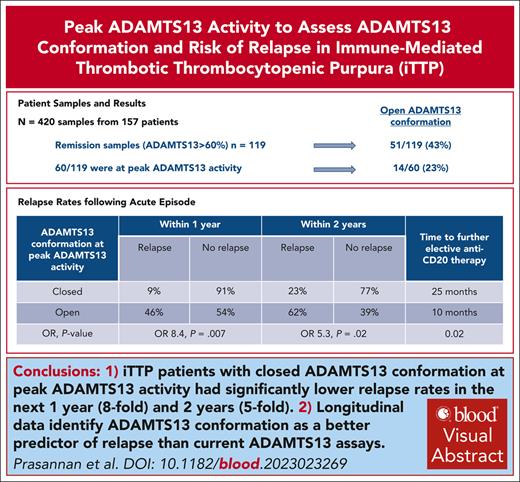
Key Points
Closed ADAMTS13 conformation at peak ADAMTS13 activity significantly reduced relapse rates by eightfold in 1 year and fivefold 2 years.
Longitudinal data identifies ADAMTS13 conformation as a better predictor of relapse than the current ADAMTS13 assays.
Visual Abstract
Previous studies have demonstrated that >38% of patients with immune-mediated thrombotic thrombocytopenic purpura in remission with activity >50% had an open ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13) conformation. We assessed ADAMTS13 conformation in remission (ADAMTS13 activity >60%), focusing on peak ADAMTS13 activity levels and longitudinal assessment in 420 samples across 157 patients. Fewer cases had an open conformation at peak ADAMTS13 activity than unselected remission samples with ADAMTS13 activity >60% (23% vs 43%). Patients with a closed ADAMTS13 conformation at peak ADAMTS13 activity had an eightfold lower relapse rate in the subsequent year (9% vs 46%) and a fivefold lower relapse rate within 2 years (23% vs 62%) compared with cases with an open conformation. Patients with an open conformation at peak ADAMTS13 activity required preemptive anti-CD20 treatment earlier than those with a closed conformation (median, 10 vs 25 months). Longitudinally, an open conformation was evident at, and often preceded relapse. When the conformation was already open before relapse, an increase in the conformation index at relapse was seen despite the undetectable anti-ADAMTS13 immunoglobulin G (IgG) antibody. In cases with detectable anti-ADAMTS13 IgG antibody, these became undetectable before achieving a closed conformation, highlighting the relapse risk even with undetectable anti-ADAMTS13 IgG antibody and the clinical utility of open/closed during monitoring. To our knowledge, this is the first study to show an association between relapse risk and ADAMTS13 conformation when activity levels are at a peak. The open conformation identifies antibody-mediated subclinical disease that is not detectable by the current ADAMTS13 testing.
Introduction
Thrombotic thrombocytopenic purpura (TTP) is a rare microangiopathic hemolytic anemia associated with microvascular thrombosis and thrombocytopenia. It is characterized by a severe deficiency of the von Willebrand factor (VWF) cleaving protease called ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13) of <10 IU/dL. Immune-mediated TTP (iTTP) is associated with autoantibodies, usually immunoglobulin G (IgG), against ADAMTS13. Established management of iTTP consists of daily plasma exchange (PEX), which has reduced untreated mortality significantly.1,2 PEX aims to replenish ADAMTS13 and reduce the autoantibodies. Immunosuppressive regimens consist primarily of steroids and rituximab.3,4 More recently, caplacizumab has become an integral part of the acute management of iTTP.5,6
The diagnosis and ongoing monitoring of iTTP are dependent on ADAMTS13 activity, which is determined through the gold standard fluorescence resonance energy transfer VWF73 assay.7 iTTP is a chronic disease consisting of acute phases and relapses that occur between periods of clinical remission and vary in duration across patients and is therefore accompanied by a risk of unpredictable relapse.8 This necessitates lifelong ADAMTS13 monitoring.8
Although clinical relapses are usually accompanied by positive symptomatology, thrombocytopenia, and microangiopathic hemolytic anemia, ADAMTS13 relapses are more variable in presentation and may be asymptomatic. In the United Kingdom, a 40% relapse rate at 5 years of follow-up has been shown, with 60% of these attributed to ADAMTS13 relapse. The same study showed lower rates of clinical relapse with regular ADAMTS13 monitoring and preemptive rituximab.9
ADAMTS13 is a multidomain protein, and the interaction between the spacer and complement C1r/C1s, Uegf, Bmp1 (CUB) domains ensures that ADAMTS13 adopts a closed/folded conformation in the absence of VWF binding. It has been shown that an open conformation can be achieved in vitro by the addition of among others,10 anti-CUB antibodies.11 A murine monoclonal antibody called 1C4 that binds to the cryptic site of the spacer domain has been developed.12 The results of the 1C4 enzyme-linked immunosorbent assay (ELISA) corrected for the ADAMTS13 antigen allow the conformation index (CIND) to be calculated and can thus be used to detect the open conformation. An open ADAMTS13 conformation was identified in acute iTTP and it was subsequently reported that anti-ADAMTS13 antibodies from patients with iTTP could also induce the same. It has been suggested that open conformation is a sensitive biomarker for subclinical iTTP, which is defined as changes in ADAMTS13 parameters during remission.13
We investigated ADAMTS13 conformation in iTTP during acute episode and remission (ADAMTS13 activity >60%), focusing on the peak ADAMTS13 activity level in remission. We assessed relapse risk in relation to ADAMTS13 conformation. ADAMTS13 conformation in preemptive anti-CD20 treated patients and as a predictor of the requirement for elective treatment were analyzed. We recently showed that a subgroup of patients treated in the caplacizumab era had delayed normalization of ADAMTS13.14 Therefore, we investigated ADAMTS13 conformation in patients treated with and without caplacizumab, as well as in the subgroup with delayed normalization of ADAMTS13. Longitudinal follow-up, including patients with persistently low ADAMTS13 activity, was performed.
Methods
Patient samples
A total of 420 samples were analyzed at different time points from 157 patients who had ADAMTS13 activity <10% at diagnosis with evidence of anti-ADAMTS13 IgG antibody.
CIND was determined in the following groups:
Acute episode, n = 98. This includes pretreatment admission samples in cases in which ADAMTS13 antigen was adequate (≥0.03 μg/mL) to test ADAMTS13 conformation, or the first available sample with an adequate ADAMTS13 antigen after starting treatment in cases in which initial antigen levels were below the detection limit. In the latter case, it was not possible to differentiate the patient’s own ADAMTS13 antigen from that acquired through PEX.
Remission (ADAMTS13 activity >60%), n = 119 patient samples, includes serial measurements from 87 patients and peak ADAMTS13 activity in 60 patient episodes.
Patients treated with preemptive anti-CD20:
Pre–anti-CD20 samples, n = 58; and
Post–anti-CD20 treatment samples, n = 60.
Samples from caplacizumab-treated patient, n = 82 samples across 67 patients (note that all these samples coincide with the remission samples mentioned above).
Longitudinal follow-up, n = 148 samples across 20 patients (note that 63 of these samples coincide with other subgroups and 85 were additional samples) over 11 to 64 months (February 2016 to December 2022).
Treatment/response definitions
Remission samples had ADAMTS13 activity >60% and included sequential samples with increasing ADAMTS13 activity over time in individual patients.
Peak ADAMTS13 activity was defined as the highest ADAMTS13 activity achieved before any ADAMTS13 activity decline and included samples >1 year from diagnosis/acute presentation if no further treatment was required.
Post–anti-CD20 samples with ADAMTS13 activity >60% were measured 3 months after anti-CD20 or at peak ADAMTS13 activity.
ADAMTS13 assays
In-house fluorescence resonance energy transfer-VWF73 method was used to measure ADAMTS13 activity (normal range [NR], 60-146 IU/dL).7 Previously described in-house ELISAs were used to quantify ADAMTS13 antigen levels15 (NR, 74%-134%) and anti-ADAMTS13 IgG antibody (NR <6.1%).16 Median ADAMTS13 activity, ADAMTS13 antigen, and anti-ADAMTS13 IgG antibody levels are expressed as % (± range).
ADAMTS13 conformation
CIND was determined using 1C4-ELISA.12 Minor modifications were made to this assay to optimize the assay in-house. Instead of the F96 Maxisorp Nunc-Immuno plate, a v-bottom polypropylene plate with low protein adsorption was used as an additional plate to preincubate the samples before transfer to the 1C4 coated plate. After the transfer of the preincubated plasma to the 1C4 coated plate, all incubation steps were performed in a plate shaker incubator to provide optimum mixing of samples during incubation. The coloring step was prolonged for 20 minutes to produce higher optical density values. After validating these modifications, the confirmatory step involving the addition of 17G2 to all patient plasma samples to induce an open conformation was omitted to allow extra samples to be run on the plate. As per the original protocol, 17G2 was used for the standard curve and positive control (normal human plasma with 17G2). Samples with ADAMTS13 antigen <0.03 μg/mL were excluded and the next available sample with ADAMTS13 antigen ≥0.03 μg/mL was used. As per the original assay,12 an open ADAMTS13 conformation was determined with CIND >0.5, whereas a closed conformation was determined with CIND ≤0.5. This was also confirmed with our normal controls (supplemental Table 1, available on the Blood website).
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 9. Continuous data were summarized as median and interquartile range/range using the Mann-Whitney test to compare ranks across the 2 groups. Gaussian distribution was not assumed for the comparison between the groups. Numbers and percentages were used to summarize categorical data. The χ2/Fisher exact test was used to assess the statistical significance of categorical variables. The odds ratio (OR) and 95% confidence interval were estimated using logistic regression models. A P value <.05 was considered statistically significant.
The study was approved by medical research ethics committee (08/H0810/54 and 08/H0716/72).
Results
ADAMTS13 conformation at acute presentation
A total of 98 patient episodes were analyzed. The male to female ratio was 29:69 (30%:70%), with a median age of 46 years (range, 12-78), 86 de novo cases and 12 relapses. Race was also examined: White 55, Black 29, Asian 13, and mixed-race 1.
A total of 97 of 98 (99%) patients with acute iTTP had an open ADAMTS13 conformation with the first available sample that was adequate for testing, with a median ADAMTS13 antigen of 11.8% (3%-77.2%) and a median anti-ADAMTS13 IgG antibody of 36.5% (2%-170%). All patients showed evidence of anti-ADAMTS13 IgG antibody to confirm the diagnosis of iTTP. Ten of these samples had a negative anti-ADAMTS13 IgG antibody in the initial sample. However, subsequent samples within this admission had positive anti-ADAMTS13 IgG antibody results in all except 1 patient who had known iTTP with a positive anti-ADAMTS13 IgG antibody detected on previous admission.
In the patient with a closed ADAMTS13 conformation at diagnosis, ADAMTS13 activity was <5%, ADAMTS13 antigen was 86.4%, and anti-ADAMTS13 IgG antibody was 19%. The conformation remained closed during retesting and did not open with 17G2.
Of the 98 patient samples analyzed, 43 (44%) had ADAMTS13 conformation determined from samples after PEX was initiated, as the presenting antigen level was below the detection limit of the assay (supplemental Table 2). From the date of diagnosis, the median time taken to test the CIND in the next available sample with adequate ADAMTS13 antigen was median 6 days (range, 1-95). Two patients were excluded because of the prolonged time needed to achieve adequate antigen, which skewed the results (134 and 160 days).
ADAMTS13 conformation in remission (ADAMTS13 >60%)
A total of 119 patient episodes achieved ADAMTS13 activity >60%. The male to female ratio was 34:85 (29%:71%), with a median age of 45 years (range, 12-77), 106 de novo cases, and 13 relapses. Race was also examined: White 64, Black 36, and Asian 19. No association was found between CIND in remission samples and race (median CIND in White 0.38, Black 0.45, and Asian 0.55; P = .3).
Fifty-one of the 119 (43%) remission samples with ADAMTS13 activity levels >60% had an open ADAMTS13 conformation. Despite normal ADAMTS13 activity, cases with an open conformation had lower ADAMTS13 activity and antigen levels than 68 of 119 cases with a closed conformation (Table 1).
Of the 51 samples with an open conformation in remission, only 18 (35%) had a positive anti-ADAMTS13 IgG antibody, median 11% (7.8%-21.5%). Thirty-two of 51 (65%) had no demonstrable anti-ADAMTS13 IgG antibody. There was no difference between CIND in patients with and without demonstrable anti-ADAMTS13 IgG antibody in the open conformation (0.94 vs 0.92; P = .79). Ten of the 68 (15%) patients with a closed ADAMTS13 conformation had detectable anti-ADAMTS13 IgG antibody.
Association between peak ADAMTS13 activity and CIND
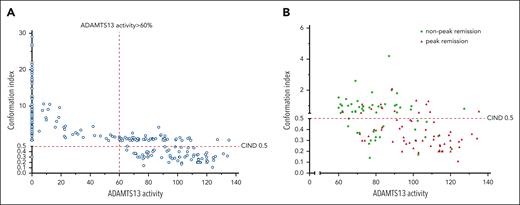
ADAMTS13 activity and CIND were analyzed in all remission samples with ADAMTS13 activity of >60%. A higher ADAMTS13 activity was associated with a lower CIND (Figure 1A). However, there was wide variation in ADAMTS13 activity in remission (60%-135.2%), which prompted analysis of conformation at peak ADAMTS13 levels achieved in the normal range.
ADAMTS13 activity and CIND. (A) Analysis of ADAMTS13 activity and CIND in remission. ADAMTS13 activity is plotted against CIND and includes samples at diagnosis (n = 98) and remission (n = 119). ADAMTS13 activity >60% and <60%, and CIND >0.5 and <0.5 are shown. A CIND >0.5 is considered an open conformation, indicative of the presence of autoantibodies. The right upper quadrant shows samples with an open conformation, despite ADAMTS13 activity >60%. (B) Proportion of patients with open and closed conformations when comparing peak and nonpeak ADAMTS13 activity in remission. Most patients with peak ADAMTS13 activity had a closed ADAMTS13 conformation.
ADAMTS13 activity and CIND. (A) Analysis of ADAMTS13 activity and CIND in remission. ADAMTS13 activity is plotted against CIND and includes samples at diagnosis (n = 98) and remission (n = 119). ADAMTS13 activity >60% and <60%, and CIND >0.5 and <0.5 are shown. A CIND >0.5 is considered an open conformation, indicative of the presence of autoantibodies. The right upper quadrant shows samples with an open conformation, despite ADAMTS13 activity >60%. (B) Proportion of patients with open and closed conformations when comparing peak and nonpeak ADAMTS13 activity in remission. Most patients with peak ADAMTS13 activity had a closed ADAMTS13 conformation.
Sixty of the 119 (50%) remission samples were analyzed for peak ADAMTS13 activity (Table 2; Figure 1B). No association was found between CIND in peak ADAMTS13 samples and race (median CIND in White 0.31, Black 0.41, Asian 0.3; P = .08). Fourteen of 60 (23%) had an open conformation at peak ADAMTS13 activity with normal but significantly lower ADAMTS13 activity than 46 of 60 with a closed conformation (P = .02). ADAMTS13 antigen and anti-ADAMTS13 IgG antibody levels showed no difference between these 2 groups.
ADAMTS13 conformation and relapse
Of the 60 samples that had ADAMTS13 conformation tested at peak ADAMTS13 activity, 4 patients were lost to follow-up and therefore excluded, leaving 56 samples for analysis: 13 with open conformation and 43 with closed conformation. There was a difference in relapse rates between open and closed ADAMTS13 conformations at peak ADAMTS13 activity within 2 years but no significant difference thereafter. The relapse rate was significantly reduced at 1 and 2 years in patients with a closed conformation (Table 3). Patients with a closed ADAMTS13 conformation at peak ADAMTS13 activity had an eightfold lower relapse rate in the subsequent year (9% vs 46%; OR, 8.4; P = .007) and a fivefold lower relapse rate within 2 years (23% vs 62%; OR, 5.3; P = .02) compared with cases with an open conformation.
Median time to treatment with preemptive anti-CD20 was 10 months (range, 8-19) and 25 months (range, 16-34; P = .02) in patients with open and closed ADAMTS13 conformations at peak ADAMTS13 activity, respectively.
ADAMTS13 conformation in patients treated with preemptive anti-CD20
Fifty-eight and 56 patient episodes had ADAMTS13 conformation tested before and after preemptive treatment with anti-CD20, respectively. Two patients received ofatumumab and the remainder received rituximab. At ADAMTS13 relapse, all had an open ADAMTS13 conformation, median ADAMTS13 activity 11% (range, <5%-35%), ADAMTS13 antigen 34.5% (range, 3.6%-110%), and anti-ADAMTS13 IgG antibody 5% (range, 1%-89%).
Fifty-six patient episodes of remission after anti-CD20 therapy were analyzed (Table 4). Thirty-three samples were analyzed at peak ADAMTS13 activity; the median ADAMTS13 activity was 98.9% (range, 62.5%-181.1%), and the median time from ADAMTS13 relapse to peak ADAMTS13 activity was 4 months (range, 1-18). Fifteen samples were analyzed 3 months after treatment with preemptive anti-CD20, with a median ADAMTS13 activity of 94.1% (range, 51%-101.8%). Two patients had ADAMTS13 conformation tested at 3 months and additionally peak remission. A total of 85% of patients with peak ADAMTS13 activity had a closed conformation, compared with 20% at 3 months after preemptive treatment (P < .0001). In 8 cases, peak ADAMTS13 activity coincided with 3 months; median ADAMTS13 activity was 81.4% (range, 59.8%-103.8%), and 75% had a closed conformation.
Associations between relapse rates within 1 and 2 years and ADAMTS13 conformation at peak ADAMTS13 activity after anti-CD20 treatment were investigated. There was no difference in relapse rates or time to further treatment (Table 5).
ADAMTS13 conformation in caplacizumab-treated patients
ADAMTS13 conformation was tested at remission (ADAMTS13 activity >60%) in caplacizumab (n = 82) and noncaplacizumab (n = 37) treated patient episodes (Table 6). Thirty-two of 82 (39%) caplacizumab-treated patient episodes and 19 of 37 (51%) noncaplacizumab-treated patients had an open conformation in remission. As expected, there was no difference in the proportion of patients with an open conformation between those treated with and without caplacizumab (P = .2).
We have previously shown that 28% of caplacizumab-treated patients have delayed normalization of ADAMTS13 activity to >30% within 30 + 28 days of completing PEX.14 Fifteen samples (including some serial repeats) from this group had CIND determined across 9 patients once ADAMTS13 activity had normalized. This was compared with 67 samples from 45 caplacizumab-treated patients who did not have delayed normalization of ADAMTS13 activity. Delayed normalization of ADAMTS13 was not associated with an increase in the proportion of patients remaining in the open conformation once ADAMTS13 activity was normalized (Table 7).
Longitudinal follow-up
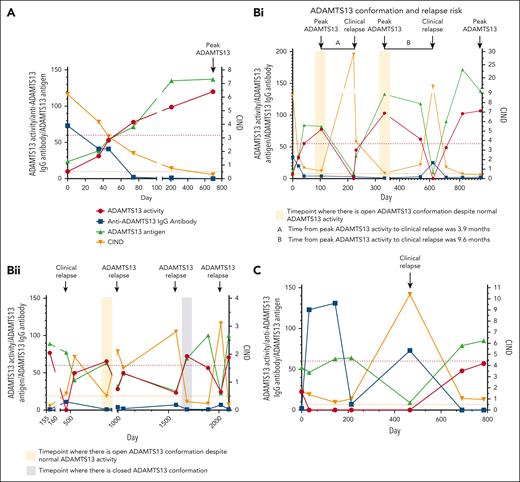
Twenty patients had ADAMTS13 conformation tested during detailed longitudinal follow-up with 5 to 12 serial time points after an acute TTP episode. These were analyzed in 3 groups with examples in each: remission with no further relapse (Figure 2A), remission and subsequent clinical/ADAMTS13 relapse (Figure 2B), and low ADAMTS13 activity (Figure 2C).
Examples of changes in ADAMTS13 conformation tested during longitudinal follow-up in 3 groups. (A) ADAMTS13 conformation at remission with no further relapse. Longitudinal follow-up of a patient from acute iTTP episode to remission reveals an open ADAMTS13 conformation consistent with severe ADAMTS13 deficiency. CIND decreases as ADAMTS13 activity recovers. The conformation remains open for some time despite the normalization of ADAMTS13 activity. A closed ADAMTS13 conformation is achieved at the peak ADAMTS13 activity. A normal anti-ADAMTS13 IgG antibody level is achieved by ADAMTS13 normalization and occurs before achieving a closed ADAMTS13 conformation. Increasing ADAMTS13 antigen levels correlate well with ADAMTS13 activity. To date, this patient has not had any further relapses. (B) Changes in ADAMTS13 conformation associated with clinical/ADAMTS13 relapse. (Bi) Longitudinal follow-up of a patient with multiple clinical relapses. ADAMTS13 conformation is open with peak ADAMTS13 activity before relapse, despite normal ADAMTS13 activity and negative anti-ADAMTS13 IgG antibody level at the same time point. At clinical relapse, there is a significant increase in CIND with severe ADAMTS13 deficiency. It took 3.9 months from peak ADAMTS13 activity to clinical relapse. With ADAMTS13 activity recovery, the CIND falls but remains open (CIND, 0.57) despite a peak ADAMTS13 activity of 103%. Over time, ADAMTS13 activity and antigen levels fall leading to another clinical relapse and is accompanied by a significant increase in CIND. The time from peak ADAMTS13 activity to clinical relapse was 9.6 months. The patient received ofatumumab instead of rituximab for this episode. With treatment, ADAMTS13 activity normalizes and ADAMTS13 conformation becomes closed at the peak ADAMTS13 activity. (Bii) This patient has an open ADAMTS13 conformation during clinical relapse. It remains borderline (CIND, 0.5) despite normalization of ADAMTS13 with treatment. This is followed by an ADAMTS13 relapse, which was treated at an ADAMTS13 activity of 28.3% due to severe symptoms in this patient. CIND increases further; however, the same is not seen with anti-ADAMTS13 IgG antibody. After first course of rituximab, ADAMTS13 activity improves but does not normalize, and the CIND improves but remains open (CIND, 1.5). This is followed by another ADAMTS13 relapse, accompanied by a further fall in ADAMTS13 activity and a rise in CIND value to 2.8. No significant rise in anti-ADAMTS13 IgG antibody is seen. With further elective rituximab, a closed ADAMTS13 conformation is achieved. However, it does not predict ADAMTS13 relapse within 2 years. (C) ADAMTS13 conformation in patient with low ADAMTS13 activity. This patient has persistent severe ADAMTS13 deficiency with a persistent open ADAMTS13 conformation. ADAMTS13 antigen levels remain adequate despite ADAMTS13 activity <10%, except for clinical relapse, when antigen levels fall significantly and are accompanied by a significant rise in CIND. Anti-ADAMTS13 IgG antibody levels are high, which explains the persistent severe ADAMTS13 deficiency and open ADAMTS13 conformation.
Examples of changes in ADAMTS13 conformation tested during longitudinal follow-up in 3 groups. (A) ADAMTS13 conformation at remission with no further relapse. Longitudinal follow-up of a patient from acute iTTP episode to remission reveals an open ADAMTS13 conformation consistent with severe ADAMTS13 deficiency. CIND decreases as ADAMTS13 activity recovers. The conformation remains open for some time despite the normalization of ADAMTS13 activity. A closed ADAMTS13 conformation is achieved at the peak ADAMTS13 activity. A normal anti-ADAMTS13 IgG antibody level is achieved by ADAMTS13 normalization and occurs before achieving a closed ADAMTS13 conformation. Increasing ADAMTS13 antigen levels correlate well with ADAMTS13 activity. To date, this patient has not had any further relapses. (B) Changes in ADAMTS13 conformation associated with clinical/ADAMTS13 relapse. (Bi) Longitudinal follow-up of a patient with multiple clinical relapses. ADAMTS13 conformation is open with peak ADAMTS13 activity before relapse, despite normal ADAMTS13 activity and negative anti-ADAMTS13 IgG antibody level at the same time point. At clinical relapse, there is a significant increase in CIND with severe ADAMTS13 deficiency. It took 3.9 months from peak ADAMTS13 activity to clinical relapse. With ADAMTS13 activity recovery, the CIND falls but remains open (CIND, 0.57) despite a peak ADAMTS13 activity of 103%. Over time, ADAMTS13 activity and antigen levels fall leading to another clinical relapse and is accompanied by a significant increase in CIND. The time from peak ADAMTS13 activity to clinical relapse was 9.6 months. The patient received ofatumumab instead of rituximab for this episode. With treatment, ADAMTS13 activity normalizes and ADAMTS13 conformation becomes closed at the peak ADAMTS13 activity. (Bii) This patient has an open ADAMTS13 conformation during clinical relapse. It remains borderline (CIND, 0.5) despite normalization of ADAMTS13 with treatment. This is followed by an ADAMTS13 relapse, which was treated at an ADAMTS13 activity of 28.3% due to severe symptoms in this patient. CIND increases further; however, the same is not seen with anti-ADAMTS13 IgG antibody. After first course of rituximab, ADAMTS13 activity improves but does not normalize, and the CIND improves but remains open (CIND, 1.5). This is followed by another ADAMTS13 relapse, accompanied by a further fall in ADAMTS13 activity and a rise in CIND value to 2.8. No significant rise in anti-ADAMTS13 IgG antibody is seen. With further elective rituximab, a closed ADAMTS13 conformation is achieved. However, it does not predict ADAMTS13 relapse within 2 years. (C) ADAMTS13 conformation in patient with low ADAMTS13 activity. This patient has persistent severe ADAMTS13 deficiency with a persistent open ADAMTS13 conformation. ADAMTS13 antigen levels remain adequate despite ADAMTS13 activity <10%, except for clinical relapse, when antigen levels fall significantly and are accompanied by a significant rise in CIND. Anti-ADAMTS13 IgG antibody levels are high, which explains the persistent severe ADAMTS13 deficiency and open ADAMTS13 conformation.
Remission with no further relapse (n = 5)
A closed ADAMTS13 conformation was more likely at the peak ADAMTS13 activity. Anti-ADAMTS13 IgG antibody levels normalized earlier than the achievement of a closed ADAMTS13 conformation in all 5 patients. ADAMTS13 antigen levels changed in parallel with changes in the CIND in all patients. In 1 patient, an open conformation was evident with peak ADAMTS13 activity >1 year after acute iTTP, despite no further relapses.
Clinical and ADAMTS13 relapse (n = 11)
Relapse was often but not always preceded by an open ADAMTS13 conformation. However, not all analyses were performed at peak ADAMTS13 activity. Clinical relapse was associated with a significant fall in ADAMTS13 antigen, although a rise in anti-ADAMTS13 IgG antibody was not always evident. ADAMTS13 relapse was associated with a more variable fall in ADAMTS13 antigen. However, an open ADAMTS13 conformation was always observed at the point of both clinical and ADAMTS13 relapse, with an increase in CIND compared with before relapse. A rise in CIND to approximately double the baseline open levels was seen despite no increase in the anti-ADAMTS13 IgG response in most cases (9/11). Therefore, an open ADAMTS13 conformation often precedes a clinical/ADAMTS13 relapse. In 8 of the 11 patients, ADAMTS13 did not become closed or remained borderline (CIND, ∼0.5) after the previous acute TTP episode.
Low ADAMTS13 activity (n = 4)
In patients with persistent ADAMTS13 deficiency (ADAMTS13 activity <60% and includes patients with persistent severe ADAMTS13 deficiency <10%), even in clinical remission, ADAMTS13 conformation was persistently open despite adequate ADAMTS13 antigen levels that were within or near normal in some cases. With clinical relapse, the ADAMTS13 antigen dropped significantly along with a significant further rise in CIND and a simultaneous anti-ADAMTS13 IgG antibody response. When ADAMTS13 activity was >10 and <60%, a fall in ADAMTS13 activity was accompanied by a rise in CIND and occasionally a fall in ADAMTS13 antigen but no significant anti-ADAMTS13 IgG antibody response.
Discussion
There is increasing interest in using the ADAMTS13 conformation to identify patients with anti-ADAMTS13 antibody presently not identified with current ADAMTS13 assays.11-13,17 The interaction between CUB and the spacer domain of ADAMTS13 results in a closed conformation.11-13,18-20 An open ADAMTS13 conformation has been found to be the hallmark of acute iTTP.12,13,21 Approximately 38% of patients with ADAMTS13 activity in the normal range had an open conformation,13 which was confirmed in our patient cohort.
We examined ADAMTS13 conformation in a very large cohort of iTTP cases, including longitudinal assessment, to better understand changes in remission and relapse and how these could be translated into clinical practice. We focused on the peak ADAMTS13 activity levels in remission and demonstrated the clinical utility of the open ADAMTS13 conformation at peak ADAMTS13 activity in predicting relapse rates within 2 years of achieving peak ADAMTS13 activity.
There was an inverse correlation between CIND and ADAMTS13 activity. The median ADAMTS13 activity in unselected remission samples was toward the lower limit of normal; therefore, further analysis was performed on peak ADAMTS13 activity samples. Using peak ADAMTS13 activity rather than ADAMTS13 activity within the normal range, we found that fewer patients (23% vs 43%) had an open conformation. Peak ADAMTS13 activity was normal but significantly lower in patients with an open conformation in remission than in those with a closed conformation. Furthermore, no demonstrable anti-ADAMTS13 IgG antibody was found in most (65%) of remission samples with an open ADAMTS13 conformation. ADAMTS13 antigen testing is currently limited; however, anti-ADAMTS13 IgG antibody testing is more widely available and has clinical importance in acute TTP but may not be increased in ADAMTS13 relapses. This signifies the added value of the open/closed assay in confirming the presence of ongoing antibody-mediated disease, which is not evident with current ADAMTS13 testing.
Almost half of the patients with an open ADAMTS13 conformation at peak ADAMTS13 activity relapsed within 1 year but 91% with a closed conformation did not relapse. The risk of ADAMTS13/clinical relapse within 1 year was 8 times less likely with a closed ADAMTS13 conformation at peak remission. Extending the analysis to 2 years, the rate of relapse was higher (62% vs 39%) with an open conformation. In the closed conformation, the risk of relapse was significantly reduced. The time to preemptive anti-CD20 treatment was shorter in patients with an open ADAMTS13 conformation (10 months vs 25 months). ADAMTS13 conformation at peak ADAMTS13 activity was found to predict relapse only after an acute episode of TTP and not after an ADAMTS13 relapse, highlighting in which ADAMTS13 conformation may be of clinical relevance. However, the ADAMTS13 IgG antibody assay remains useful for the diagnosis of iTTP during the acute phase, especially given the number of cases in which ADAMTS13 antigen is too low to allow ADAMTS13 conformation testing.
It has been shown that TTP has a 5-year relapse rate of 40%9 and that the median time to achieve peak ADAMTS13 activity after ofatumumab and obinutuzumab was 120 days.22 This highlights the long duration of relapse and supports long-term monitoring of patients with TTP. When a fall in ADAMTS13 activity is demonstrated, the frequency of clinical and laboratory monitoring is increased accordingly.
It has recently been shown that a CIND >0.645 and >0.835, respectively, have over three- and fourfold higher risks for earlier ADAMTS13 and clinical relapse.23 However, to our knowledge, this is the first analysis to show an association between ADAMTS13 conformation at peak ADAMTS13 activity and the risk of relapse and time to preemptive treatment. This allows clinicians to identify a subgroup of patients in whom the relapse risk is higher, thereby allowing closer monitoring and provision of preemptive anti-CD20 treatment to prevent relapse. A closed conformation at the peak ADAMTS13 activity is protective from relapse for up to 2 years; however, an open conformation at the peak ADAMTS13 activity does not signify a higher risk of relapse within 1 year (46% with relapse vs 54% with no relapse).
Studies have examined the use of preemptive therapy with falling ADAMTS13 activity to prevent relapse.4,24 In our patient cohort treated with preemptive anti-CD20 therapy, 85% had a closed ADAMTS13 conformation with peak ADAMTS13 activity. After anti-CD20 treatment, there was no overall difference in relapse rates or time to further treatment, based on the open or closed ADAMTS13 conformation.
The use of caplacizumab did not affect ADAMTS13 conformation results and the subgroup of patients with delayed normalization of ADAMTS13 was not more likely to have an open conformation once ADAMTS13 activity remission was achieved. However, as previously shown,13 when ADAMTS13 activity is <50%, conformation is open; therefore, these patients are at an increased risk of relapse during the period when ADAMTS13 activity is not normalized.
We presented multiple time points from a range of patients with iTTP during longitudinal follow-up, from acute TTP episodes achieving remission with no relapses to cases in which conformation becomes closed with treatment after acute TTP, followed by a switch to open conformation before further relapse (usually ADAMTS13 relapse). To our knowledge, this is the first study to show longitudinal data with a range of patterns, particularly in patients with persistent ADAMTS13 deficiency, in which ADAMTS13 conformation is persistently open and shows a further increase in CIND during relapse. In this group of patients, the ADAMTS13 antigen along with CIND may be a better marker of disease activity and relapse risk. Our longitudinal data also highlight the limitations of anti-ADAMTS13 IgG antibody in relapses, as it does not always increase significantly. A significant rise in CIND with an open conformation preceding relapse, despite no anti-ADAMTS13 IgG, suggests that an open ADAMTS13 conformation represents antibodies that are not detected with our current anti-ADAMTS13 IgG ELISA. Longitudinally, a closed conformation may be identified, but repeat testing would be required as an open antibody status occurs before there is a drop in ADAMTS13 activity despite negative anti-ADAMTS 13 IgG antibody levels.
Some insight into the pathophysiology of the evolving anti-ADAMTS13 response is gained from these observations. ADAMTS13 is in an open conformation in clinical remission in the cohort with low ADAMTS13 activity, but with well-preserved antigen levels, suggesting that anti-ADAMTS13 antibodies predominantly inhibit the enzyme at that stage. The rise in CIND and anti-ADAMTS13 IgG and fall in antigen at clinical relapse indicates that the antibody response progresses to additionally clear ADAMTS13 from the circulation.
Only 1 patient had a closed conformation at diagnosis of acute iTTP and this was accompanied by a normal ADAMTS13 antigen level of 86% and has been similarly reported.13 Interestingly, 17G2 did not induce an open conformation in this patient and future epitope mapping studies may provide insights into this finding.
In samples in which the ADAMTS13 antigen level was below the detection limit of the assay at diagnosis, pre-PEX samples from subsequent days were used. The CIND will be influenced by the ADAMTS13 parameters present in the donor plasma; however, not all patients were undergoing PEX at the time of analysis. Therefore, native ADAMTS13 parameters would be accountable for the measured CIND in these cases.
Roose et al13 have shown that conformational change in ADAMTS13 can be induced by anti-ADAMTS13 autoantibodies and that an open ADAMTS13 conformation may occur before a substantial drop in ADAMTS13 activity, which is also demonstrated in our analysis. Furthermore, we have established that conformational changes can occur without an anti-ADAMTS13 IgG detected, suggesting that changes may occur before a measurable anti-ADAMTS13 IgG response. Whether the anti-ADAMTS13 IgG antibody starts to exert an effect on ADAMTS13 conformation when its response has been initiated but before measurable levels are seen, is a possibility. At present, we have no reliable means of measuring this but more light may be shed on this theory in the future. We found that some iTTP samples at diagnosis did not have a positive anti-ADAMTS13 in the initial sample with ADAMTS13 activity <10% (but did mount a response in subsequent samples), which may support this theory.
In summary, to our knowledge, this is the first report to identify peak ADAMTS13 activity as an optimal time point to assess ADAMTS13 conformation in remission and to show the risk of relapse within 2 years of achieving peak ADAMTS13 activity. This emphasizes multiple changes in ADAMTS13 conformation with a longitudinal follow-up. This focuses the subgroup of patients who require closer monitoring, allowing earlier detection and preemptive treatment to prevent relapse. The reason why some patients remain at a higher risk of relapse is not clear and, as shown, is not always accompanied by a rise in measurable anti-ADAMTS13 IgG antibody level. However, our data help to differentiate patients who have a significantly lower risk of relapse within 2 years by achieving a closed ADAMTS13 conformation at peak ADAMTS13 activity. Furthermore, we showed that patients who had an open ADAMTS13 conformation with peak ADAMTS13 activity after treatment had a shorter time to require preemptive anti-CD20 treatment.
Authorship
Contribution: N.P. designed the research, conducted laboratory testing, analyzed the data, and wrote the manuscript; B.D., M. Subhan, and D.S. conducted laboratory testing and reviewed the manuscript; M.T. designed the research and reviewed the manuscript; R.d.G. advised on laboratory testing and reviewed the manuscript; K.V. reviewed the manuscript; and M. Scully designed the research, reviewed the manuscript, and is a senior author.
Conflict-of-interest disclosure: M. Scully received speaker fees and served on advisory boards for Alexion, Novartis, Takeda, Sanofi, and Octapharma; and received research grants from Shire and Alexion. M.T. received speaker fees from Bayer, Sanofi Genzyme, and Anthos; served on advisory boards for Ablynx, Sanofi Genzyme, and Bayer; provided consultancy for Bayer and Anthos; and received educational grants from Anthos. The remaining authors declare no competing financial interests.
Correspondence: Nithya Prasannan, Department of Haematology, University College London Hospital, 250 Euston Rd, London NW1 2PG, United Kingdom; email: n.prasannan@nhs.net.
References
Author notes
Data are available on request from the corresponding author, Nithya Prasannan (n.prasannan@nhs.net).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.