Abstract
Outcomes for patients with acute myeloid leukemia (AML) remain poor due to the inability of current therapies to fully eradicate disease initiating leukemia stem cells (LSCs) (Shlush et al., 2017). Oxidative phosphorylation (OXPHOS) is a highly targetable and essential process for LSC energy production and survival (Lagadinou et al., 2013). Sirtuin 3 (SIRT3), a mitochondrial deacetylase with a multi-faceted role in metabolic regulation (Finley et al., 2016), has been specifically shown to regulate OXPHOS in cancer models (Li et al., 2019, Xu et al., 2019). Thus, we sought to identify if SIRT3 is essential for LSC function.
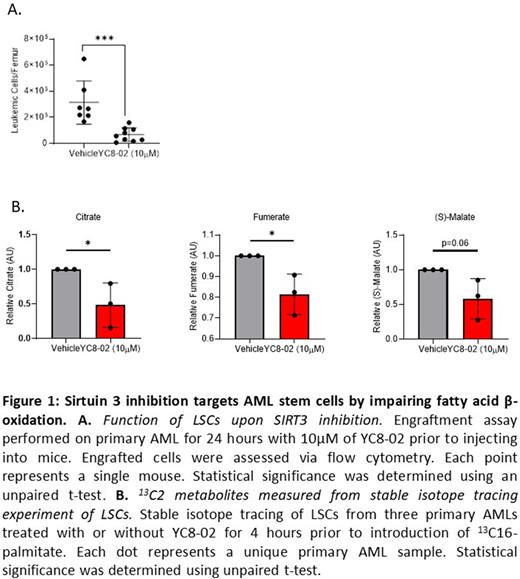
To determine if SIRT3 is essential for LSCs, we measured cell viability, colony forming potential, and engraftment potential of primary AML specimens upon perturbation of SIRT3 function using both RNAi and a small molecule inhibitor (YC8-02) (Li et al., 2019). SIRT3 reduction significantly decreased viability, colony forming potential and engraftment of LSCs (Fig. 1A), suggesting that SIRT3 inhibition decreases LSC function. Importantly, neither SIRT3 knockdown nor inhibition impacted the viability, colony forming potential, or engraftment potential of normal hematopoietic stem and progenitor cells (HSPCs). Altogether, these data suggest that SIRT3 is essential for LSCs and not HSPCs.
To determine the molecular mechanisms by which SIRT3 is essential for LSC function, we performed proximity-dependent biotin labeling (BioID) in 293 T-Rex cells to map the interactome of SIRT3. Strikingly, our BioID analysis identified several fatty acid oxidation (FAO) enzymes as proximity interactors including CPT2, ACADVL, ACADM, ECHI, HADH, and ACAA2. To corroborate these findings in LSCs, we performed RNA-sequencing upon SIRT3 inhibition in primary LSCs derived from three primary AML specimens. Gene set enrichment analysis revealed a significant decrease in OXPHOS and FAO gene signatures upon SIRT3 inhibition. Importantly, FAO is known to support OXPHOS which suggests that SIRT3 may regulate OXPHOS through mediation of FAO.
To determine if protein interactions and gene expression changes result in functional changes in mitochondrial biology, we measured OXPHOS and mitochondrial ATP production in LSCs upon SIRT3 perturbation in LSCs using a seahorse assay. This analysis revealed that SIRT3 perturbation resulted in decreased OXPHOS and ATP production. To determine if SIRT3 regulates OXPHOS via FAO mediation, we performed mass spectrometry-based lipidomic and metabolic interrogation on LSCs from three AML samples treated with YC8-02, which showed an accumulation of fatty acid upon SIRT3 inhibition. To interrogate fatty acid metabolism further, we performed stable isotope tracing analysis using 13C16-palmitate on LSC enriched from three patient specimens upon SIRT3 inhibition. Tracing studies revealed a reduction in 13C enrichment in tricarboxylic acid (TCA) cycle metabolites in the YC8-02-treated LSCs (Fig. 1B), suggesting an impairment to fatty acid utilization upon SIRT3 inhibition. Decreasing availability of TCA products impairs OXPHOS and ultimately causes cell death. Importantly, viability of LSCs was partially rescued through addition of the TCA intermediate α-ketoglutarate, demonstrating reduced FAO to be an essential part of the mechanism by which SIRT3 targets LSCs.
In addition to decreasing OXPHOS, the accumulation of fatty acids should induce cell death by causing lipotoxicity. However, phenocopy experiments revealed that LSCs are protected from lipotoxicity unlike AML blasts or AML cell lines. Lipidomic analysis suggested that fatty acids may be converted to cholesterol esters, which can then be stored or excreted from cells, specifically in LSCs and not in AML cell lines. Therefore, we postulated that targeting cholesterol homeostasis using dipyridamole would enhance the effect of SIRT3 inhibition (Esquejo et al., 2021). The combination treatment significantly reduced the viability of LSCs compared to individual agents, indicating that co-inhibition of SIRT3 and cholesterol homeostasis enhances the toxic effects of FAO inhibition on LSCs.
In all, we identify SIRT3 as a key regulator of LSC survival, through the mediation of lipid metabolism. We further demonstrate that LSCs are tolerant to FAO inhibition which can be overcome by concurrent inhibition of FAO and cholesterol metabolism, which has the potential to lead to improved treatment response.
Disclosures
Dick:Celgene/BMS: Research Funding; Trillium Therapeutics/Pfizer: Patents & Royalties: patent licencing; Graphite Bio: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.