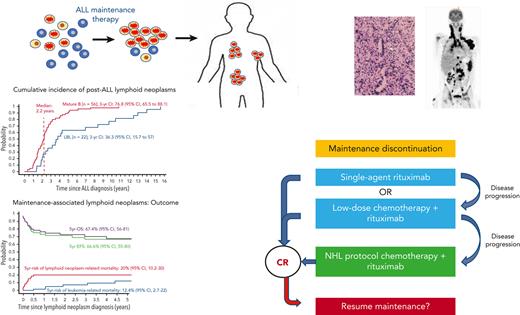
Key Points
A large proportion of post-ALL lymphoid neoplasms are associated with an immunodeficient state precipitated by ALL maintenance therapy.
Treatment strategies for these lymphoid neoplasms are distinct from sporadic NHL because they may respond to reduction of immunosuppression.
Abstract
The development of a second malignancy after the diagnosis of childhood acute lymphoblastic leukemia (ALL) is a rare event. Certain second malignancies have been linked with specific elements of leukemia therapy, yet the etiology of most second neoplasms remains obscure and their optimal management strategies are unclear. This is a first comprehensive report of non-Hodgkin lymphomas (NHLs) following pediatric ALL therapy, excluding stem-cell transplantation. We analyzed data of patients who developed NHL following ALL diagnosis and were enrolled in 12 collaborative pediatric ALL trials between 1980-2018. Eighty-five patients developed NHL, with mature B-cell lymphoproliferations as the dominant subtype (56 of 85 cases). Forty-six of these 56 cases (82%) occurred during or within 6 months of maintenance therapy. The majority exhibited histopathological characteristics associated with immunodeficiency (65%), predominantly evidence of Epstein-Barr virus–driven lymphoproliferation. We investigated 66 cases of post-ALL immunodeficiency-associated lymphoid neoplasms, 52 from our study and 14 additional cases from a literature search. With a median follow-up of 4.9 years, the 5-year overall survival for the 66 patients with immunodeficiency-associated lymphoid neoplasms was 67.4% (95% confidence interval [CI], 56-81). Five-year cumulative risks of lymphoid neoplasm– and leukemia-related mortality were 20% (95% CI, 10.2-30) and 12.4% (95% CI, 2.7-22), respectively. Concurrent hemophagocytic lymphohistiocytosis was associated with increased mortality (hazard ratio, 7.32; 95% CI, 1.62-32.98; P = .01). A large proportion of post-ALL lymphoid neoplasms are associated with an immunodeficient state, likely precipitated by ALL maintenance therapy. Awareness of this underrecognized entity and pertinent diagnostic tests are crucial for early diagnosis and optimal therapy.
Introduction
The cure rate of childhood acute lymphoblastic leukemia (ALL) is now above 90%. This has been achieved by an intensive remission induction therapy, followed by consolidation therapy, and finally, an oral maintenance treatment with daily mercaptopurine and weekly methotrexate until 2 to 3 years from leukemia diagnosis.1 The development of a second malignancy after the diagnosis of childhood ALL is a rare event, associated with significant morbidity and mortality.2-4 Certain second malignancies have been linked with specific elements of leukemia therapy, including an association between cranial irradiation and subsequent central nervous system (CNS) neoplasms5 and a significantly elevated risk for myeloid leukemia after intensive epipodophyllotoxin therapy.6 Nevertheless, the etiology of most second neoplasms remains obscure, and their optimal management strategies are unclear. A previous international study reported 642 patients with second malignant neoplasms diagnosed after childhood ALL.7 To our knowledge, this is the first comprehensive study of the characteristics, treatment, and outcome of non-Hodgkin lymphomas (NHLs) diagnosed after pediatric ALL therapy, excluding hematopoietic stem cell transplantation (HSCT). We describe a previously underrecognized disorder of immunodeficiency-associated (IA) lymphoid neoplasms occurring during ALL maintenance therapy or shortly thereafter, whose management strategies are distinct from sporadic NHL because these lymphoid neoplasms may recede in response to reduction of immunosuppression.
Patients and methods
Study design
The study integrates 2 phases of data capture. The first phase focuses on the epidemiology and features of NHL that occurred after ALL diagnosis. The second phase investigates the characteristics, treatment, and outcome of IA–lymphoid neoplasms following pediatric ALL.
Phase 1: epidemiology and characteristics of non-Hodgkin lymphoid neoplasms arising after pediatric ALL diagnosis
Data on NHL following ALL therapy were collected from the databases of pediatric ALL cooperative study groups belonging to the Ponte di Legno childhood ALL consortium, an international network established to investigate rare subsets of patients with childhood ALL. Twelve international consortia participated in this study, encompassing patients aged 0 to 21 years when enrolled onto pediatric ALL clinical trials between 1980 and 2007 and were subsequently diagnosed with NHL. Nine of the cooperative groups extended the time frame of the study up to 31 December 2018 (supplemental Table 1, available on the Blood website). The investigators classified cases as mature B-cell lymphoproliferations, lymphoblastic lymphomas (LBLs), peripheral T-cell lymphomas, follicular lymphomas, NHL with no further classification, or other lymphoid neoplasms. Data collection from patients with ALL who did not develop NHL was not part of the study. Patients who underwent HSCT before a lymphoid neoplasm diagnosis were excluded from further analysis.
Phase 2: characteristics and outcome of IA–lymphoid neoplasms after pediatric ALL therapy
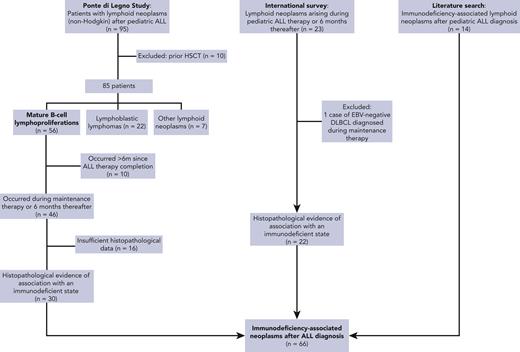
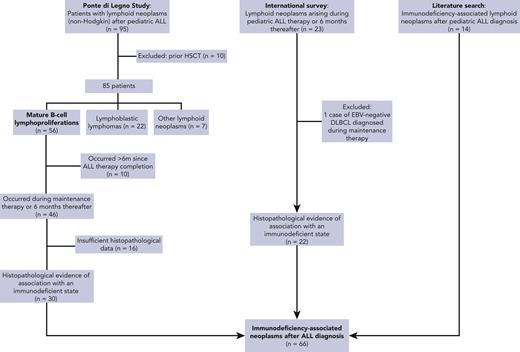
Data on IA–lymphoid neoplasms following pediatric ALL were extracted from 3 sources (Figure 1):
Ponte di Legno Childhood ALL Working Group study of lymphoid neoplasms after childhood ALL (phase 1). Only patients whose tumors harbored evidence of association with an immunodeficient state were included.
International survey: a case survey was conducted through professional pediatric ALL networks, including the International BFM Study Group and St. Jude Global, disseminated through emails and conferences, seeking patients diagnosed with lymphoid neoplasms that harbored evidence of association with an immunodeficient state and occurring during ALL therapy or within 6 months from the cessation of therapy that included methotrexate/thiopurine maintenance therapy. Patients aged 0 to 21 years who started ALL therapy between 1980 and 2020 and subsequently developed lymphoid neoplasms (excluding Hodgkin lymphoma) were eligible. Patients who received methotrexate/thiopurine maintenance therapy in the context of relapsed ALL therapy were included, provided they had not undergone previous HSCT.
Literature search: a literature search was conducted to identify additional reports of IA–lymphoid neoplasms after ALL diagnosis. A computer-aided search of MEDLINE was performed using the following terms: “lymphoproliferative”, “lymphoma”, or “lymphomatoid,” combined with ALL. The search was restricted to English language publications between 1980 and 2020.
Cases were carefully reviewed to exclude double reporting from different sources.
Patient evaluation
Patient data included clinical and biological characteristics of leukemia and the second neoplasm, treatment details, and outcome. In cases where data on leukemia therapy, drug doses, or schedule were unavailable, the patients were assigned the treatment regimen described in the ALL protocols they were enrolled in.
The classification of lymphoid neoplasms was based on histopathology, according to the contemporary World Health Organization (WHO) classification system8 (supplemental Table 2). Epstein-Barr virus (EBV) association was determined in biopsy samples using EBV-encoded RNA in situ hybridization or latent membrane protein immunohistochemical detection. When available, the results of the quantitative polymerase chain reaction for EBV DNA in the blood or serum were reported. The following characteristics were considered as evidence of association with an immunodeficient state: tumor EBV positivity and/or distinctive histopathological entities such as lymphomatoid granulomatosis9 or histopathological subtypes defined by the current WHO classification under IA lymphoid neoplasms.8-13 The tumor stage was defined according to the St Jude childhood and adolescent NHL staging system.14
Ethical approval
All patients were treated after obtaining a written informed consent from the patient, the patient’s parents, or legal guardians. All clinical trials had been approved by review boards or ethics committees. Patient data for the Ponte di Legno Childhood ALL Working Group study were collected as part of each trial and shared with the investigators at the University Hospital Rigshospitalet, Copenhagen, Denmark. The international survey was conducted by the investigators at Schneider Medical Center of Israel with the approval of review boards or ethics committees of all participating centers.
Statistical analysis
Lymphoid neoplasm–related death was defined as any death occurring when NHL was not in complete remission or resulting from lymphoid neoplasm therapy. Leukemia-related death was defined as any death resulting from leukemia progression or because of leukemia therapy, provided that the lymphoid neoplasm was in remission at the time. The overall survival (OS) was calculated from the time of lymphoid neoplasm diagnosis to death, whereas event-free survival (EFS) was defined as the time from lymphoid neoplasm diagnosis to leukemia relapse or death from any cause; time was censored at the date of last patient contact if no event had occurred. The Kaplan–Meier method was used to estimate survival rates; curves were compared using the two-sided log-rank test. Cumulative incidence functions were constructed using the method of Kalbfleisch and Prentice and compared with the Gray test after adjusting for competing risks of other events. A Cox proportional hazards regression model was used to estimate hazard ratios (HR) and their 95% confidence intervals (CI) for prognostic factors. Cause-specific hazard rates were estimated by the Fine-Gray subdistribution hazard model15 to assess the association of groups with the 2 competing events (lymphoma- and leukemia-related death). Other comparisons were performed using the Fisher exact test and the Brown-Mood median test for comparing medians. A two-sided P value of ≤ .05 was considered statistically significant. All analyses were performed with the R Project for Statistical Computing, version 3.4.2.
Results
Epidemiology of non-Hodgkin lymphoid neoplasms after pediatric ALL
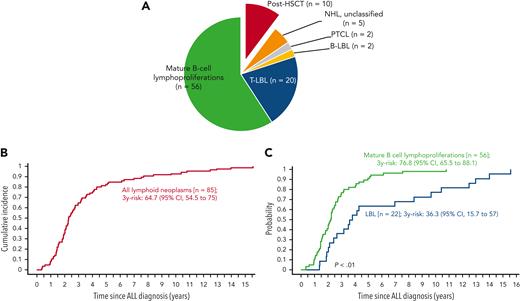
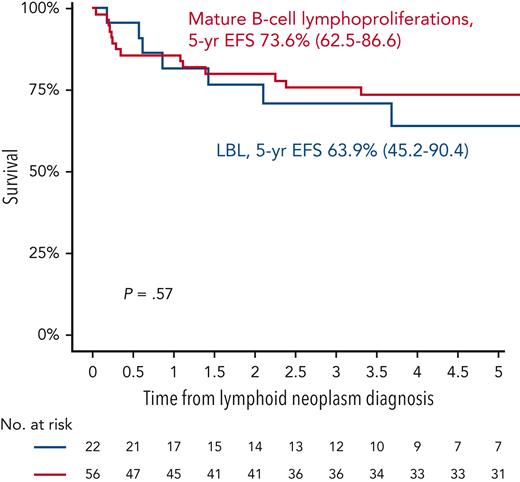
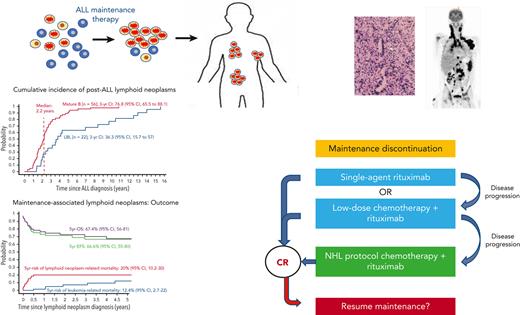
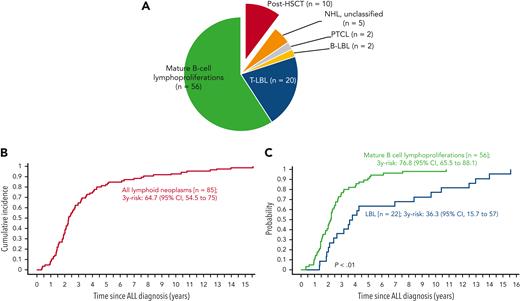
In phase 1, 12 international collaborative groups reported 95 patients who were diagnosed with lymphoid neoplasms after an ALL diagnosis (supplemental Table 1). Ten cases were excluded because of an HSCT before the lymphoid neoplasm. The remaining 85 cases included 56 mature B-cell lymphoproliferations (66%), 22 LBLs (26%), 2 peripheral T-cell lymphomas (2%), and 5 NHLs with no further classification (6%) (Figure 2A; Table 1). The median interval between ALL and lymphoid neoplasm diagnoses was 2.3 years (Figure 2B), with mature B-cell lymphoproliferations occurring after a significantly shorter interval than those with LBLs (median: 2.2 years vs 3.7 years, respectively; P < .01) (Table 1; Figure 2C and supplemental Figure 1). Five-year EFS did not differ significantly between patients diagnosed with mature B-cell lymphoproliferations and those diagnosed with LBLs (Figure 3). Among the 56 cases classified as mature-B cell lymphoproliferations, 46 (82%) occurred during, or within 6 months from completion of maintenance therapy, vs 8 out of 22 (36%) in patients with LBLs (P < .01) (Table 1). Thirty of the mature-B cell lymphoproliferations (65%) that occurred during this time frame exhibited histopathological characteristics similar to lymphoid neoplasms that arise in the setting of immunodeficiency, including polymorphic lymphoproliferative disorders (LPDs) (n = 12), lymphomatoid granulomatosis (n = 5), EBV-positive diffuse large B-cell lymphoma (n = 11), and LPD with no further classification (n = 2). Overall, EBV positivity was reported in 23 of these 30 cases. For the other 16 cases occurring during, or within 6 months from the completion of maintenance therapy, more elaborate histopathological data had not been recorded and tissue EBV-association analyses had not been performed. None of the cases that occurred more than 6 months after maintenance therapy completion were reported to harbor characteristics associated with immunodeficiency.
Lymphoid neoplasms after ALL. (A) Classification. (B) Cumulative incidence of lymphoid neoplasms after ALL. (C) Comparison of cumulative incidences of mature B-cell lymphoproliferations and LBL after ALL. B-LBL, B-cell lymphoblastic lymphoma; PTCL, peripheral T-cell lymphoma; T-LBL, T-cell lymphoblastic lymphoma.
Lymphoid neoplasms after ALL. (A) Classification. (B) Cumulative incidence of lymphoid neoplasms after ALL. (C) Comparison of cumulative incidences of mature B-cell lymphoproliferations and LBL after ALL. B-LBL, B-cell lymphoblastic lymphoma; PTCL, peripheral T-cell lymphoma; T-LBL, T-cell lymphoblastic lymphoma.
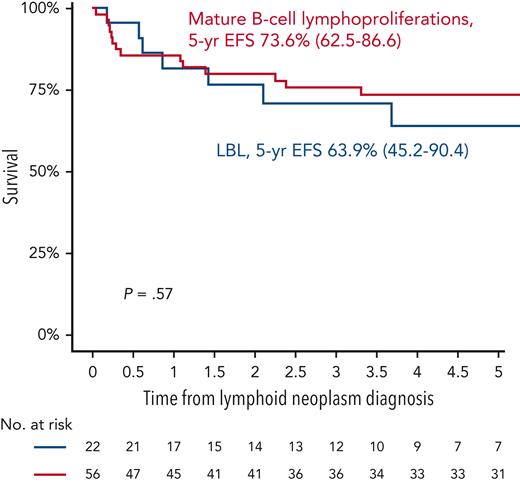
Outcomes of lymphoid neoplasms after ALL. 5-year EFS of mature B-cell lymphoproliferations vs LBL.
Outcomes of lymphoid neoplasms after ALL. 5-year EFS of mature B-cell lymphoproliferations vs LBL.
IA–lymphoid neoplasms after pediatric ALL
Data of this cohort were obtained from 3 sources:
Patients whose lymphoid neoplasms exhibited evidence of association with an immunodeficient state from the Ponte di Legno ALL Working Group study described in “Phase 1” (n = 30).
The international survey yielded 22 additional cases exhibiting evidence of association with an immunodeficient state. Nine of these had not been reported to their respective national databases as secondary malignancies. Of note, 2 patients with primary LBL who had been treated with an ALL therapy that included methotrexate/thiopurine maintenance and had developed LPDs during maintenance therapy were included. Histopathological specimens from both cases were reviewed by international experts, and the subsequent malignancies were determined to be molecularly and pathologically distinct from the primary LBL.
Finally, a literature search identified 14 additional case reports of IA–lymphoid neoplasms occurring after ALL (summarized in supplemental Table 4), providing a total cohort of 66 patients.
Altogether, 66 patients with IA–lymphoid neoplasms occurring during ALL maintenance therapy or within 6 months of its completion were identified (Figure 1).
For the whole IA–lymphoid neoplasm cohort (n = 66), the median follow up from ALL and lymphoid neoplasm diagnoses were 7.1 years (range, 1.1-22.7 years) and 4.9 years (range, 0.3-20.4 years), respectively.
Demographics and lymphoid neoplasm characteristics of the IA–lymphoid neoplasm cohort (n = 66)
Patient characteristics are summarized in Table 2. The median ages at leukemia and lymphoid neoplasm diagnoses were 4.5 years (range, 0.2-29 years) and 6.3 years (range, 2.5-31 years), respectively. The male-to-female ratio was 1.4:1. All patients diagnosed with IA–lymphoid neoplasms had prior exposure to the maintenance thiopurine/methotrexate combination. The median duration of exposure to the maintenance therapy before lymphoid neoplasm diagnosis was 14 months (range, 0.5-30 months). In 40% of cases, maintenance regimens included intermittent vincristine and corticosteroid therapy. Six cases (9%) occurred in individuals with Down syndrome. No other known genetic predisposition syndromes were reported for any of the patients, nor was there any significant history of familial cancer or immunodeficiency.
Lymphoid neoplasm characteristics are depicted in Table 3. Histopathology encompassed a wide spectrum of lymphoid neoplasms, including polymorphic B–LPDs (n = 24), monomorphic B-cell LPD (n = 16), monomorphic T-cell LPD (n = 2), lymphomatoid granulomatosis (n = 15), and EBV-positive mucocutaneous ulcer (n = 2). Of 55 cases with available data regarding tumor EBV status, 53 cases (96%) exhibited EBV positivity, and 2 cases were negative. Nondestructive (early) lesions had not been reported.
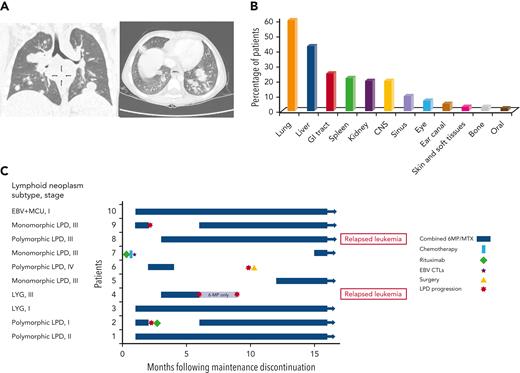
Lymphoid neoplasms were often diagnosed at advanced III/IV stages (n = 49; 82% of 60 patients with available data), with frequent extranodal involvement, including pulmonary, gastrointestinal, and CNS involvement in 60%, 25%, and 20% of patients, respectively (Figure 4A-B). Bone marrow involvement was not reported. In 49 cases, fever was reported as a presenting symptom, and in 18, an infectious mononucleosis-like prodrome was noted days to weeks before the lymphoid neoplasm diagnosis. Three cases were diagnosed post mortem. Eight of 15 patients with gastrointestinal tract involvement presented with multiple bowel perforations. In 5 cases (8%), lymphoid neoplasms were accompanied by hemophagocytic lymphohistiocytosis (HLH). The median time from symptom onset to the diagnosis of lymphoid neoplasm (data available for 36 of the patients) was 5 weeks (range, 1 day-11 months).
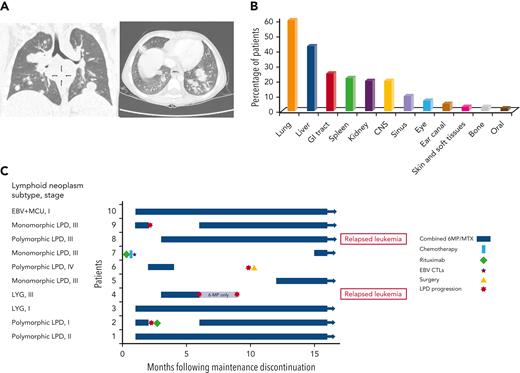
IA lymphoid neoplasms after ALL. (A) Coronal (left) and axial (right) chest CT scans of a 4-year-old boy with persistent fever and cough during ALL maintenance therapy, demonstrating multiple nodular pulmonary lesions, subsequently diagnosed with EBV-positive DBCL. (B) Percentage of patients with involvement of different extranodal sites (data available for 60 patients). (C) A swimmer’s plot summarizing treatment and outcome of 10 patients for whom maintenance therapy had been reinitiated after achieving remission from lymphoid neoplasm. Patient numbers, lymphoid neoplasm subtype, and stage are depicted on the left. CT, computed tomography; CTL, cytotoxic T lymphocytes; LYG, lymphomatoid granulomatosis; MCU, mucocutaneous ulcer; MTX, methotrexate; 6MP, mercaptopurine.
IA lymphoid neoplasms after ALL. (A) Coronal (left) and axial (right) chest CT scans of a 4-year-old boy with persistent fever and cough during ALL maintenance therapy, demonstrating multiple nodular pulmonary lesions, subsequently diagnosed with EBV-positive DBCL. (B) Percentage of patients with involvement of different extranodal sites (data available for 60 patients). (C) A swimmer’s plot summarizing treatment and outcome of 10 patients for whom maintenance therapy had been reinitiated after achieving remission from lymphoid neoplasm. Patient numbers, lymphoid neoplasm subtype, and stage are depicted on the left. CT, computed tomography; CTL, cytotoxic T lymphocytes; LYG, lymphomatoid granulomatosis; MCU, mucocutaneous ulcer; MTX, methotrexate; 6MP, mercaptopurine.
Treatment
Treatment strategies are summarized in Table 3 (full data available for 58 patients) and supplemental Tables 3-4. Maintenance therapy was discontinued in all but 1 patient with surgically resected localized disease (50 of 51 patients with on-therapy lymphoid neoplasms). Fourteen study patients received rituximab monotherapy. Fourteen additional patients received rituximab with systemic chemotherapy; 8 of these were treated with NHL protocols (supplemental Table 3) and 6 received low-dose chemotherapeutic regimens (cyclophosphamide, vincristine, and corticosteroids, as previously described16,17). Overall, 28 study patients (48%) were treated with rituximab. Nine patients received systemic chemotherapy without rituximab; 7 of these were treated with NHL protocols and 2 with low-dose regimens. Overall, 23 of 58 patients (40%) were treated with systemic chemotherapy. Additional treatment modalities included surgery (n = 14), radiotherapy (n = 3), and EBV-specific cytotoxic T lymphocytes (n = 3). HSCT was performed in 5 cases because of a presumed, yet undiagnosed, genetic predisposition to lymphoid malignancies. In 10 cases, maintenance therapy was resumed after lymphoid neoplasm remission, leading to lymphoid neoplasm recurrence in 4 cases (Figure 4C). Two of these 4 patients resumed maintenance once again after an additional period of therapy discontinuation or rituximab treatment. Overall, 8 patients successfully resumed maintenance therapy.
Outcome
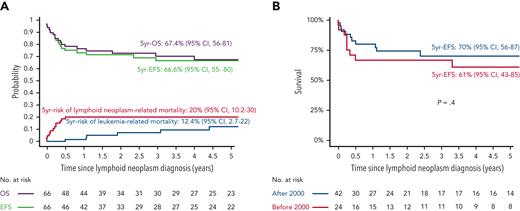
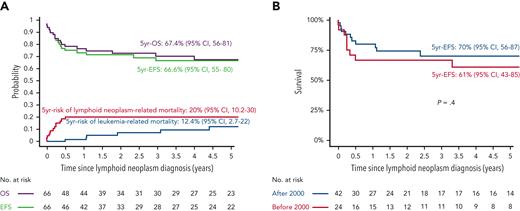
The 5-year EFS and OS from the lymphoid neoplasm diagnosis for the IA–lymphoid neoplasm cohort (n = 66) were 66.6% (95% CI, 55.3-80.1) and 67.4% (95% CI, 56-81.1), respectively (Figure 5A). Five-year cumulative risks of lymphoid neoplasm– and leukemia-related mortality were 20.2% (95% CI, 10.2-30) and 12.4% (95% CI, 2.8-22), respectively. In total, 13 patients died because of lymphoid neoplasm–related factors: progressive lymphoid neoplasm (pulmonary, n = 7; CNS, n = 1), concurrent HLH (n = 4), and therapy-related toxicity (n = 1). The median time to lymphoid neoplasm–related death was 1.6 months (range, 0-6.1 months) from diagnosis. Of those who attained remission from their lymphoid neoplasms, 7 patients experienced leukemia relapse, 6 of whom died. For patients who experienced leukemia relapse after lymphoid neoplasm diagnosis (n = 7; 5 of them aged >10 years or <1 year), the median duration of administered maintenance therapy before lymphoid neoplasm diagnosis was 7 months, vs 14.5 months for those who did not experience leukemia relapse (P = .08). The 5-year EFS did not differ significantly between patients diagnosed with lymphoid neoplasm before the year 2000 (n = 24) and those diagnosed after the year 2000 (n = 42); P = .4 (Figure 5B). In multivariate analysis, including age, sex, treatment era, duration of maintenance therapy before lymphoid neoplasm diagnosis, histological subtype, CNS and pulmonary involvement, and concurrent HLH (performed in 58 patients with sufficient data), concurrent HLH was the single variable associated with increased mortality (HR, 7.32; 95% CI, 1.62-32.98; P = .01) (Table 4; supplemental Figures 2-4). In this cohort, a significant impact of different therapeutic strategies upon lymphoid neoplasm–related mortality or upon leukemia-related mortality could not be shown (supplemental Figures 3-5).
Outcome of 66 patients with IA-lymphoid neoplasms after ALL. (A) EFS, OS, and cumulative incidence of lymphoid neoplasm- and leukemia-related mortality (B) Comparison of EFS for patients diagnosed with IA-lymphoid neoplasms after ALL diagnosis before and after the year 2000.
Outcome of 66 patients with IA-lymphoid neoplasms after ALL. (A) EFS, OS, and cumulative incidence of lymphoid neoplasm- and leukemia-related mortality (B) Comparison of EFS for patients diagnosed with IA-lymphoid neoplasms after ALL diagnosis before and after the year 2000.
Discussion
To our knowledge, this is the first comprehensive report regarding lymphoid neoplasms following pediatric ALL diagnosis. Here, we report that most lymphoid neoplasms occurring after ALL are mature B-cell lymphoproliferations, 82% of which occurred during maintenance therapy or shortly thereafter. These lymphoid neoplasms were predominantly EBV-driven or harbored additional histopathological characteristics associated with immunodeficiency. All were diagnosed after prior exposure to the maintenance thiopurine/methotrexate combination. Our findings suggest that these lymphoid neoplasms are associated with an immunodeficient state, likely precipitated by ALL maintenance therapy.
We further report the characteristics and outcome of an international cohort of 66 patients diagnosed with a range of IA–lymphoid neoplasms, generally EBV-related, during (or shortly after) pediatric ALL maintenance therapy. Several distinctive clinical features characteristic of this disorder have emerged because of our study. Patients often presented with an aggressive clinical picture (stage III/IV disease in 82%) with frequent extranodal involvement, including prominent pulmonary (60%), bowel (25%), and CNS (20%) involvement. Concurrent HLH was associated with an increased risk of mortality (HR, 7.32; 95% CI, 1.62-32.98; P = .01). Lymphoid neoplasms were diagnosed well into maintenance therapy (median 14 months), suggesting a cumulative, or delayed, effect of maintenance medication exposure. The predominance of advanced-stage (III/IV) disease and the absence of reported nondestructive (early) lesions suggest that maintenance-associated lymphoid neoplasms may be underdiagnosed as well as underreported. Furthermore, the lack of recognition of this clinical entity might have contributed toward its belated diagnosis (median 5 weeks from symptom initiation), high incidence of intestinal perforations (8 out of 15 with gastrointestinal involvement), and fatality rates, which have not significantly improved over the years. With the decreased use of radiotherapy and epipodophyllotoxins in contemporary ALL protocols, leading to a decreased rate of their associated secondary malignancies, maintenance-associated lymphoid neoplasms may now represent a significant subtype of subsequent malignancies.18
Lymphoproliferations that arise in patients treated with immunosuppressive drugs for autoimmune disease or conditions other than in the post-transplant setting are classified by the WHO (supplemental Table 2) as IA-LPDs, specifically as other iatrogenic IA-LPDs, 8 a distinct category that is very rarely reported in a pediatric population. Both methotrexate and thiopurines have previously been associated with LPD in the adult population: in methotrexate therapy of autoimmune disorders19,20 and in patients treated with thiopurines due to inflammatory bowel disease.21,22 In the contemporary WHO classification, the full range of lymphoproliferations uncovered in our study are not encompassed under 1 category; most subtypes are formally recognized in the post-transplant setting only.13 Additional entities encountered in our study, lymphomatoid granulomatosis9 and EBV-positive mucocutaneous ulcer23 (together accounting for 26% of cases in the IA–lymphoid neoplasm cohort), both predominantly EBV-driven, are also known for their association with an immunodeficient state. Thus, the data emerging from this study refine and expand our knowledge regarding the full repertoire of histopathological findings that may be found in this specific immunodeficient setting.
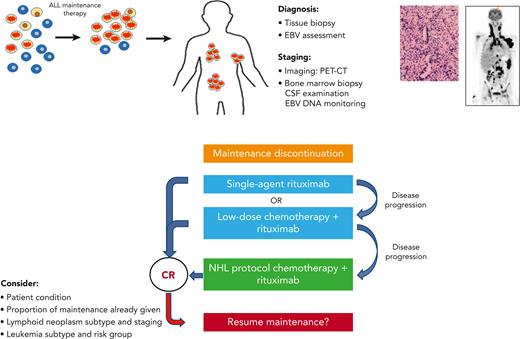
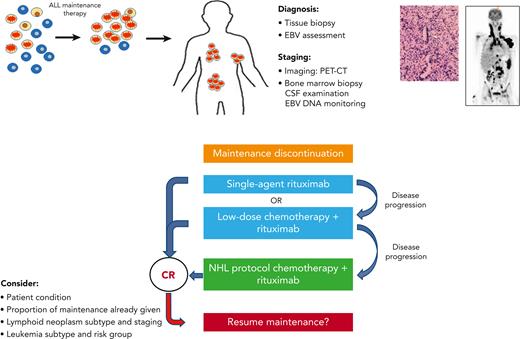
The crucial importance of recognizing IA–lymphoid neoplasms lies in the therapeutic implications: these neoplasms may regress in response to a reduction in immunosuppression. This has a major effect upon treatment strategies (Figure 6). Reduction in immunosuppression, namely maintenance therapy discontinuation, is a primary step toward control of IA–lymphoid neoplasms by the restoration of cellular immunity.24,25 In our study, systemic chemotherapy was administered only to 38% of the cohort, a third of whom received low-dose, de-escalated chemotherapeutic regimens, whereas rituximab was administered to 45% of the patients. Our data support a sequential, stepwise treatment strategy in selected patients, similar to that used in post-transplant LPDs.16,17,26-29 This strategy may include, as a first step, maintenance discontinuation and rituximab administration.30-32 Low-dose chemotherapy16,17 may be given in combination with rituximab, or added sequentially, if the tumor response to rituximab is insufficient. Standard NHL therapeutic regimens may be used as a salvage therapy for progressive disease. Adoptive immunotherapy using third-party EBV-specific cytotoxic T lymphocytes may be utilized.33-36 Rare histopathological subtypes of IA–lymphoid neoplasms, such as Burkitt lymphoma37 and natural killer T-cell lymphoid neoplasms,38 may be treated with histology-specific treatment regimens.39 Novel immunotherapeutic approaches, including checkpoint inhibitors,40 chimeric antigen receptor T cells,41 daratumumab,42 and brentuximab,43 are currently being explored in the post-transplant setting. When a patient is diagnosed with a lymphoid neoplasm after ALL, tumor assessment for EBV is an integral part of the standard workup, and communication with the pathologist is of paramount importance to ensure the delivery of optimal tailored therapy. Although the prospective monitoring of EBV DNA is recommended in certain high-risk post-transplant populations,44,45 routine surveillance during maintenance therapy of pediatric ALL does not appear justified because of the rarity of this disorder and a potentially low yield of such an approach.
Suggestions for the clinical management of maintenance-associated lymphoid neoplasms. CR, complete remission; CSF, cerebrospinal fluid; PET-CT, positron emission tomography-computed tomography.
Suggestions for the clinical management of maintenance-associated lymphoid neoplasms. CR, complete remission; CSF, cerebrospinal fluid; PET-CT, positron emission tomography-computed tomography.
Early truncation of maintenance therapy may adversely affect leukemia outcome.46,47 However, the optimal duration of the maintenance phase of therapy, which differs between leukemia subtypes,48 has not yet been determined.49 Fortunately, a large proportion of IA–lymphoid neoplasms occur at advanced stages of maintenance therapy (median, 14 months). Here, maintenance therapy was successfully reinitiated in 8 of 10 attempted cases. The decision to restart maintenance therapy must be carefully weighed considering potentially life-threatening recurrences of lymphoid neoplasms. Maintenance therapy may be reinitiated in patients who have not completed a significant portion of their maintenance therapy, provided that complete remission from lymphoid neoplasm has been attained and that the patient is in good clinical condition. The lymphoid neoplasm subtype and staging as well as leukemia subtypes should also be considered. Maintenance reinitiation, if undertaken, should be closely monitored, both clinically and radiologically. Longitudinal monitoring of EBV viral loads in these cases is recommended.
The limitations of this study include its retrospective design and the absence of a central review of histopathological specimens. The size of the cohort and heterogeneity of lymphoid neoplasms preclude the drawing of statistically definitive conclusions regarding therapy. The overall risk for subsequent hematological malignancies after ALL diagnosis is reportedly 1% to 2% in current collaborative protocols,50 with NHL accounting for 16% of these cases. The majority of subsequent hematological malignancies (80%) occur within the first 5 years from ALL diagnosis.7 However, the exact incidence of maintenance-associated IA–lymphoid neoplasms is difficult to estimate because of underreporting, even among patients treated on clinical trials. Here, 30 of 46 post-ALL mature B-cell lymphoproliferations (65%) occurring during maintenance therapy or shortly thereafter harbored a histopathological evidence of association with immunodeficiency, yet the other 16 cases lacked sufficient recorded data, suggesting that the extent of this phenomenon may be greater than estimated. Future prospective studies may enable a true assessment of its incidence, dose-response relationship, and latency from maintenance therapy and may lead to the identification of patients who are at risk for this rare complication.
The majority of EBV infections have a self-limited clinical course in immunocompetent patients. In certain settings, patients may have an impaired ability to control EBV infection, with ensuing life-threatening complications, including malignancy and HLH.51 EBV-driven lymphoid neoplasms result from the proliferation of infected B cells in the setting of deficient cytotoxic T-cell surveillance. Our findings suggest that the pathogenesis of post-ALL IA–lymphoid neoplasms is related to an immune dysregulation occurring specifically during or after the exposure to maintenance therapy. Maintenance therapy exerts cytotoxic and immunosuppressive effects, both of which may play a role in inducing lymphoid neoplasms. Thiopurine-induced strand breakage, through displacement of purine nucleotide incorporation into DNA, may predispose to carcinogenesis.52-56 Multiple mechanisms have been postulated for the immunomodulatory effects of methotrexate57 and thiopurines, including the unique role of thiopurines in the induction of T-cell apoptosis.58-61 Several studies have reported the immune effects of ALL therapy.62-69 Although significant neutropenia is rare during maintenance therapy, lymphopenia is common, persisting months after treatment completion.63-66 B-cell counts are severely suppressed during maintenance therapy, with differential reconstitution rates of various B-cell subpopulations after therapy cessation. The extent of T-cell suppression during ALL therapy varies between different reports.62,67-69 A slow reconstitution of the T-cell compartment following therapy completion has been reported, with a prolonged T-cell subset imbalance.62,68 Whether a synergistic effect of combined thiopurine and methotrexate therapy or an additive immunomodulatory effect of previous intensive chemotherapy contributes to the pathogenesis of IA–lymphoid neoplasms 62,70 remains to be established.
Despite a low incidence of sporadic NHL71-73 in patients with Down syndrome74 and although this patient subset accounts for only 1% to 3% of patients in childhood ALL clinical studies,75 in our study, 6 out of 66 (9%) patients were individuals with Down syndrome. This relatively high incidence suggests that an inherent underlying immune dysregulation may further predispose these individuals to this disorder. Indeed, patients with Down syndrome have a significantly higher risk for treatment-related mortality during maintenance therapy,75,76 yet the mechanisms underlying their particular vulnerability to this phase of therapy are poorly understood. None of the other study patients were reported to have any known genetic predisposition syndrome or history of immunodeficiency. However, most children who receive ALL maintenance therapy do not develop IA lymphoid neoplasms, suggesting a role for host genetic predisposition,77-80 which may be further investigated in the future. Thus, although maintenance therapy plays a pivotal role in contemporary pediatric ALL therapy,48 our report of maintenance therapy-associated lymphoproliferations further illustrates that the precise nature of the prolonged immune dysregulation associated with this phase of ALL therapy remains unclear and requires further investigations.
In conclusion, this is the first comprehensive report regarding immunodeficiency-associated lymphoid neoplasms after pediatric ALL diagnosis and their association with maintenance therapy. Physicians engaged in the treatment of pediatric ALL should be aware of this life-threatening, but treatable, complication of ALL maintenance therapy.
Acknowledgments
The authors thank the patients, families, and clinical teams who participated in the treatment of these patients; the members of the ALL and NHL Committees of the I-BFM Study Group for their contributions to this study; and Naomi Litichever and Dina Kugel, data managers of the Israeli Study Group of Childhood ALL and Gioia Blayer for assistance with the statistical analysis.
Authorship
Contribution: S.E., S.I., and K.S. were responsible for conception and design of the study; S.E., B.B., R.N., A.B., and K.S. performed data analysis and interpretation; S.E. provided administrative support; S.E. and K.S. wrote the manuscript; all authors collected and assembled data; and all authors approved the final manuscript.
This work was supported by the Israel Cancer Association, the Chaim Association, and the Israel Childhood Cancer Fund (New York) (S.E. and S.I.); and by the Dora and Giorgio Professorship at Tel Aviv University (S.I.).
Conflict-of-interest disclosure: H.I. received research grant from Servier and is on the advisory board of Jazz Pharmaceuticals. A.M. is a consultant and has received honoraria from Clinigen and BTG. K.S. is a speaker and/or on the advisory Board and received honoraria from Illumina (2021), Jazz Pharmaceuticals (2020, 2021), and Servier (2020, 2021); has received speaker fee from Amgen (2020, 2021) and Medscape (2020, 2021); has received educational grant from Servier (2020, 2021); and has received research grant from Novo Nordisk Foundation. The remaining authors declare no competing financial interests.
Correspondence: Sarah Elitzur, Department of Pediatric Hematology and Oncology, Schneider Children’s Medical Center, 14 Kaplan St, Petach Tikva 4920235, Israel; e-mail: sarhae@clalit.org.il.
References
Author notes
Data are available on request from the corresponding author, Sarah Elitzur (sarhae@clalit.org.il).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.