Background: An inflammatory bone marrow microenvironment with overactive immune stimulation mediated by cytotoxic T cells and defective T regulatory cells contribute to acquired bone marrow failure (BMF) syndromes, including aplastic anemia (AA), primary myelofibrosis (PMF) and hypoplastic myelodysplasia (MDS). Adoptive therapy with allogeneic cord blood (CB) T-regulatory (Treg) cells has shown safety in humans and clinical benefit in graft vs. host disease. Derived from CB, these Treg cells enjoy immune privilege, demonstrate high proliferative index, lack plasticity, and have been shown to consistently suppress inflammation. Therefore, we hypothesized that adoptive therapy with CB Tregs can be utilized as treatment for the inflammatory BMF disorders.
Study Design and Methods: This is a phase I clinical trial examining the role of a single infusion of CK0801, an allogeneic, fresh CB Treg product for the treatment of BMF. CK0801 was produced utilizing novel process development that consists of well-defined qualification criteria for the starting material (CB units), parameters for manufacturing and culture-expansion; and well-defined analytic testing and lot release criteria. Three dose-levels were examined: i) 1x106 cells/kg; ii) 3x106 cells/kg and iii) 10x106 cells/kg (NCT03773393). No immune suppression or lymphodepletion was administered. Patients were allowed to continue their ongoing treatment at stable doses. The study follows 3+3 phase I design. Patients aged ≥ 18 yrs with aplastic anemia, myelodysplastic syndrome or myelofibrosis, and available (HLA 3 out of 6) matched CB unit were eligible. The primary endpoint is to determine the maximum tolerated dose (MTD) and dose limiting toxicity (DLT) of the Treg infusion defined as any of the 3 events: i) severe (grade 3 or 4) infusion toxicity within 24 hours (NCI-CTCAE V4.0), ii) regimen related death within 30 days, or iii) severe (grade 3 or 4) cytokine release syndrome (CRS) within 30 days. Secondary endpoints include exploration of efficacy, PB and BM immune reconstitution, and inflammatory cytokines.
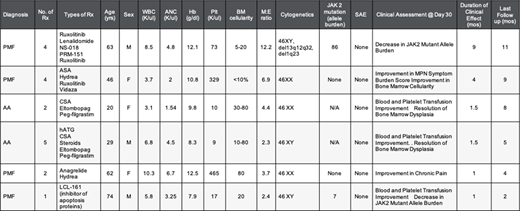
Results: Six patients (Pts) have been treated, with a median age of 54 (range, 20-74). Four pts (67%) had a diagnosis of PMF and 2 patients (33%) had a diagnosis of AA. Overall, the patients had received a median of 4 prior therapies (2-5). There were no infusion related reactions and not DLTs in any of the patients. The patient characteristics and outcomes are summarized in Table 1. Three patients were treated at dose level 1 where the clinical effect was measured at day 30 post CK0801 infusion: Pt#1 (PMF): decrease in JAK2 mutant allele burden; Pt#2 (Triple negative PMF): improvement in MPN symptom burden and improved BM cellularity; and Pt#3 (AA): improvement in blood and platelet transfusion requirement and resolution of BM dysplasia. Additional three patients were treated at dose level 2, where the clinical effect was measured at day 30 post CK0801 infusion: Pt#4 (AA): improvement in blood and platelet transfusion requirement and resolution of BM dysplasia; Pt#5 (PMF): improvement in chronic pain; Pt#6 (PMF) improvement in blood and platelet transfusion requirement and decrease in JAK2 mutant allele burden. The durability of the clinical effect was variable, ranging from 1 month to 9 months. No DLTs were encountered and the MTD has not been reached. Enrollment on dose level 3 has started.
Conclusion: This is the first study to show that a single infusion of allogeneic CB derived Treg cells (CK0801) is well tolerated, feasible, and may be associated with clinical improvement in patients with immune related bone marrow disorders. Determining the optimal dose and schedule of the Treg cell infusion is ongoing.
Kadia:Amgen: Research Funding; Novartis: Honoraria; Pfizer: Honoraria, Research Funding; Celgene: Research Funding; Cyclacel: Research Funding; Ascentage: Research Funding; Cellenkos: Research Funding; Astra Zeneca: Research Funding; Astellas: Research Funding; Pulmotec: Research Funding; BMS: Honoraria, Research Funding; Incyte: Research Funding; Abbvie: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; JAZZ: Honoraria, Research Funding. Pemmaraju:Daiichi Sankyo: Research Funding; Cellectis: Research Funding; AbbVie: Honoraria, Research Funding; Samus Therapeutics: Research Funding; MustangBio: Honoraria; Blueprint Medicines: Honoraria; DAVA Oncology: Honoraria; LFB Biotechnologies: Honoraria; Stemline Therapeutics: Honoraria, Research Funding; Celgene: Honoraria; Incyte Corporation: Honoraria; Novartis: Honoraria, Research Funding; Plexxikon: Research Funding; Affymetrix: Other: Grant Support, Research Funding; Pacylex Pharmaceuticals: Consultancy; SagerStrong Foundation: Other: Grant Support; Roche Diagnostics: Honoraria. Yilmaz:Pfizer: Research Funding; Pint Pharma: Honoraria; Daicho Sankyo: Research Funding. Parmar:Cellenkos Inc.: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding. DiNardo:Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Jazz: Honoraria; Novartis: Consultancy; Celgene: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; MedImmune: Honoraria; Calithera: Research Funding; Takeda: Honoraria; ImmuneOnc: Honoraria; Notable Labs: Membership on an entity's Board of Directors or advisory committees; Syros: Honoraria; Agios: Consultancy, Honoraria, Research Funding. Daver:Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novimmune: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Trovagene: Research Funding; Fate Therapeutics: Research Funding; ImmunoGen: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Trillium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Syndax: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Research Funding; Servier: Research Funding; Genentech: Research Funding. Issa:Novartis: Membership on an entity's Board of Directors or advisory committees; Syndax: Research Funding; Celegene: Research Funding. Jabbour:AbbVie: Other: Advisory role, Research Funding; Amgen: Other: Advisory role, Research Funding; BMS: Other: Advisory role, Research Funding; Takeda: Other: Advisory role, Research Funding; Adaptive Biotechnologies: Other: Advisory role, Research Funding; Genentech: Other: Advisory role, Research Funding; Pfizer: Other: Advisory role, Research Funding. Borthakur:Oncoceutics: Research Funding; Novartis: Research Funding; BioTherix: Consultancy; FTC Therapeutics: Consultancy; Curio Science LLC: Consultancy; Jannsen: Research Funding; Argenx: Consultancy; BioLine Rx: Consultancy; BioLine Rx: Research Funding; Cyclacel: Research Funding; GSK: Research Funding; Polaris: Research Funding; AstraZeneca: Research Funding; BMS: Research Funding; Nkarta Therapeutics: Consultancy; Treadwell Therapeutics: Consultancy; Abbvie: Research Funding; PTC Therapeutics: Research Funding; Incyte: Research Funding; Xbiotech USA: Research Funding; PTC Therapeutics: Consultancy. Verstovsek:Incyte Corporation: Consultancy, Research Funding; PharmaEssentia: Research Funding; AstraZeneca: Research Funding; ItalPharma: Research Funding; Protagonist Therapeutics: Research Funding; Celgene: Consultancy, Research Funding; Roche: Research Funding; CTI Biopharma Corp: Research Funding; Genentech: Research Funding; Sierra Oncology: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Blueprint Medicines Corp: Research Funding; Gilead: Research Funding; NS Pharma: Research Funding; Promedior: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.