Abstract
Background: The oral Bcl-2 inhibitor venetoclax (Ven) has preclinical activity in acute lymphoblastic leukemia (ALL) and has shown encouraging response rates in combination with the Bcl-xL inhibitor navitoclax in patients (pts) with relapsed/refractory (R/R) ALL. We hypothesized that the addition of Ven to low-intensity chemotherapy with mini-hyper-CVD may improve outcomes in pts with ALL.
Methods: Pts ≥60 years of age with newly diagnosed Philadelphia chromosome (Ph)-negative B- or T-cell ALL/lymphoblastic lymphoma (LBL) or ≥18 years of age with R/R Ph-negative B- or T-cell ALL/LBL were eligible. Pts were required to have a PS of ≤3, total bilirubin ≤1.5 mg/dl, AST/ALT ≤3 x ULN and creatinine ≤2 mg/dl. Pts received mini-hyper-CVD alternating with methotrexate and cytarabine (cyclophosphamide and dexamethasone at 50% dose reduction, no anthracycline, methotrexate at 75% dose reduction, cytarabine at 0.5 g/m 2 x 4 doses) for up to 8 cycles. Ven was given at a dose of 400 mg daily on days 1-14 of cycle 1 and on days 1-7 of cycles 2-8. Rituximab (if CD20+ B-cell ALL) and prophylactic IT chemotherapy x 8 doses were given for the first 4 cycles. Pts with T-cell ALL received an additional 2 cycles of nelarabine (650 mg/m 2 daily on days 1-5) and peg-asparaginase (1,500 IU/m 2 [capped at 3750 IU] on day 5), without Ven, during consolidation and another 2 cycles of nelarabine plus peg-asparaginase during maintenance. Responding pts received vincristine and prednisone maintenance with venetoclax daily on days 1-14 of each 28-day cycle for up to 2 years.
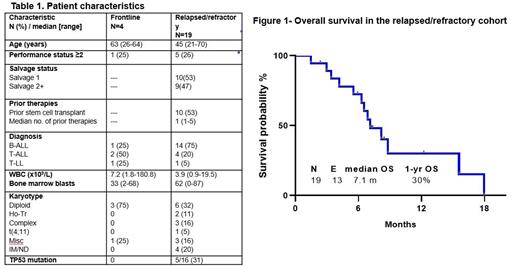
Results: Between 6/2019 and 12/2020, 23 pts have been treated (4 frontline and 19 R/R). Pt characteristics are summarized in Table 1. The median age in the frontline cohort was 63 yrs (range, 26-64) and in the R/R cohort was 45 (range, 21-70). In the frontline cohort 1 pt had B-ALL, 2 pts had T-cell ALL and 1 pt had T-cell LBL. In the R/R cohort, 14 had B-cell ALL, 4 had T-cell ALL, and 1 had T-cell LBL, including 2 pts with ETP ALL. Among the 19 R/R pts, the median number of prior therapies was 1 (range, 1-5) and 10 (53%) had undergone prior allogeneic stem cell transplant (alloSCT). Among the 14 R/R B-cell pts, 12 (86%) had received prior blinatumomab and 5 (36%) had received prior inotuzumab ozogamicin.
Among the 4 frontline pts, 3 (75%) achieved CR. The 4 th pt achieved brief PR as best response. All responders achieved their best response after cycle 1. One pt achieved MRD negativity after cycle 1, and all 3 responders achieved MRD negativity at some point during therapy.
Among the 19 R/R pts, 2 were in CR at enrollment and 17 were evaluable for morphologic response. 11 pts (65%) responded to the regimen (CR, n=8; CRp, n=2; CRi, n=1). An additional pt achieved PR. Among the 11 responders, 8 achieved best response after 1 cycle and 3 after cycle 2. 2 pts achieved MRD negativity after cycle 1, and 3 pts achieved MRD negativity at some point during therapy. Responses were similar among pts with B-cell ALL (64%) and T-cell ALL/LBL (66%) and among pts with adverse-risk karyotype (40%) and non-adverse risk karyotype (60%) [P=0.46].
The median duration of follow-up is 12.2 months (range, 2-18 months). Among the 3 frontline pts who achieved remission, 1 underwent alloSCT and is still in remission and the other 2 remain on therapy in remission. Among the 13 R/R pts who achieved remission, 6 (55%) relapsed, 4 (31%) underwent alloSCT (2 are still alive without relapse and 2 subsequently relapsed), 2 (18%) died in remission, and 1 (9%) is in remission without alloSCT or relapse. In the R/R cohort, the median PFS and OS were 6.2 and 7.1 months, respectively, and the estimated 1-year PFS and OS rates were 14% and 30%, respectively (Figure 1). Outcomes were inferior for those with adverse-risk karyotype versus others (median OS 6.0 vs 10.7 months; 1-yr OS rate: 17% vs 45%; P=0.04).
Treatment was overall well-tolerated. In cycle 1, the median time to platelet recovery was 27 days (range, 0-81 days) and neutrophil recovery was 20 days (range, 0-36); in cycle, median times to recovery were 26 days (range, 17-41) and 19 days (range, 0-26), respectively. In the entire cohort, the 30-day and 60-day mortality rates were 0% and 4%, respectively. One pt in the R/R cohort died from refractory disease and sepsis on day 43.
Conclusion: Low-intensity chemotherapy with hyper-CVD plus venetoclax was safe and effective in pts with Ph-negative ALL. Continued evaluation of venetoclax-based regimens in ALL, including in the frontline setting, are warranted.
Kantarjian: Ascentage: Research Funding; Immunogen: Research Funding; Jazz: Research Funding; Ipsen Pharmaceuticals: Honoraria; Pfizer: Honoraria, Research Funding; Astra Zeneca: Honoraria; Astellas Health: Honoraria; KAHR Medical Ltd: Honoraria; AbbVie: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; NOVA Research: Honoraria; BMS: Research Funding; Daiichi-Sankyo: Research Funding; Aptitude Health: Honoraria; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Short: AstraZeneca: Consultancy; NGMBio: Consultancy; Jazz Pharmaceuticals: Consultancy; Astellas: Research Funding; Takeda Oncology: Consultancy, Research Funding; Novartis: Honoraria; Amgen: Consultancy, Honoraria. Thompson: Amgen: Other: Institution: Honoraria, Research Grant/Funding; Gilead: Other: Institution: Advisory/Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Pharmacyclics: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Adaptive Biotechnologies: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding, Expert Testimony; Genentech: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; AbbVie: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding. Pemmaraju: Springer Science + Business Media: Other; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Celgene Corporation: Consultancy; MustangBio: Consultancy, Other; Incyte: Consultancy; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Protagonist Therapeutics, Inc.: Consultancy; Affymetrix: Consultancy, Research Funding; Roche Diagnostics: Consultancy; DAVA Oncology: Consultancy; Clearview Healthcare Partners: Consultancy; CareDx, Inc.: Consultancy; Aptitude Health: Consultancy; Cellectis S.A. ADR: Other, Research Funding; Daiichi Sankyo, Inc.: Other, Research Funding; Plexxicon: Other, Research Funding; Samus: Other, Research Funding; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Sager Strong Foundation: Other; LFB Biotechnologies: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Blueprint Medicines: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Jain: Precision Biosciences: Honoraria, Research Funding; Incyte: Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; TG Therapeutics: Honoraria; Janssen: Honoraria; Aprea Therapeutics: Research Funding; Beigene: Honoraria; AstraZeneca: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Servier: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Pfizer: Research Funding; ADC Therapeutics: Honoraria, Research Funding; Fate Therapeutics: Research Funding; Cellectis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Pharmacyclics: Research Funding. Wierda: Janssen: Research Funding; AstraZeneca: Research Funding; Xencor: Research Funding; Miragen: Research Funding; Juno Therapeutics: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; KITE Pharma: Research Funding; Gilead Sciences: Research Funding; Loxo Oncology, Inc.: Research Funding; GSK/Novartis: Research Funding; Cyclacel: Research Funding; Karyopharm: Research Funding; Acerta Pharma Inc.: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Genentech: Research Funding; Sunesis: Research Funding; Genzyme Corporation: Consultancy; AbbVie: Research Funding. Borthakur: GSK: Consultancy; ArgenX: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; University of Texas MD Anderson Cancer Center: Current Employment; Astex: Research Funding; Ryvu: Research Funding; Protagonist: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees. Ravandi: Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Taiho: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astex: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Agios: Honoraria, Research Funding; Prelude: Research Funding; AstraZeneca: Honoraria; Xencor: Honoraria, Research Funding; Novartis: Honoraria; AbbVie: Honoraria, Research Funding; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding. Kadia: Dalichi Sankyo: Consultancy; Cellonkos: Other; Ascentage: Other; Genfleet: Other; Sanofi-Aventis: Consultancy; Pulmotech: Other; Astellas: Other; Genentech: Consultancy, Other: Grant/research support; AstraZeneca: Other; Pfizer: Consultancy, Other; Jazz: Consultancy; Novartis: Consultancy; Liberum: Consultancy; Aglos: Consultancy; Cure: Speakers Bureau; BMS: Other: Grant/research support; Amgen: Other: Grant/research support; AbbVie: Consultancy, Other: Grant/research support. Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding.