients present with more favorable disease than multi-hit TP53 patients: they are less cytopenic, have lower bone marrow blasts (median 4 vs. 9%, p<0.0001) and are enriched in low risk WHO subtypes. We show that the established association between mutated TP53 and complex karyotype is specific to the multi-hit TP53 state (OR=66, CI: 33-141, p<0.0001). Critically, we show that multi-hit TP53 associates with worse overall survival as compared to mono-allelic TP53 (HR=3.7, CI: 2.7-5.1, p<0.0001; Figure 1a) and more pronounced AML transformation (HR=5.3, CI: 3.1-8.9, p<0.0001; Figure 1b). Patients with mono-allelic TP53 mutations have a similar survival to that of wild type TP53 patients and track overall IPSS-R, whereas multi-hit TP53 stratifies adverse prognostic subgroups independent of the IPSS-R. We formally test this using multivariate models that consider age, peripheral blood counts, blasts and IPSS-R cytogenetic score and show that multi-hit TP53 state is an independent prognostic factor for overall survival and AML transformation, whilst mono-allelic TP53 state is not significant. We also observe a significant difference in overall survival between TP53 states in the context of therapy-related MDS (HR=3.1, CI: 1.2-7.9, p=0.03). Last, analyses of 12 serial samples identify multi-hit targeting of the TP53 locus as a critical driver of AML transformation in the context of TP53-mutated MDS. These findings are replicated in the validation cohort.
Conclusions
TP53 is a natural candidate for incorporation in molecularly informed risk stratification schemas (molecular IPSS-R). We show that TP53 state rather than mutation alone is an independent diagnostic and prognostic biomarker in MDS. We propose that ascertainment of TP53 state is critical in prospective clinical sequencing for risk estimation, disease monitoring and future correlative research into predictors of response to established and investigational therapies.
Bernard:Celgene: Research Funding. Hasserjian:Jazz Pharmaceuticals: Consultancy; Promedior, Inc.: Consultancy. Germing:Celgene: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz Pharmaceuticals: Honoraria; Amgen: Honoraria. Cargo:Celgene: Research Funding. Santini:Acceleron: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Menarini: Membership on an entity's Board of Directors or advisory committees; Johnson & Johnson: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kotsianidis:Celgene: Research Funding. Takaori-Kondo:Pfizer: Honoraria; Chugai: Research Funding; Janssen: Honoraria; Kyowa Kirin: Research Funding; Takeda: Research Funding; Ono: Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Honoraria. Savona:Selvita: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm Therapeutics: Consultancy, Equity Ownership, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Patents & Royalties; Celgene Corporation: Membership on an entity's Board of Directors or advisory committees; Incyte Corporation: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sunesis: Research Funding. Ades:Takeda: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Silence Therapeutics: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Helsinn Healthcare: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding. Neuberg:Pharmacyclics: Research Funding; Madrigal Pharmaceuticals: Equity Ownership; Celgene: Research Funding. Stevenson:Celgene: Research Funding. Fenaux:Jazz: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Aprea: Research Funding; Celgene Corporation: Honoraria, Research Funding. Platzbecker:Novartis: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Celgene: Consultancy, Honoraria. Heuser:Synimmune: Research Funding; Bayer Pharma AG, Berlin: Research Funding. Valent:Blueprint: Research Funding; Pfizer: Honoraria; Celgene: Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Deciphera: Honoraria, Research Funding. Miyazaki:Nippon-Shinyaku: Honoraria; Dainippon-Sumitomo: Honoraria; Otsuka: Honoraria; Chugai: Research Funding; Novartis: Honoraria; Kyowa-Kirin: Honoraria. Finelli:Novartis: Consultancy, Speakers Bureau; Celgene Corporation: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Speakers Bureau. Atsuta:CHUGAI PHARMACEUTICAL CO., LTD.: Honoraria; Kyowa Kirin Co., Ltd: Honoraria. Gattermann:Novartis: Honoraria; Takeda: Research Funding; Alexion: Research Funding. Ebert:Broad Institute: Other: Contributor to a patent filing on this technology that is held by the Broad Institute.; Celgene: Research Funding; Deerfield: Research Funding. Bejar:Celgene: Consultancy; Takeda Pharmaceuticals: Research Funding; AbbVie/Genentech: Consultancy, Honoraria; Astex/Otsuka: Consultancy; Modus Outcomes: Consultancy; Daiichi-Sankyo: Consultancy. Greenberg:Notable Labs: Research Funding; Celgene: Research Funding; Genentech: Research Funding; H3 Biotech: Research Funding; Aprea: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees. Ogawa:Qiagen Corporation: Patents & Royalties; ChordiaTherapeutics, Inc.: Consultancy, Equity Ownership; RegCell Corporation: Equity Ownership; Dainippon-Sumitomo Pharmaceutical, Inc.: Research Funding; Kan Research Laboratory, Inc.: Consultancy; Asahi Genomics: Equity Ownership. Papaemmanuil:Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.