Introduction: Lineage switch (LS) refers to the transformation of acute leukemia from one cell lineage to another (e.g., lymphoid (ALL) to myeloid (AML)). This rare phenomenon, distinct from treatment induced malignancy (i.e., t-AML) and mixed phenotype acute leukemia (MPAL), is increasingly being observed following cell surface antigen targeting. Given challenges in the diagnosis and treatment of LS, Project EVOLVE was developed to globally identify cases of LS following immunotherapy.
Methods: A protocol and data collection form for this IRB exempt study was widely disseminated to capture deidentified LS case information across multiple centers and cancer consortia. This included collection of data from published reports of LS, with authors contacted to obtain additional information.
For this analysis, LS was defined by the emergence of cell-surface markers warranting reclassification of the original leukemia as a different lineage derivation based on the immunophenotype, including cases of AML emerging alongside the original ALL. “Confirmed” LS cases included those where retention of original cytogenetic or molecular aberrations and/or the clonal immunoglobulin rearrangement patterns were verified to confirm the clonal relationship. “Suspected” LS cases included those where samples to confirm clonality marker retention could not be obtained, but the clinical presentation was consistent with LS. The term “transition” was applied when immunophenotypic shifts were transient. Cases with new cytogenetic abnormalities unrelated to the original diagnosis and compatible with t-AML were excluded. Statistical analysis was primarily descriptive. Data cut-off was July 26, 2023.
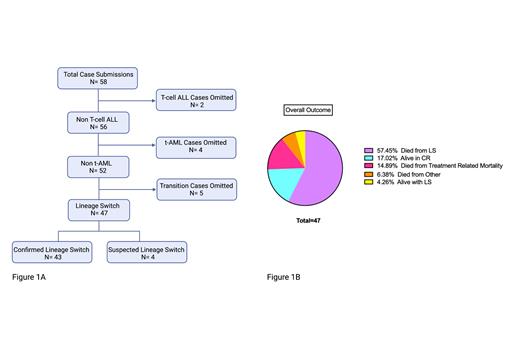
Results: A total of 58 cases were identified, including 19 cases referenced in prior publications. Of the 58, 11 cases were excluded from this initial analysis, including: 4 cases of t-AML, 2 cases of T-ALL to AML, and 5 “transition” cases. Collectively, 47 cases of LS (43 “confirmed”; 4 “suspected”), spanning across 3 continents and 8 countries, were analyzed. (Fig. 1A)
Cases included transition from B-ALL to AML in 36 (76.6%) and to MPAL/biphenotypic/ambiguous lineage in 11 (23.4%). Two likely had MPAL at diagnosis and a third with chronic myeloid leukemia. The median age at initial diagnosis was 8.4 years (range, 1 day-76.5 years); median age at LS presentation was 11.0 years (range 0.4-77.3 years). Twenty-three (48.9%) were male and 19 (40.4%) had prior stem cell transplant (SCT). Baseline cytogenetics showed KMT2A rearrangement in 27 (57.4%). Baseline myeloid antigen co-expression was seen in 22 of 23 patients with submitted data, but variation in degree of expression across patients and antigens was substantial.
The most proximal immunotherapy prior to development of LS was CAR T-cells in 23 (48.9%) and blinatumomab in 24 (51.1%). The median time from the most proximal immunotherapy to development of LS was 1.6 months (range, 7 days-36.5 months). In 7 (29.2%) patients receiving blinatumomab, LS developed during the infusion. LS presented as isolated marrow relapse in 27 (57.4%), isolated CNS in 2 (4.3%), isolated non-CNS extramedullary disease in 3 (6.4%), combined relapse in 14 (29.8%) and unreported in 1.
First line treatment for LS was chemotherapy induction in 31 (66.0%), palliative care/no therapy in 8 (17.0%), and alternative therapies in the remaining 8 (17.0%). In 35 (74.5%) patients, remission induction was the goal of first line therapy. Fifteen (31.9%) achieved a complete remission (CR) of whom 2 were treated with palliative intent.
Across all patients, 8 (17.0%) are alive in remission, the longest being 4.7 years from LS diagnosis; 2 (4.3%) are alive with active LS and 37 (78.7%) died from LS, their original disease, or complications of treatment of LS (Fig 1B). For those alive in CR (n=8), 7 (87.5%) proceeded to a consolidative SCT for treatment of LS and 1 patient, an octogenarian remains in remission after AML directed therapy.
Conclusions: LS is emerging as an important mechanism of immune escape following immunotherapy. Project EVOLVE provides the largest dataset of patients with LS to date. This systematic, international effort reveals substantial complexity and variability in diagnosing and managing LS. Unfortunately, outcomes following LS are grim, underscoring the critical need to identify risk factors of and optimal therapies for this leukemia transformation.
Disclosures
Aldoss:Takeda: Consultancy; Amgen: Consultancy, Honoraria; Pfizer: Consultancy; Jazz: Consultancy; Sobi: Consultancy; KiTE: Consultancy. Vatsayan:Pfizer: Current equity holder in publicly-traded company; Illumina: Current equity holder in publicly-traded company. Dickens:Syndax: Research Funding; Tempus Inc.: Honoraria; Amgen: Honoraria; Iowa Cancer Consortium: Membership on an entity's Board of Directors or advisory committees; American Society of Pediatric Hematology Oncology: Membership on an entity's Board of Directors or advisory committees; American Academy of Pediatrics: Membership on an entity's Board of Directors or advisory committees. El Chaer:Association of Community Cancer Centers: Consultancy; Arog Pharmaceuticals: Research Funding; Novartis: Research Funding; MEI Pharma: Research Funding; BioSight: Research Funding; PharmaEssentia: Research Funding; Sanofi: Research Funding; Sumitomo Pharma Oncology: Consultancy, Research Funding; Fibrogen: Research Funding; Amgen: Consultancy, Research Funding; Bristol Myers Squib: Research Funding; Celgene: Research Funding; DAVA Oncology: Other: Travel grant. Reed:Sumitomo Pharma America: Other: reimbursement of time presenting or training related to the BBI-TP-3654-102 study. Abdel-Azim:Adaptive: Research Funding. Fabrizio:Adaptimmune: Consultancy. Ghorashian:Novartis: Honoraria; UCLB: Patents & Royalties. Shah:Immunoadoptive Cell Therapy Private Limited: Consultancy, Other: Scientific Advisory Board; VOR: Consultancy, Research Funding; CARGO: Consultancy; Lentigen: Research Funding.