Background: Inotuzumab ozogamicin (INO) and blinatumomab both improve overall survival (OS) in relapsed or refractory B-cell acute lymphoblastic leukemia (ALL). Use of these effective monoclonal antibodies in the frontline setting may lead to deep and durable remissions in older adults with newly diagnosed Philadelphia chromosome (Ph)-negative B-cell ALL.
Methods: Patients (pts) ≥60 years of age with newly diagnosed Ph-negative pre-B-cell ALL, including pts who had received no more than 1 prior cycle of chemotherapy, were eligible. Pts were required to have a performance status of ≤3, total bilirubin ≤1.5 mg/dl, AST/ALT ≤3x ULN and creatinine ≤2 mg/dl. Pts received mini-hyper-CVD (cyclophosphamide and dexamethasone at 50% dose reduction, no anthracycline, methotrexate at 75% dose reduction, cytarabine at 0.5 g/m2 x 4 doses) for up to 8 cycles. INO was initially given at a dose of 1.3-1.8mg/m2 on day 3 of cycle 1 and 0.8-1.3mg/m2 on day 3 of cycles 2-4. Rituximab (if CD20+) and prophylactic IT chemotherapy were given for the first 4 cycles. Responding pts received POMP maintenance for up to 3 years. In order to decrease the risk of veno-occlusive disease (VOD), the protocol was amended in 3/2017 (pts 50+) to give INO in fractionated doses each cycle (0.6 mg/m2 on day 2 and 0.3 mg/m2 on day 8 of cycle 1; 0.3 mg/m2 on day 2 and 8 of cycles 2-4) and to administer 4 cycles of blinatumomab following 4 cycles of hyper-CVD plus INO, followed by maintenance with 12 cycles of POMP and 4 cycles of blinatumomab (1 cycle of blinatumomab after every 3 cycles of POMP). The cumulative dose of INO given before and after this most recent amendment was 4.3 mg/m2 and 2.7 mg/m2, respectively.
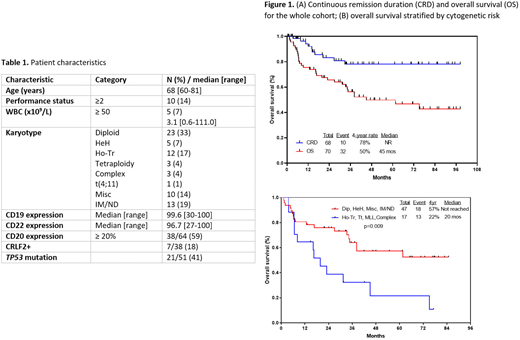
Results: 73 pts have been treated, 70 of whom are evaluable for efficacy (3 pts too early for response assessment). 6 pts were in complete remission (CR) at enrollment and unevaluable for morphological response. Pt characteristics of the 70 evaluable pts are summarized in Table 1. Median age was 68 years (range, 60-81 years); 29 pts (41%) were ≥70 years. 41% were positive for TP53 mutation, 18% were CRLF2 positive by flow cytometry, and 27% had adverse-risk karyotype. 38/64 pts (59%) were CD20+ and received rituximab.
Among 64 pts evaluable for morphologic response, 63 (98%) responded (CR, n=56; CRp, n=6; CRi, n=1). MRD negativity by flow cytometry was achieved in 53/66 pts (80%) after 1 cycle and 65/68 pts (96%) overall. There were no early deaths, and the 30-day and 60-day mortality rates were 0% and 3%, respectively.
Among 69 pts who achieved remission, 9 (13%) relapsed, 3 (4%) underwent allogeneic SCT in first remission (1 of whom subsequently relapsed), 35 (51%) remain on treatment or have completed therapy, and 21 (32%) died in CR/CRp. Notably, 6 pts (9%) developed VOD, 1 after subsequent allogeneic SCT. The rate of VOD was 6/70 (9%) with no difference in rate of VOD in pts who did or did not receive fractionated INO and blinatumomab.
With a median follow-up of 45 months (range, 2-98 months), the 4-year continuous remission and OS rates were 78% and 50%, respectively (Figure 1A). Age and cytogenetic risk were the primary factors associated with OS. The 4-year OS rate was 61% in pts 60-69 years vs. 34% in pts ≥70 years (P=0.06), driven by higher rates of death in remission in the older pts (13/29 [45%] vs. 8/41 [20%] in pts 60-69 years of age; P=0.03). These remission deaths in pts ≥70 years were primarily due to infection (n=7) or development of MDS/AML (n=3). Pts with high-risk cytogenetic features (e.g. KMT2A rearranged, low hypodiploidy/near triploidy, complex cytogenetics) had a 4-year OS rate of 22% vs. 57% for patients without high-risk cytogenetic features (Figure 1B; P=0.009). Neither CRLF2 positivity by flow cytometry nor the presence of a TP53 mutation significantly impacted OS.
Conclusion: Reduced-intensity chemotherapy with hyper-CVD plus INO, with or without blinatumomab, is safe and effective in older adults with newly diagnosed Ph-negative ALL, with an overall response rate of 98% and 4-year OS rate of 50%. This novel regimen leads to durable remissions and apparent cure in the majority of pts age 60-69 years of age and in those without poor-risk cytogenetic features. To decrease treatment-related mortality, the protocol has been amended to eliminate chemotherapy for pts ≥70 years of age.
Short:Amgen: Honoraria; Takeda Oncology: Consultancy, Honoraria, Research Funding; Astellas: Research Funding; AstraZeneca: Consultancy. Kantarjian:Janssen: Honoraria; Abbvie: Honoraria, Research Funding; Immunogen: Research Funding; Oxford Biomedical: Honoraria; Delta Fly: Honoraria; Adaptive biotechnologies: Honoraria; Aptitute Health: Honoraria; Daiichi-Sankyo: Honoraria, Research Funding; BioAscend: Honoraria; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ascentage: Research Funding; Amgen: Honoraria, Research Funding; Jazz: Research Funding; Sanofi: Research Funding; Pfizer: Honoraria, Research Funding; BMS: Research Funding; Novartis: Honoraria, Research Funding. Ravandi:Celgene: Consultancy, Honoraria; Orsenix: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Xencor: Consultancy, Honoraria, Research Funding; Macrogenics: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding. Jain:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Research Funding; Aprea Therapeutics: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Bioscienes: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; BMS: Research Funding; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Research Funding; Incyte: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Kadia:JAZZ: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; Novartis: Honoraria; Astra Zeneca: Research Funding; Cyclacel: Research Funding; Ascentage: Research Funding; Genentech: Honoraria, Research Funding; Amgen: Research Funding; Cellenkos: Research Funding; Celgene: Research Funding; Astellas: Research Funding; Incyte: Research Funding; Pulmotec: Research Funding. Alvarado:FibroGen: Research Funding; Astex Pharmaceuticals: Research Funding; Daiichi-Sankyo: Research Funding; Sun Pharma: Research Funding; MEI Pharma: Research Funding; Tolero Pharmaceuticals: Research Funding; BerGenBio ASA: Research Funding; Jazz Pharmaceuticals: Research Funding. Burger:Janssen Pharmaceuticals: Consultancy, Speakers Bureau; TG Therapeutics: Research Funding, Speakers Bureau; AstraZeneca: Consultancy; Pharmacyclics, an AbbVie company: Consultancy, Research Funding, Speakers Bureau; Beigene: Research Funding, Speakers Bureau; Gilead Sciences: Consultancy, Research Funding. Daver:Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees. Borthakur:Abbvie: Research Funding; Jannsen: Research Funding; Xbiotech USA: Research Funding; Polaris: Research Funding; BMS: Research Funding; Oncoceutics: Research Funding; Curio Science LLC: Consultancy; AstraZeneca: Research Funding; Novartis: Research Funding; Incyte: Research Funding; PTC Therapeutics: Consultancy; Argenx: Consultancy; BioTherix: Consultancy; Nkarta Therapeutics: Consultancy; Treadwell Therapeutics: Consultancy; PTC Therapeutics: Research Funding; GSK: Research Funding; FTC Therapeutics: Consultancy; Cyclacel: Research Funding; BioLine Rx: Consultancy; BioLine Rx: Research Funding. DiNardo:Novartis: Consultancy; ImmuneOnc: Honoraria; Syros: Honoraria; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; MedImmune: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Calithera: Research Funding; Notable Labs: Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Jazz: Honoraria. Konopleva:Forty-Seven: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Cellectis: Research Funding; Stemline Therapeutics: Consultancy, Research Funding; AstraZeneca: Research Funding; Amgen: Consultancy; Ablynx: Research Funding; Agios: Research Funding; Ascentage: Research Funding; Eli Lilly: Research Funding; Calithera: Research Funding; Sanofi: Research Funding; Genentech: Consultancy, Research Funding; Kisoji: Consultancy; Rafael Pharmaceutical: Research Funding; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; F. Hoffmann La-Roche: Consultancy, Research Funding. Pemmaraju:Plexxikon: Research Funding; Cellectis: Research Funding; Daiichi Sankyo: Research Funding; Samus Therapeutics: Research Funding; Incyte Corporation: Honoraria; Pacylex Pharmaceuticals: Consultancy; Celgene: Honoraria; AbbVie: Honoraria, Research Funding; Blueprint Medicines: Honoraria; Affymetrix: Other: Grant Support, Research Funding; SagerStrong Foundation: Other: Grant Support; Stemline Therapeutics: Honoraria, Research Funding; LFB Biotechnologies: Honoraria; DAVA Oncology: Honoraria; Novartis: Honoraria, Research Funding; Roche Diagnostics: Honoraria; MustangBio: Honoraria. Garcia-Manero:Jazz Pharmaceuticals: Consultancy; Bristol-Myers Squibb: Consultancy, Research Funding; Acceleron Pharmaceuticals: Consultancy, Honoraria; Helsinn Therapeutics: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Amphivena Therapeutics: Research Funding; Merck: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Research Funding; Astex Pharmaceuticals: Consultancy, Honoraria, Research Funding; Novartis: Research Funding; H3 Biomedicine: Research Funding; Onconova: Research Funding. O'Brien:Gilead, Pharmacyclics, TG Therapeutics, Pfizer, Sunesis: Consultancy, Research Funding; Amgen, Astellas, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences Inc. Vaniam Group, AbbVie, Alexion, Verastem, Eisai, Juno Therapeutics, Vida Ventures: Consultancy; Kite, Regeneron, Acerta: Research Funding. Jabbour:Amgen: Other: Advisory role, Research Funding; BMS: Other: Advisory role, Research Funding; Genentech: Other: Advisory role, Research Funding; AbbVie: Other: Advisory role, Research Funding; Adaptive Biotechnologies: Other: Advisory role, Research Funding; Takeda: Other: Advisory role, Research Funding; Pfizer: Other: Advisory role, Research Funding.
Inotuzumab ozogamicin and blinatumomab - frontline treatment of ALL
Author notes
Asterisk with author names denotes non-ASH members.