Key Points
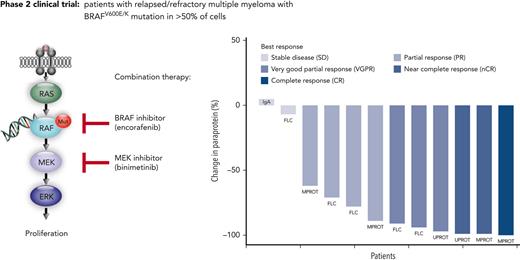
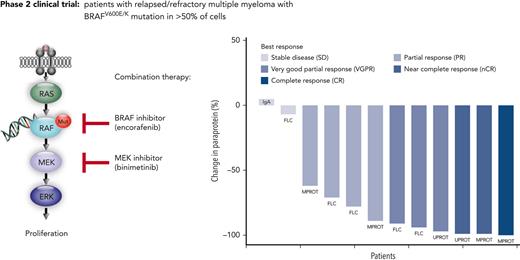
Combined BRAF/MEK inhibition shows high response rates in relapsed refractory multiple myeloma with activating BRAF mutations.
RAS mutations and structural variants involving the BRAF locus may drive resistance to therapy.
Abstract
Activating BRAF mutations are found in a small subset of patients with newly diagnosed multiple myeloma, but prevalence increases in late-stage, refractory disease, and the mutations are associated with adverse outcome. This prospective single-arm, open-label, multicenter phase 2 trial assessed the efficacy and safety of combined BRAF/MEK inhibition, using encorafenib and binimetinib, in patients with relapsed/refractory multiple myeloma (RRMM) carrying a BRAFV600E mutation. Patients received 450 mg encorafenib once daily and binimetinib 45 mg twice daily. The primary end point was the overall response rate achieved within the first year after start of treatment according to International Myeloma Working Group criteria. Twelve RRMM patients with a median of 5 prior lines of therapy were enrolled. The overall response rate was 83.3%, with 10 patients achieving at least a partial response. The median progression-free survival was 5.6 months, and overall survival was 55% at 24 months. Emerging resistance to therapy was driven by RAS mutations and structural variants involving the BRAF locus. This is the first prospective clinical trial to demonstrate that combined BRAF/MEK inhibition is highly effective in patients with BRAFV600E-mutated RRMM, and it represents a successful targeted precision medicine approach in this disease. This trial was registered at www.clinicaltrials.gov as #NCT02834364.
Introduction
Inhibitors of BRAF kinase (BRAFi) alone and in combination with MEK inhibitors are standard of care (SOC) in advanced melanoma. In multiple myeloma (MM), activating BRAF mutations are rarely seen in newly diagnosed patients, but their prevalence increases to up to 8% in relapsed/refractory MM (RRMM) patients, and they are associated with extramedullary disease and inferior survival.1-3 Previous case studies reported clinical responses to BRAF inhibition as monotherapy in MM.1,4 In this prospective multicenter phase 2 trial, we investigated the efficacy and safety of combination therapy with a BRAFi, encorafenib, and a MEK inhibitor (MEKi), binimetinib, in RRMM patients with a BRAFV600E mutation.
Study design
The BRAF/MEK inhibition in relapsed/refractory multiple myeloma (BIRMA) study is a prospective, single-arm, open-label, nonrandomized multicenter phase 2 study to evaluate the efficacy and safety of the combination treatment of encorafenib and binimetinib. Patients were eligible after at least 2 prior lines of therapy including an immunomodulatory drug and a proteasome inhibitor if a BRAFV600E mutation was confirmed by immunohistochemistry using a mutation-specific antibody and DNA sequencing in ≥50% of MM cells. The study was approved by local ethics committees. After providing written informed consent, patients received encorafenib 450 mg once daily and binimetinib 45 mg twice daily as continuous treatment in cycles of 28 days until disease progression or unacceptable toxicity. Response to treatment was evaluated by International Myeloma Working Group (IMWG) criteria.5 The primary end point tested the 1-sided null hypothesis of overall response rate (ORR) ≤20% using a 1-slided binomial test at the significance level of 5%. For all time-to-event end points, Kaplan-Meier curves together with 95% confidence intervals (CIs) were calculated. The statistical analyses were performed using R version 4.0.2 (www.r-project.org).6 All study details can be found in the protocol (supplemental Appendix on the Blood website). Correlative scientific analyses were based on whole genome sequencing (WGS) and RNA sequencing of primary tumor cells.
Results and discussion
From November 2017 to August 2020, 12 patients were enrolled at academic MM centers in Germany. All patients were heavily pretreated with a median of 5 (range, 2-14) prior therapies (supplemental Table 1). All patients were refractory to proteasome inhibitors, 11 (92%) patients to immunomodulatory drugs, and 4 (33%) patients to anti-CD38 monoclonal antibodies.
Overall, 111 adverse events (AEs) were recorded as unique AEs (supplemental Table 2). The most common organ-related AEs were eye disorders (15.3%) with blurred vision, visual impairment, or subretinal fluid, all reversible within a few days by dose adjustments. Gastrointestinal disorders constituted 14.4% of AEs, and 6.8% of AEs were infections. Other main recurrent study drug-related AEs (n > 4) were hypertension (n = 6) and elevated creatinine kinase (n = 5). Only 10.1% of AEs were recorded as Common Terminology Criteria for Adverse Events grade 3/4.
Dose modifications and temporary treatment interruptions resulted in a median drug exposure per patient of approximately 70% of the starting dose of each study drug (supplemental Tables 3 and 4). Despite the overall high number of temporary treatment interruptions or dose reductions, the majority of these occurred early after treatment initiation and were well manageable and only short-lived in nature. Notably, none of the study drug-related AEs led to permanent treatment discontinuation.
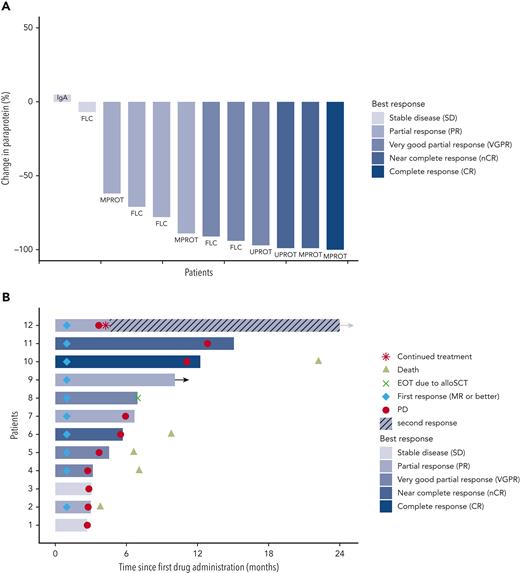
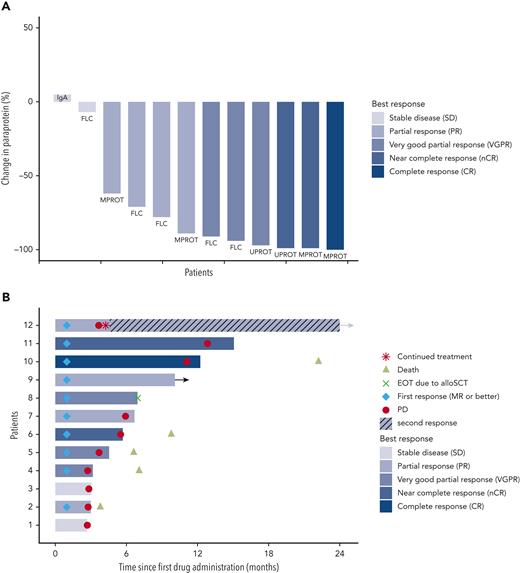
The ORR (primary efficacy endpoint) was 83.3%, with 10 of 12 patients achieving at least a partial response (PR) (Figure 1A). As further outlined in supplemental Table 5, at least a very good partial response was reached in 6 patients (50%), a near complete response in 2 patients (17%), and complete response in 1 patient (8%). The 83.3% ORR with lower 95% CI of 56.4% clearly exceeded the null hypothesis ORR of 20% (P < .001). All responding patients showed an objective response already within the first therapy cycle (supplemental Figure 1).
Depth of response and time to event analysis in patients undergoing combined BRAF/MEK inhibition. (A) Paraprotein reductions after start of treatment for all patients enrolled in the trial. Percentage of paraprotein reduction compared with the baseline value is provided. The individual measurable paraprotein test, according to IMWG response criteria and guidelines, is provided for each patient. Patients are ordered from left to right based on best overall response achieved. The color code indicates the IMWG response category for each patient. FLC, free-light-chain test; IgA, nephelometric detection of quantitative immunoglobulin A; MPROT, M protein detected by serum protein electrophoresis; UPROT, M protein detected by urine protein electrophoresis from 24-hour urine. (B) Swimmer plot displays time to events for enrolled patients by patient number (y-axis) and time since first study drug administration (x-axis). The color code provides information on the best overall response achieved for each patient as per IMWG criteria. The lengths of the horizontal bars indicate the durations of treatment with encorafenib and binimetinib. Additional information provided in the plot per patient: the time points of death events, first response, and progression. The arrow added to the horizontal bar of patient 9 indicates that this patient continued therapy after the database lock and had an uninterrupted partial response. The treatment discontinuation in patient 8 was due to an allogeneic transplantation as indicated. Refer to the main text for details on patient 12 with continued therapy despite formal progressive disease, resulting in a second response, as indicated. alloSCT, allogenic stem cell transplantation; EOT, end of treatment; MR, minimal response; PD, progressive disease.
Depth of response and time to event analysis in patients undergoing combined BRAF/MEK inhibition. (A) Paraprotein reductions after start of treatment for all patients enrolled in the trial. Percentage of paraprotein reduction compared with the baseline value is provided. The individual measurable paraprotein test, according to IMWG response criteria and guidelines, is provided for each patient. Patients are ordered from left to right based on best overall response achieved. The color code indicates the IMWG response category for each patient. FLC, free-light-chain test; IgA, nephelometric detection of quantitative immunoglobulin A; MPROT, M protein detected by serum protein electrophoresis; UPROT, M protein detected by urine protein electrophoresis from 24-hour urine. (B) Swimmer plot displays time to events for enrolled patients by patient number (y-axis) and time since first study drug administration (x-axis). The color code provides information on the best overall response achieved for each patient as per IMWG criteria. The lengths of the horizontal bars indicate the durations of treatment with encorafenib and binimetinib. Additional information provided in the plot per patient: the time points of death events, first response, and progression. The arrow added to the horizontal bar of patient 9 indicates that this patient continued therapy after the database lock and had an uninterrupted partial response. The treatment discontinuation in patient 8 was due to an allogeneic transplantation as indicated. Refer to the main text for details on patient 12 with continued therapy despite formal progressive disease, resulting in a second response, as indicated. alloSCT, allogenic stem cell transplantation; EOT, end of treatment; MR, minimal response; PD, progressive disease.
The median duration of response was 4.8 months (95% CI, 2.76 to not available). One patient (Figure 1B patient 9) was still on study treatment at 10 months at the time of database lock. Follow-up of this patient at time of submission confirmed an ongoing PR at 30 months. Another patient (patient 12) remained on treatment after a particular clinical course: After the patient achieved a PR within the first cycle, treatment was interrupted owing to an infection resulting in biochemical progressive disease, and thus the patient came off the study. However, treatment with the combined inhibitors was restarted 2 months later, and the patient remained on treatment for another 32 months, with PR as the best second response. The second response and its duration were not included in the formal efficacy analyses of the study.
The median progression-free survival was 5.6 months (95% CI, 3.4-11.3). The Kaplan-Meier estimate for progression-free survival at 12 months was 17% (95% CI, 0%-43%) (supplemental Figure 2A). Considering all available data, 4 out of 12 patients remained on BRAFi/MEKi combination therapy for 12 months or longer. The median OS has not been reached and was 66% (95% CI, 38%-93%) at 12 months and 55% (95% CI, 25%-85%) at 24 months (supplemental Figure 2B).
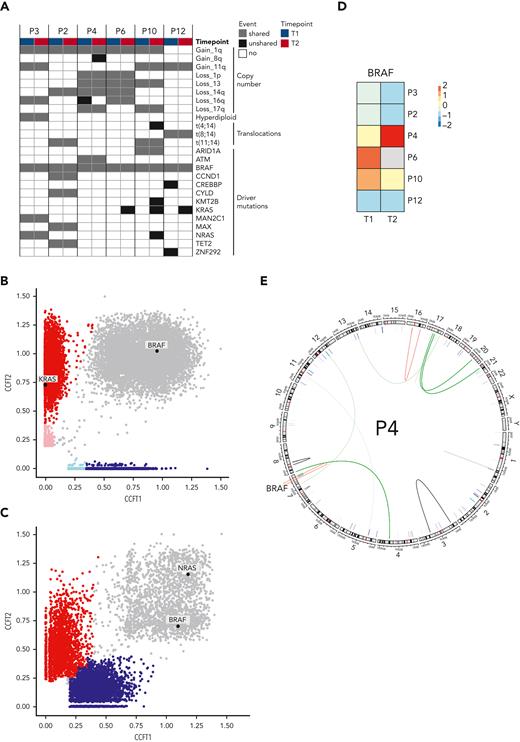
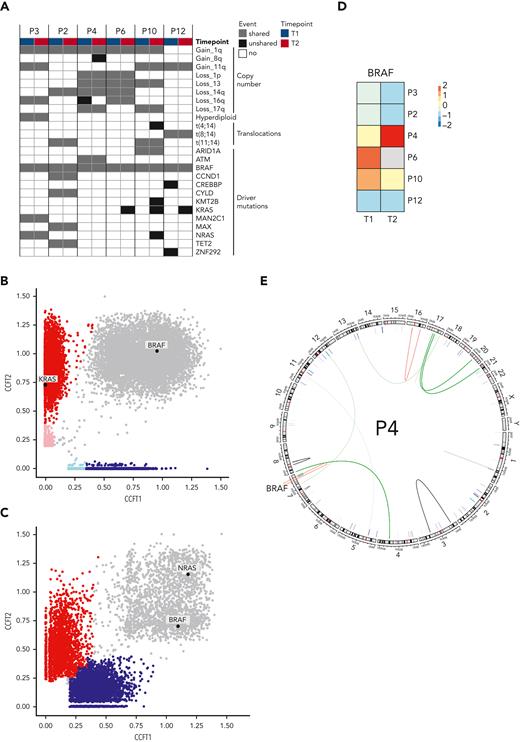
To identify the mechanisms of resistance to combined BRAFi/MEKi in MM, 6 patients with sufficient sample material underwent WGS and RNA sequencing at baseline (T1) and time of relapse (T2). Copy number variations, translocations, and MM driver mutations are shown in Figure 2A.The BRAFV600E mutation was clonal at least in the investigated bone marrow samples at both time points in all patients. Resistance mechanisms to BRAF-directed therapy in melanoma include mutations of NRAS, KRAS, or MEK and BRAF amplifications or splicing variants.7 At T2, we observed selection of a mutant NRAS subclone in 1 patient and mutant KRAS subclones in 3 patients (Figure 2B and supplemental Figure 3). One patient (patient 3) already harbored a clonal NRAS mutation at T1 and did not respond to study treatment (Figure 2B). This strongly supports an important role of mutant RAS in resistance to BRAFi/MEKi in MM. BRAF expression was reduced in most patients at T2, with the exception of patient 4, who had a fivefold increased expression (Figure 2C). This patient displayed an acquired translocation within the BRAF gene (chr7:140493911 [intron 8]; 140618414 [intron 1]) at T2 (Figure 2D). Together, we observed emerging mutations in NRAS and KRAS and a BRAF translocation at relapse, which resulted in significantly increased BRAF expression, as resistance mechanisms to BRAFi/MEKi in MM.
Molecular determinants of resistance. (A) Copy number alterations, translocations, and driver mutations in MM in 6 patients with available WGS and RNA sequencing are indicated at baseline (T1) and at relapse (T2). Cancer clonal fractions (CCF) with enrichment of a KRAS mutation at T2 in responding patient P6 (B) (patients P10 and P12 are shown in supplemental Figure 3), in contrast with presence of an NRAS mutation shared between T1 and T2 in a nonresponding patient (patient P3) (C). (D) BRAF expression in MM cells decreased at T2 compared with T1 in most patients, with the notable exception of patient P4 who showed a fivefold increase. (E) Patient P4 harbored an acquired translocation within the BRAF gene.
Molecular determinants of resistance. (A) Copy number alterations, translocations, and driver mutations in MM in 6 patients with available WGS and RNA sequencing are indicated at baseline (T1) and at relapse (T2). Cancer clonal fractions (CCF) with enrichment of a KRAS mutation at T2 in responding patient P6 (B) (patients P10 and P12 are shown in supplemental Figure 3), in contrast with presence of an NRAS mutation shared between T1 and T2 in a nonresponding patient (patient P3) (C). (D) BRAF expression in MM cells decreased at T2 compared with T1 in most patients, with the notable exception of patient P4 who showed a fivefold increase. (E) Patient P4 harbored an acquired translocation within the BRAF gene.
In conclusion, the GMMG-BIRMA study is the first prospective clinical trial to demonstrate the significant antimyeloma activity of BRAFi/MEKi combination therapy in RRMM patients with BRAFV600E mutations. The observed ORR of 83.3% in this heavily pretreated population exceeds recently reported response rates for BRAFi alone.4 Moreover, the response rate to encorafenib/binimetinib combination therapy exceeded those achieved with other available therapies in patients with more than 4 prior therapies, with the exception of novel T-cell–engaging therapies.2,8-17 Whether the combination regimen translates into improved survival rates compared with single-agent BRAFi, as reported in the VE-BASKET study, cannot be answered owing to the limited patient numbers in both trials.4 Likely, a combination with SOC medication in MM would further improve outcome in this patient population.18
The observed safety profile was quite manageable and was in line with what has been reported in other diseases.19,20 No permanent treatment discontinuations due to AEs occurred. This supports the further development of this combination therapy together with SOC compounds in MM. We recommend to include the assessment of BRAF status to molecular testing in patients with RRMM.
Acknowledgments
The authors thank the investigators, the study nurses, and all the members of the study teams at the participating trial sites, the teams of the myeloma research laboratories at the University Hospital Heidelberg and the DKFZ, the Coordination Centre for Clinical Trials (KKS) in Heidelberg, the participating patients, and their families. The authors also thank the DKFZ Genomics and Proteomics Core Facility for sequencing services and Patrick J. Hayden for proofreading and expert advice.
The trial was supported by Array Pharmaceuticals, a subsidiary of Pfizer, which provided study medication and funding. The study was also in part supported by the DKFZ-Heidelberg Center for Personalized Oncology (DKFZ-HIPO) project HIPO K08K and the NCT PoC funds. Further support was provided by the Dietmar Hopp Foundation.
Authorship
Contribution: N.G., M.C., H.G., A.B., and M.S.R. designed the study and analyzed the data; N.G., M.C., C.S., B.B., I.M., C.K., K.T.G., K.M.K., C.M.T., H.G., K.W., and M.S.R. provided patients and collected data; N.B. and A.S.G. conducted data management and study monitoring; K.M. and A.B. performed statistical analyses; G.M., B.G., and A.S. performed pathological studies; A.M.P., N.W., and M.S.R. performed sequencing studies; N.G., K.M., A.B., T.M., and M.S.R. wrote the manuscript; and all authors critically reviewed and approved of the final manuscript.
Conflict-of-interest disclosure: M.S.R. received funding from Array Pharmaceuticals for study conduct. The remaining authors declare no competing financial interests.
Correspondence: Marc S. Raab, Deptartment of Medicine V, Heidelberg University Hospital, Heidelberg, Germany; e-mail: marc.raab@med.uni-heidelberg.de.
References
Author notes
∗N.G. and M.C. contributed equally to this study.
Raw molecular data have been deposited at the European Genome-Phenome Archive (EGAS00001005973).
Data are available on request from the corresponding author, Marc S. Raab (marc.raab@med.uniheidelberg.de).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.