In this issue of Blood, Goldsmith et al report that the use of posttransplantation cyclophosphamide (PTCy) doubled the risk of cytomegalovirus (CMV) infection among CMV-seropositive recipients in both haploidentical (HaploCy) and matched sibling donor (SibCy) hematopoietic stem cell transplantation (HSCT) compared with calcineurin inhibitor–based sibling donor transplantation (SibCNI). In patients receiving PTCy, donor CMV seropositivity (R−/D+) was also associated with higher incidence of CMV infection compared with SibCNI. In addition, among seropositive patients or those with CMV infection, the HaploCy cohort had the worst nonrelapse mortality (NRM) and overall survival (OS), confirming results of earlier studies.1
Multivariate analyses of the combined impact of CMV serostatus, donor source, and PTCy on OS. See Figure 3B in the article by Goldsmith et al that begins on page 3291.
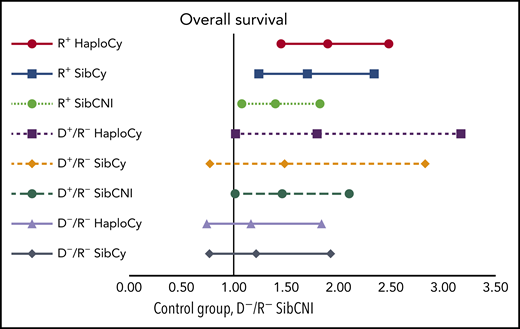
Multivariate analyses of the combined impact of CMV serostatus, donor source, and PTCy on OS. See Figure 3B in the article by Goldsmith et al that begins on page 3291.
This study analyzed a large number of patients (N = 2765) with acute myeloid leukemia, acute lymphoid leukemia, or myelodysplastic syndrome reported to the Center of International Blood and Marrow Transplant Research (CIBMTR) between 2012 and 2017 (HaploCy, n = 757; SibCy, n = 403; SibCNI, n = 1605). The low number of unrelated donors using PTCy precluded inclusion of this group in the study, but it is hoped this group will be studied later. Three additional subpopulations were created according to patient/donor CMV serostatus: seropositive recipient (R+), seropositive donor (D+/R−), and seronegative recipient and donor (D−/R−). An interesting feature included in this report by the authors is the analysis of the aggregation of the 3 main variables: donor source (haploidentical vs sibling), use of PTCy and CMV serostatus (R+ vs D+/R− and D−/R−; see figure). Indeed, the creation of these triplets permits analysis approaching real-world situations rather than endless speculation regarding which may have greater impact (eg, PTCy use vs seropositivity). Their report indicates that not only do these 2 factors increase the risk of posttransplantation CMV infection, but they do so synergistically.
CMV remains the most frequent infection after HSCT. In the early days of HSCT, CMV pneumonia was a major cause of death posttransplantation. As a young trainee, I was introduced by my highly experienced attending to the reality of this infection via the following: “Concerning CMV pneumonia, be warned that your ready-to-be-discharged patient may very well start a mild cough tomorrow at 10 am, be intubated at 6 pm, and have a 50% chance of being dead on the following morning.” Unfortunately, he was not exaggerating; CMV pneumonia remained for many of us the nightmare of long and discouraging nights. In recent years, thanks to the development of pharmacologic treatments and adoptive T-cell transfer therapy, direct CMV mortality has decreased dramatically. Consequently, CMV infection has become somewhat misconstrued as an annoying but unavoidable and manageable event with the appropriate meticulous monitoring for infection and prompt treatment. However, the Goldsmith et al study and other contemporary analyses2,3 have set the record straight: CMV infection is still associated with high NRM and reduced OS. In addition, the report by Goldsmith et al emphasizes the risk of PTCy regarding both occurrence and severity of this viral infection.
Although the HSCT adventure began >60 years ago, the following decades have each been the setting of numerous and sometimes unexpected achievements. The widespread use of transplants from alternative donors, more specifically from 1-haplotype mismatched donors, has greatly expanded therapeutic options for patients. From 2008 to 2018, according to European Society for Blood and Marrow Transplantation and CIBMTR registries, the number of allogeneic transplantations doubled, with alternative donors increasing from 53% to >70% of total donors.4 Haploidentical transplantations greatly contributed to this expansion, increasing by >600% during this period of time and now exceeding 16% of reported HSCTs on both sides of the Atlantic.4,5
The recent enthusiasm for haploidentical HSCT, after years of failure or difficult-to-reproduce sophisticated approaches, is related to the relative simplicity of the method developed in Baltimore, Maryland.6 Based on the administration of high doses of cyclophosphamide on days 3 and 4 after HSCT, PTCy results in both respectable rates of engraftment and graft-versus-host disease (GVHD) control in mismatched transplantation because of the selective toxicity of cyclophosphamide for proliferating alloreactive T cells. The subsequent skewing of T-cell repertoires favors delayed T-cell reconstitution.7 This likely explains the dampened cellular immunity against CMV and may explain the findings reported by Goldsmith et al. Logically, because of the simplicity of PTCy and encouraging results achieved in the complex case of mismatched transplantation, it has also been used after HLA-matched HSCT. Both uncontrolled and controlled studies8 have indicated that PTCy achieves efficient GVHD prevention without increasing relapse, leading to improved GvHD-free, relapse-free survival. However, it is important to note that to date, neither NRM nor OS seem to have dramatically improved.1,8 Furthermore, in these studies, it seems that when PTCy was used, infection-related death was the leading cause of nonrelapse death, instead of GVHD.1,8 Goldsmith et al also suggest that the protective effect of PTCy on chronic GVHD was compromised in patients developing CMV infection, an effect deserving further investigation.
The report by Goldsmith et al suggests that when using PTCy, one must be aware of the hidden danger of CMV infection and act accordingly. The possibility of promoting letermovir prophylaxis,9 as suggested by the authors, is appealing but needs to be evaluated in the PTCy context. Indeed, large prophylactic strategies should be carefully assessed to predict possible unexpected consequences.10 In the Goldsmith et al analysis and others,8 contrary to what has been sometimes suggested, it is reassuring to note that neither CMV infection nor serostatus affected relapse, which supports the idea of prophylaxis. In any case, one should understand that transplantation remains a difficult game of pickup sticks, still full of unanticipated consequences.
Conflict-of-interest disclosure: D.B. has received honoraria from Jazz Pharmaceuticals and expertise fees from Molmed.