INTRODUCTION. CD19-targeting CAR-T cell therapy has changed the treatment landscape of refractory/relapsed large B cell lymphoma, including transformed from FL (tFL). Axicabtagene ciloleucel (axi-cel), lisocabtagene maraleucel (liso-cel), and tisagenlecleucel (tisa-cel) are now FDA-approved for the treatment of tFL, but limited data are available on their clinical activity in patients (pts) with tFL since outcomes in those pts were not separately reported in registrational studies. Here, we report a single center's experience with CD19-directed CAR-T cell therapy in pts with tFL.
METHODS. We performed a retrospective analysis of 54 pts with tFL treated with CAR-T cell therapy from January 2018 to December 2022. Pathological evidence of histologic transformation (HT) was confirmed. The main clinical-biological characteristics of the pts were extracted from the electronic medical records. Cytokine release syndrome (CRS) and neurotoxicity were graded according to ASTCT guidelines. Progression-free (PFS) and overall survival (OS) were measured from the time of CAR-T cell infusion; PFS events were death, relapse, and disease progression.
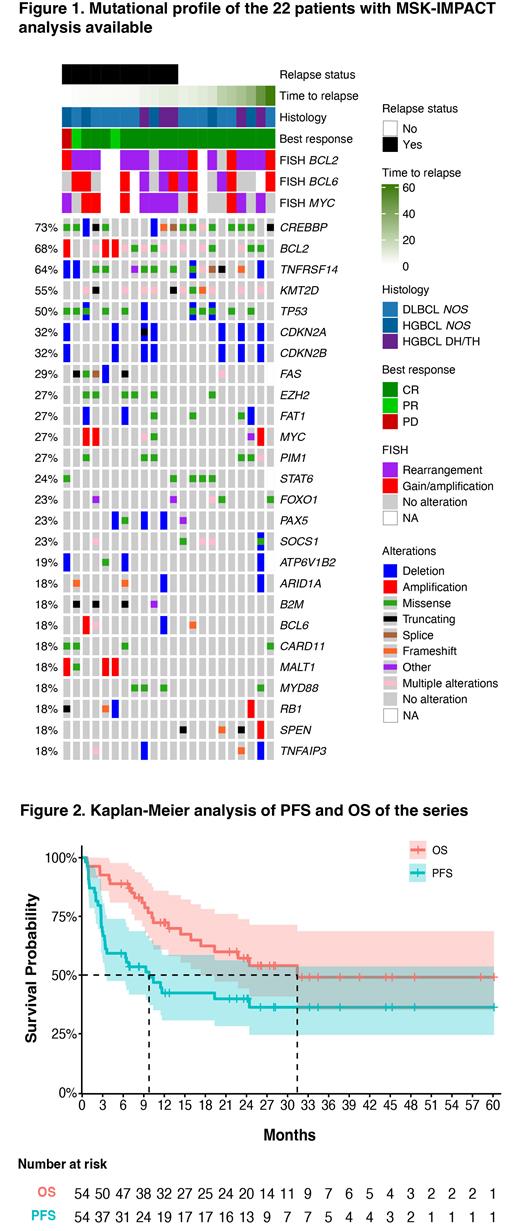
RESULTS. Within our cohort of patients with tFL, the median age was 67 years (range 42-81), 65% were male and 77% had stage III-IV disease. Eight of the pts (15%) had an ECOG Performance Status (ECOG-PS) ≥2. Median time to transformation was 27 months from original FL diagnosis (range 0 to 223), including 6 cases with composite lymphoma (FL + aggressive histology) and 4 with synchronous transformation. Twenty percent presented with high-grade B-cell lymphoma (HGBCL) with MYC and BCL2 and/or BCL6 rearrangements. Using Hans' algorithm, 85% (44/52) were identified as a germinal center B-cell origin (GCB). In 22 cases, targeted sequencing using the MSK-IMPACT panel was performed at the time of HT or prior to CAR-T. TP53 mutation/deletion was present in 50% of the cases (Figure 1).
Pts received a median of 3 (range 1-9) prior lines of therapy including those for FL, and 9 pts (17%) had undergone prior autologous stem cell transplantation (SCT). Thirty-four pts (63%) were refractory to the last therapy, including 29 pts (53.7%) with primary refractory disease after HT. Forty-one pts (76%) received bridging therapy.
Seventy-two percent of pts received axi-cel, 19% tisa-cel, and 9% liso-cel. CRS of any grade was seen in 43 (80%) pts with grade >3 in 3 (6%). Neurologic toxicity of any grade was observed in 20 (37%) pts with grade >3 in 7 (13%). The median duration of follow-up was 26.2 months. The overall response rate was 87%, including 67% complete response (CR). The median response duration (DOR) was 11 months, with a 2-year DOR of 42% (95% CI: 28-61%). Objective responses were achieved in pts with high-risk features such as HGBCL, TP53 mutation, and chemotherapy-refractory disease.
Twenty-nine pts experienced relapse/progression during follow-up, with a median PFS for the entire cohort of 9.8 months and a 2-year PFS of 40% (95% CI: 28.3-56.3%) (Figure 2). Refractory disease after HT and high LDH serum levels were associated with a significantly lower PFS (median PFS not reached vs. 6.4 months, P=0.031; 24.4 vs. 3.2 months, P=0.003 respectively). Notably, all pts who achieved PR eventually progressed. In 23 pts with relapse, a biopsy was obtained, showing DLBCL or HGBCL morphology in all instances. Interestingly, all relapses occurred during the first 24 months. Twenty-six pts received salvage therapy after CAR-T progression, with a median of 1 line (range 1-7). Out of these, eight (31%) pts achieved a CR and went to allogenic SCT as a consolidative strategy.
Twenty-two pts died during follow-up, 19 due to progression and 3 of non-lymphoma causes, including 2 of COVID-19 infection. The median OS was 31 months, and the 2-year OS rate was 57% (95% CI: 44.3-73.8%) (Figure 2). Median OS was not reached among the 36 pts who achieved a complete response.
CONCLUSIONS. CD19-targeting CAR-T cell therapy is effective for pts with relapsed/refractory tFL, including patients with characteristics of poor prognosis, with efficacy and toxicity profiles comparable to that observed in previous DLBCL studies. Unfortunately, pts who relapse post-CAR-T therapy have limited effective therapeutic options available, and this currently remains an unmet medical need within the field.
Disclosures
Epstein-Peterson:Amgen: Research Funding; WebMD: Honoraria; OncLive: Honoraria; Kymera: Research Funding; Viracta: Research Funding. Falchi:Roche: Consultancy, Research Funding; Genentech: Consultancy, Other: Advisory Board, Research Funding; Genmab: Consultancy, Research Funding; Abbvie: Consultancy, Other: Advisory Board, Research Funding; Seagen: Other: Advisory Board; ADC Therapeutics: Other: Advisory Board; AstraZeneca: Consultancy. Hamlin:ADC Therapeutics: Consultancy. Johnson:Myeloid Therapeutics: Consultancy. Kumar:Genentech: Consultancy, Research Funding; Janssen: Consultancy; Kite Pharma: Consultancy; Loxo/Lily Oncology: Consultancy, Research Funding; Beigene: Research Funding; Celgene: Research Funding; Seattle Genetics: Research Funding; BridgeBio: Current equity holder in publicly-traded company; Adaptive Biotechnologies: Research Funding; Pharmacyclics: Research Funding; Astra Zeneca: Consultancy, Research Funding; Abbvie Pharmaceuticals: Research Funding. Lue:OncLive: Consultancy; Merck: Consultancy. Scordo:Amgen, Inc.: Research Funding; Omeros Corporation: Consultancy, Research Funding; Medscape, LLC: Honoraria; CancertNetwork (Intellisphere LLC): Honoraria; Angiocrine Bioscience, Inc.: Research Funding. Dogan:Seattle Genetics: Consultancy; Physicians' Education Resource: Consultancy, Honoraria; EUSA Pharma: Consultancy; Loxo: Consultancy; Peer View: Honoraria; Incyte: Consultancy; Takeda: Other: Research Funding; Roche: Other: Research Funding. Zelenetz:Abbvie: Research Funding; MEI Pharma Inc: Consultancy, Honoraria, Research Funding; Janssen Pharmaceuticals: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; SAB: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria, Research Funding; None other than mutual funds (401K): Current equity holder in publicly-traded company; BMS: Consultancy, Honoraria; Lymphoma Research Foundation: Membership on an entity's Board of Directors or advisory committees. Perales:Astellas: Consultancy, Honoraria; Allogene: Research Funding; Servier: Other; Sellas Life Sciences: Consultancy; Miltenyi Biotec: Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Kite: Consultancy, Honoraria, Research Funding; Medigene: Consultancy, Other; Karyopharm: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Exevir: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; VectivBio AG: Consultancy, Honoraria; Omeros: Consultancy, Current equity holder in publicly-traded company, Honoraria; Caribou: Consultancy, Honoraria; Equillium: Consultancy, Honoraria; DSMB: Other; BMS: Consultancy, Honoraria; Vor Biopharma: Consultancy, Honoraria; Allovir: Consultancy; Adicet: Honoraria; Celgene: Honoraria; Orcabio: Consultancy, Current equity holder in publicly-traded company, Honoraria; Cidara Therapeutics: Consultancy, Other; Syncopation: Honoraria; NexImmune: Consultancy, Current equity holder in publicly-traded company; Merck: Consultancy, Honoraria; Nektar Therapeutics: Consultancy, Honoraria, Research Funding. Palomba:BMS: Honoraria; Cellectar: Honoraria; Ceramedix: Honoraria; Juno: Honoraria, Patents & Royalties; Kite: Honoraria; MustangBio: Honoraria; GarudaTherapeutics: Honoraria; Novartis: Honoraria; Pluto Immunotherapeutics: Honoraria; Rheos: Honoraria; Seres Therapeutics: Honoraria, Patents & Royalties; Smart Immune: Honoraria; Thymofox: Honoraria; Synthekine: Honoraria. Shah:BMS: Research Funding; Beyond Spring: Research Funding; ArcellX: Other: DSMB; Janssen: Research Funding; Amgen: Research Funding. Salles:Owkin: Current holder of stock options in a privately-held company; Molecular Partners: Consultancy; Janssen: Consultancy, Research Funding; ATB Therapeutics: Consultancy; Merck: Consultancy, Honoraria; EPIZYME: Consultancy; Nordic Nanovector: Consultancy; AbbVie: Consultancy, Honoraria; Nurix: Consultancy; Novartis: Consultancy; Debiopharm: Consultancy; BMS/Celgene: Consultancy; Loxo/Lilly: Consultancy; Kite/Gilead: Consultancy; Incyte: Consultancy; Genmab: Consultancy; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; BeiGene: Consultancy; Orna: Consultancy; Ipsen: Consultancy, Research Funding.