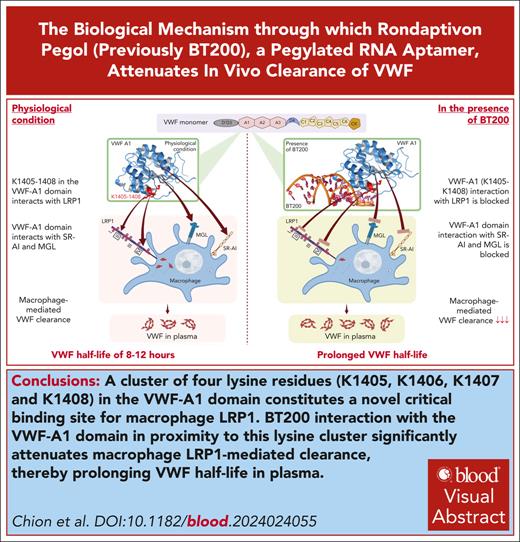
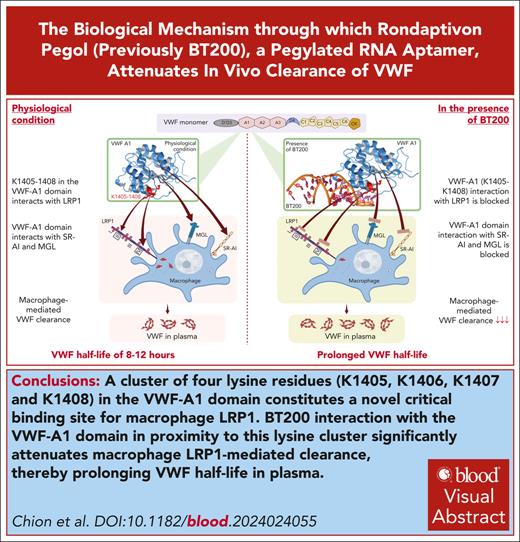
Key Points
A cluster of 4 lysine residues (K1405, K1406, K1407 and K1408) in the VWF-A1 domain constitutes a critical binding site for macrophage LRP1.
BT200 interaction with the VWF-A1 domain in proximity to this lysine cluster significantly attenuates macrophage LRP1-mediated clearance.
Visual Abstract
Rondaptivon pegol (previously BT200) is a pegylated RNA aptamer that binds to the A1 domain of von Willebrand factor (VWF). Recent clinical trials demonstrated that BT200 significantly increased plasma VWF–factor VIII levels by attenuating VWF clearance. The biological mechanism(s) through which BT200 attenuates in vivo clearance of VWF has not been defined. We hypothesized that BT200 interaction with the VWF-A1 domain may increase plasma VWF levels by attenuating macrophage-mediated clearance. We observed that full-length and VWF-A1A2A3 binding to macrophages and VWF-A1 domain binding to lipoprotein receptor–related protein 1 (LRP1) cluster II and cluster IV were concentration-dependently inhibited by BT200. Additionally, full-length VWF binding to LRP1 expressed on HEK293T (HEK-LRP1) cells was also inhibited by BT200. Importantly, BT200 interacts with the VWF-A1 domain in proximity to a conserved cluster of 4 lysine residues (K1405, K1406, K1407, and K1408). Alanine mutagenesis of this K1405-K1408 cluster (VWF-4A) significantly (P < .001) attenuated binding of VWF to both LRP1 clusters II and IV. Furthermore, in vivo clearance of VWF-4A was significantly (P < .001) reduced than that of wild-type VWF. BT200 did not significantly inhibit binding of VWF-4A to LRP1 cluster IV or HEK-LRP1 cells. Finally, BT200 interaction with the VWF-A1 domain also inhibited binding to macrophage galactose lectin and the SR-AI scavenger receptor. Collectively, our findings demonstrate that BT200 prolongs VWF half-life by attenuating macrophage-mediated clearance and specifically the interaction of K1405-K1408 in the VWF-A1 domain with macrophage LRP1. These data support the concept that targeted inhibition of VWF clearance pathways represents a novel therapeutic approach for von Willebrand disease and hemophilia A.
Introduction
In normal human plasma, von Willebrand factor (VWF) circulates with a half-life of ∼8 to 12 hours.1,2 Furthermore, an estimated 95% of plasma factor VIII (FVIII) circulates in high-affinity complex with VWF and consequently is cleared through VWF–dependent clearance pathways.3,4 Previous studies have provided significant insights into the cellular mechanisms involved in regulating VWF clearance.2 Lenting et al5 showed that radio-labeled VWF infused into VWF-deficient mice was primarily targeted to the liver, where it colocalized with CD68+ Kupffer macrophage cells.6 In addition, macrophage depletion with either gadolinium or clodronate significantly prolonged VWF survival in vivo. Subsequent in vitro studies confirmed concentration-dependent binding of VWF to human and murine macrophages.6-9 This macrophage binding was followed by VWF uptake and subsequent degradation.6,10,11 A number of specific macrophage surface receptors have been implicated in regulating VWF clearance, including low-density lipoprotein receptor–related protein 1 (LRP1) and scavenger receptor class A member 1 (SR-AI), respectively.12,13 In addition, the macrophage galactose lectin (MGL) has been reported to interact with desialylated O-glycan determinants on VWF.14,15 More recent studies have suggested that other cells including liver sinusoidal endothelial cells (ECs)16 and hepatocytes17 may also play roles in regulating VWF clearance. Improved understanding of the precise biological mechanisms underlying VWF-FVIII complex clearance may ultimately offer the opportunity to develop novel extended half-life VWF and FVIII therapies.18
Rondaptivon pegol (previously BT200) is a pegylated RNA aptamer that binds to the A1 domain of VWF with high affinity.19 Consistent with this VWF-A1 domain interaction, initial studies in cynomolgus monkeys confirmed that BT200 dose-dependently inhibited VWF interaction with platelets.19 However, subsequent studies in healthy human volunteers demonstrated that subcutaneous or IV BT200 administration also significantly increased plasma VWF and FVIII levels approximately fourfold.20 In contrast, BT200 had no significant effect on plasma VWF propeptide levels, suggesting that BT200 inhibits VWF-FVIII complex clearance in vivo.20 Importantly, the in vivo effect of BT200 in prolonging VWF-FVIII half-life was observed at concentrations below those needed to inhibit VWF interaction with platelet GPIbα.19,20 Based on these findings, recent studies have investigated BT200 as a potential novel therapeutic to increase plasma VWF and/or FVIII levels in patients with von Willebrand disease (VWD) or hemophilia A. Phase 2 clinical trials demonstrated that BT200 significantly increased plasma VWF levels and normalized platelet counts in some patients with type 2B VWD.21 Furthermore, subcutaneous BT200 administration also significantly increased plasma FVIII levels in patients with mild (median FVIII:C, 22%-48%) or moderate hemophilia A (median FVIII:C, 3%-7.5%).22 Finally, in patients with severe hemophilia A, pretreatment with BT200 significantly prolonged the half-life of infused FVIII therapy (median, 10.4-31.1 hours).22 Critically, however, despite these promising clinical data, the biological mechanism(s) through which BT200 binding to the VWF-A1 domain attenuates in vivo clearance of the VWF-FVIII complex have not been defined.
The molecular mechanisms through which individual VWF domains and glycan determinants interact with specific macrophage clearance receptors remain poorly understood.2 However, previous studies demonstrated that VWF binding to macrophages was significantly enhanced in the presence of either shear stress or ristocetin,11,12 suggesting that the VWF-A1 domain plays a key role in regulating macrophage-mediated clearance. Consequently, we hypothesized that binding of BT200 to the VWF-A1 domain may increase plasma VWF-FVIII complex levels by inhibiting macrophage-mediated clearance. Herein, we demonstrate, to our knowledge, for the first time that a cluster of 4 lysine residues (K1405, K1406, K1407, and K1408) in the VWF-A1 domain constitutes a critical binding site for macrophage LRP1. In addition, our findings show that BT200 interaction with the VWF-A1 domain in proximity to this conserved lysine cluster significantly increases plasma VWF-FVIII levels by attenuating macrophage LRP1–mediated clearance. Finally, we further show that BT200 interaction with the VWF-A1 domain also inhibits binding to the MGL and the SR-AI scavenger receptor.
Materials and methods
Expression and purification of recombinant VWF truncations and variants
Full-length recombinant human VWF with a C-terminal His-tag was expressed in HEK293T cells using the expression vector pcDNA-VWF as previously described.7 Truncated VWF fragments VWF-D’A3 (residues 764-1872), VWF-A1A2A3 (residues 1260-1874), VWF-A1 (residues 1260-1494), VWF-A2 (residues 1495-1672), and VWF-A3 (residues 1673-1874) were inserted into the vector pEXPR-IBA 42 via NheI and AgeI restriction sites. Site-directed mutagenesis of full-length VWF and the VWF truncations was performed to replace K1405, K1406, K1407, and K1408 in the VWF-A1 domain with lysine residues. As described before, mutagenesis studies were performed using the QuikChange method with KOD Hot Start DNA Polymerase (Merck, Cork, Ireland).7 All mutations were confirmed by DNA sequencing. All VWF variants were transiently expressed in HEK293T cells. For each variant, conditioned serum free medium was harvested, concentrated, and purified via nickel affinity chromatography, as described before.7
Stable expression of LRP1 in HEK293 cells
Full-length LRP1 complementary DNA (cDNA; 13.63 kilobase) was amplified with primers (5’-AACTCGAGCACACCATGCTGACCCCG-3’, 5’AAGTTTAAACTATGCCAAGGGGTCCCC-3’) on human placental cDNA (Invitrogen; Thermo Fisher Scientific) with KOD Hot Start DNA Polymerase (Merck). Polymerase chain reaction product was cloned into pJET vector (Thermo Fisher Scientific). LRP1 cDNA was excised by XhoI-PmeI digestion and ligated into pcDNA3.1-V5-HisA (Thermo Fisher Scientific). The LRP1 expression vector was then transfected into HEK293 with TansIT 293 reagent (Mirus Bio), and clonal selection was performed using G418.
VWF binding to macrophages and HEK-LRP1 in the presence or absence of BT200
Bone marrow was isolated from femurs and tibias of 8- to 14-week-old C57BL/6J mice and differentiated into macrophages by culturing in RPMI (Merck) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific), 1% penicillin/streptomycin (Merck), and 10% macrophage colony-stimulating factor derived from L929 cells for 7 to 10 days, with supplementation of media with macrophage colony-stimulating factor on days 3 and 7 to generate bone marrow–derived macrophages (BMDMs). All murine experiments were approved by the Royal College of Surgeons in Ireland Ethics Committee (REC1315) and were performed in full accordance with the Health Products Regulatory Authority of Ireland (license AE19136/P040). Blood samples were collected from healthy blood donors after obtaining written consent (St James’s Hospital Research Ethics Committee 2017/01/6 and the Irish Blood Transfusion Society IBTS-004-03-18). Human peripheral blood mononuclear cells were isolated from buffy coats by Histopaque (Sigma) gradient separation. Anti-CD14 beads (Miltenyi Biotec) were used to isolate monocytes. Isolated monocytes were differentiated into macrophages for 7 to 10 days in RPMI media supplemented with 10% human serum (Sigma).
VWF (10 μg/mL) was mixed with either BT100 or BT200 aptamer in binding buffer (1% polyvinylpyrrolidone and 3% bovine serum albumin in Tris-buffered saline with 0.1% Tween 20 [TBS-T] supplemented with 5 mM CaCl2) and incubated at 37°C for 30 minutes. VWF binding to cells was subsequently evaluated by flow cytometry. Bone marrow–derived macrophages were resuspended and counted. A total of 5 × 106 cells per mL were then incubated with VWF:aptamer complexes (1 μg/mL VWF) at 37°C for 45 minutes in the presence of ristocetin (1.5 mg/mL), followed by incubation with Fc block (6.0% volume-to-volume final) at 4°C for 30 minutes. Cells were then washed, and cell-bound VWF probed using rabbit polyclonal anti-VWF (5 μg/mL at 4°C for 30 minutes). Alexa Fluor 488 goat anti-rabbit immunoglobulin G was used to detect bound anti-VWF antibody (4 μg/mL at 4°C for 45 minutes). Live/dead stain (1/50-fold dilution final) was used to identify live cells and dead cells. For HEK cells expressing LRP1, Accutase was used (10% final) to detach cells, and VWF binding was assessed as described above, but Fc-blocking was omitted. Fluorescence intensity was measured using a BD FACSCanto (BD Biosciences) or NxT Attune flow cytometer (Thermo Fisher Scientific, Bio-Science). Each assay was performed in triplicate, and the results were analyzed using FlowJo software.
Binding of VWF to LRP1 cluster II, cluster IV, SR-AI, and MGL
LRP1 cluster II was coated at 2 μg/mL, and cluster IV was coated at 1 μg/mL, as previously described.23 SR-AI was coated at 2 μg/mL, and MGL was coated at 5 μg/mL. The binding protocol for full-length VWF and VWF fragments was as previously detailed.23 All plate-binding assays were performed in the presence of ristocetin (1.5 mg/mL). Full-length VWF binding was probed using rabbit polyclonal DAKO-HRP conjugated. Binding of truncated VWF-A1A2A3, VWF-A1, VWF-A2, and VWF-A3 proteins (with or without sequence variants) was probed with conjugated penta-HIS-HRP. For experiments involving caplacizumab (Ablynx, Sanofi, Waterford, Ireland), BT100, or BT200 aptamer binding, VWF (10 μg/mL) or VWF fragments (150 nM final) were mixed in microtubes with various amounts of aptamer or caplacizumab. Tubes were incubated at 37°C for 45 minutes. At the end of incubation, the formed complexes were loaded onto receptor-coated plates, and binding experiments were completed as above.
Data presentation and statistical analysis
GraphPad Prism 10.1.0 (GraphPad Software LLC) was used to plot and analyze data. Plate- and cell-binding curves were plotted using “nonlinear regression curve fit,” in which dots represent mean values and error bars represent standard deviations. The extra-sum-of-squares F test was used to statistically test the binding capacity of a curve or to compare the binding capacity of 2 curves. A P value <.05 was considered significant.
See the supplemental Data, available on the Blood website, for additional details regarding materials and methods.
Results
VWF binding to macrophages is significantly inhibited by BT200
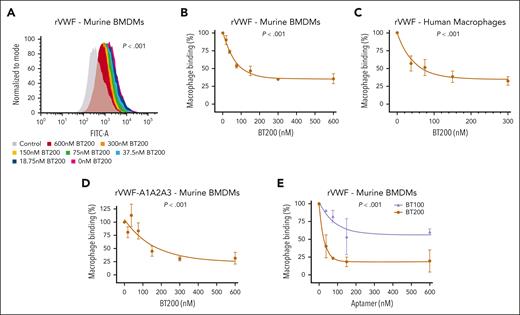
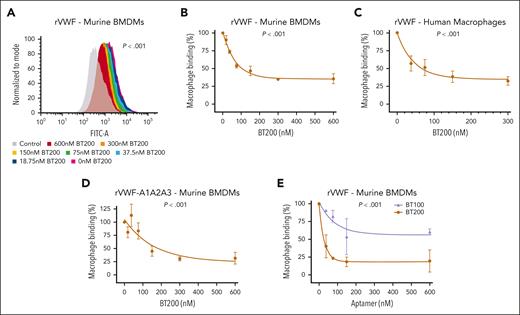
To determine the biological mechanisms through which BT200 inhibits clearance of the VWF-FVIII complex, we first investigated VWF binding to macrophages. Consistent with previous studies,6,7,9-12 we confirmed that full-length VWF bound to both human and murine macrophages in a dose-dependent manner (Figure 1A-C; supplemental Figure 1A). Interestingly, binding and uptake of recombinant human VWF to macrophages was significantly (P < .001) inhibited by BT200 in a concentration-dependent manner (Figure 1A-C; supplemental Figure 1B). In contrast, although free FVIII has also been shown to bind to macrophages, no significant effect of BT200 on FVIII-macrophage interaction was observed (supplemental Figure 2A-B). In addition, the BT100 aptamer did not bind to free FVIII, and BT200 had no inhibitory effect on the binding of FVIII to VWF (supplemental Figure 2C-D).
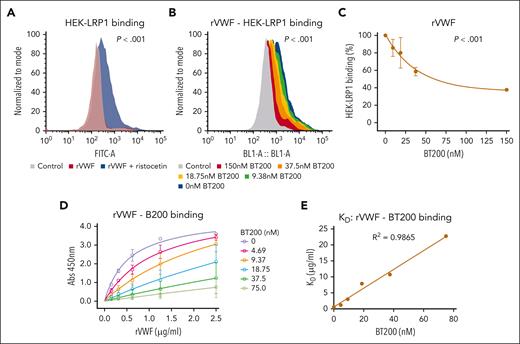
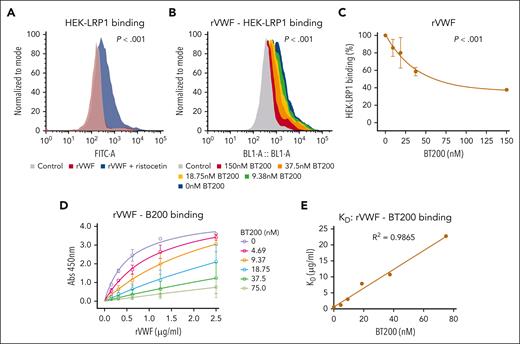
VWF binding to macrophages is concentration-dependently inhibited by BT200. (A) Binding of full-length recombinant human VWF to murine BMDMs was assessed by flow cytometry in the presence or absence of increasing concentrations of BT200. Magenta is VWF binding in the absence of BT200; gray is background in the absence of VWF; blue to red signals for increasing BT200 concentrations. The y-axis represents the binding capacity normalized to the number of cells. FITC-A on the x-axis represents the fluorescence intensity absorbance at the wavelength of 490 nm, in which higher values indicate more VWF binding. (B-E) BMDM or primary human monocyte–derived macrophage binding on the y-axis are calculated from mean fluorescent intensities at different BT200 or BT100 concentrations presented on the x-axis. Error bars depict the standard deviation of the fluorescent intensities. P values are outcomes of extra-sum-of-squares F test and represent whether the binding curves demonstrate a significant binding to BMDMs in panels B-D or to compare the binding capacity of BT100 and BT200 to BMDMs in panel E. (B) Full-length human rVWF binding (%) to BMDMs is concentration-dependently inhibited by BT200. (C) Full-length human rVWF binding (%) to primary human macrophages is concentration-dependently inhibited by BT200. (D) Binding of truncated human VWF-A1A2A3 to BMDMs is concentration-dependently inhibited by BT200. (E) Binding of full-length human rVWF to BMDMs is significantly more inhibited by the pegylated BT200 compared to the unpegylated BT100. FITC-A, fluorescein isothiocyanate; rVWF, recombinant VWF.
VWF binding to macrophages is concentration-dependently inhibited by BT200. (A) Binding of full-length recombinant human VWF to murine BMDMs was assessed by flow cytometry in the presence or absence of increasing concentrations of BT200. Magenta is VWF binding in the absence of BT200; gray is background in the absence of VWF; blue to red signals for increasing BT200 concentrations. The y-axis represents the binding capacity normalized to the number of cells. FITC-A on the x-axis represents the fluorescence intensity absorbance at the wavelength of 490 nm, in which higher values indicate more VWF binding. (B-E) BMDM or primary human monocyte–derived macrophage binding on the y-axis are calculated from mean fluorescent intensities at different BT200 or BT100 concentrations presented on the x-axis. Error bars depict the standard deviation of the fluorescent intensities. P values are outcomes of extra-sum-of-squares F test and represent whether the binding curves demonstrate a significant binding to BMDMs in panels B-D or to compare the binding capacity of BT100 and BT200 to BMDMs in panel E. (B) Full-length human rVWF binding (%) to BMDMs is concentration-dependently inhibited by BT200. (C) Full-length human rVWF binding (%) to primary human macrophages is concentration-dependently inhibited by BT200. (D) Binding of truncated human VWF-A1A2A3 to BMDMs is concentration-dependently inhibited by BT200. (E) Binding of full-length human rVWF to BMDMs is significantly more inhibited by the pegylated BT200 compared to the unpegylated BT100. FITC-A, fluorescein isothiocyanate; rVWF, recombinant VWF.
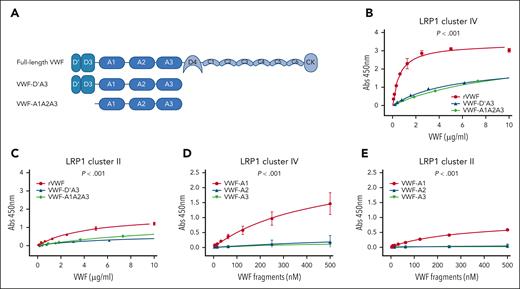
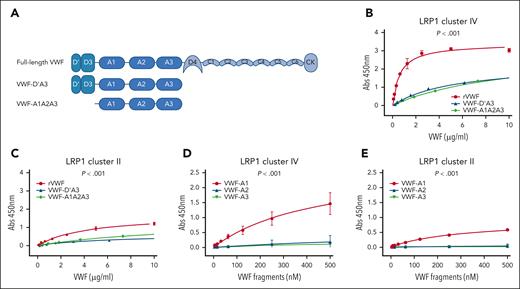
Interaction of VWF domains with LRP1 cluster II and cluster IV. (A) Overview of the recombinant truncated VWF constructs studied. (B) Binding of full-length rVWF (red), VWF-D’A3 (blue), and VWF-A1A2A3 (green) to purified LRP1 cluster IV. (C) Binding of full-length rVWF (red), VWF-D’A3 (blue), and VWF-A1A2A3 (green) to LRP1 cluster II. (D) Binding of individual VWF-A1 (red), VWF-A2 (blue), and VWF-A3 (green) domains to LRP1 cluster IV. (E) Binding of individual VWF-A1 (red), VWF-A2 (blue), and VWF-A3 (green) domains to LRP1 cluster II. Dots represent mean absorbance at 450 nm at corresponding VWF concentrations. Error bars depict standard deviations. P values are outcomes of extra-sum-of-squares F test and compare the binding capacity of different VWF constructs with LRP1 cluster IV or cluster II. Abs, absorbance.
Interaction of VWF domains with LRP1 cluster II and cluster IV. (A) Overview of the recombinant truncated VWF constructs studied. (B) Binding of full-length rVWF (red), VWF-D’A3 (blue), and VWF-A1A2A3 (green) to purified LRP1 cluster IV. (C) Binding of full-length rVWF (red), VWF-D’A3 (blue), and VWF-A1A2A3 (green) to LRP1 cluster II. (D) Binding of individual VWF-A1 (red), VWF-A2 (blue), and VWF-A3 (green) domains to LRP1 cluster IV. (E) Binding of individual VWF-A1 (red), VWF-A2 (blue), and VWF-A3 (green) domains to LRP1 cluster II. Dots represent mean absorbance at 450 nm at corresponding VWF concentrations. Error bars depict standard deviations. P values are outcomes of extra-sum-of-squares F test and compare the binding capacity of different VWF constructs with LRP1 cluster IV or cluster II. Abs, absorbance.
In keeping with the fact that the BT200 aptamer interacts specifically with the VWF-A1 domain,19 significantly (P < .001) attenuated binding of truncated VWF-A1A2A3 to macrophages was also observed in the presence of BT200 (Figure 1D). BT100 is an unpegylated RNA aptamer that shares the same nucleotide sequence as BT200.19 To assess the relative importance of aptamer sequence vs the 40-kDa polyethylene glycol (PEG) moiety in regulating inhibition of macrophage-mediated VWF clearance, binding studies were compared in the presence of BT100 or BT200. Although BT100 reduced binding of full-length VWF to macrophages, the inhibitory effect of BT200 was significantly (P < .001) more marked (Figure 1E). Collectively, these data show that BT200 binding to the VWF-A1 domain significantly attenuates VWF binding to macrophages.
VWF-A1 domain regulates interactions with LRP1 clusters II and IV
The macrophage LRP1 receptor regulates VWF clearance in a shear-dependent manner.11,12 This LRP1 receptor consists of 4 extracellular cluster repeats (I-IV) that are responsible for interacting with a diverse array of ligands.24 The majority of LRP1 ligands bind to cluster II and cluster IV.23,25,26 Using plate-binding assays, we observed that full-length recombinant VWF, VWF-D’A3, and VWF-A1A2A3 all bound to LRP cluster IV in a concentration-dependent manner (Figure 2A-B). Consistent with previous studies suggesting that several different VWF domains may interact with LRP1,9,10,23,27,28 binding of full-length VWF was significantly (P < .001) increased than that of either VWF-D’A3 or VWF-A1A2A3 (Figure 2B). Although interaction of full-length VWF with LRP1 cluster II was also seen, this was limited compared with cluster IV binding (Figure 2C). In addition, we observed that the isolated VWF-A1 domain could modulate binding to both LRP1 clusters II and IV (Figure 2D-E). In contrast, no binding was seen for isolated VWF-A2 or VWF-A3 domains. Together, these findings demonstrate that macrophage LRP1-mediated VWF clearance involves interactions with cluster II and cluster IV and further highlight a specific role for the VWF-A1 domain in modulating binding to both clusters.
BT200 concentration-dependently inhibits VWF interaction with LRP1
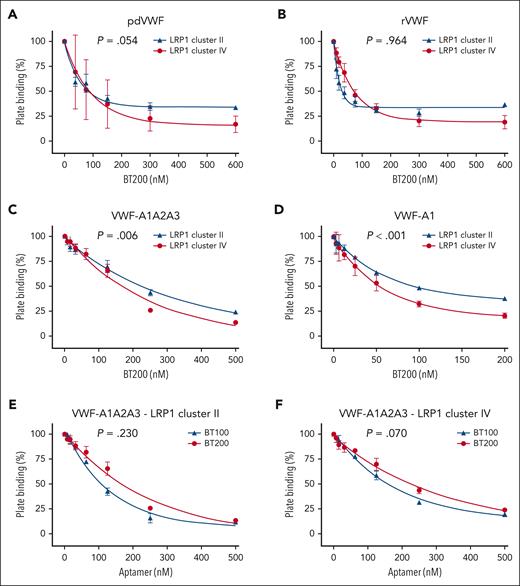
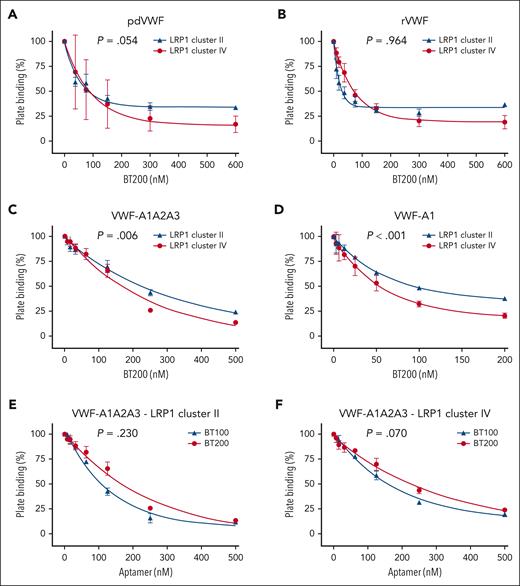
Given the role of LRP1 in regulating VWF clearance,9,11,12 we next investigated the effect of BT200 on VWF binding to LRP1 clusters II and IV. In keeping with the inhibitory effects of BT200 on VWF-macrophage binding (Figure 1), we observed that BT200 concentration-dependently reduced binding of human plasma-derived and recombinant full-length VWF to both LRP1 cluster II and cluster IV (Figure 3A-B). Consistent with the fact that BT200 is directed against the VWF-A1 domain,19 the pegylated aptamer also significantly reduced the binding of VWF-A1A2A3 and the isolated VWF-A1 domain to LRP1 cluster II and cluster IV (Figure 3C-D). In contrast to the differential effects of BT200 and BT100 aptamers in terms of their respective abilities to inhibit VWF binding to macrophages (Figure 1D), we observed that unpegylated BT100 significantly inhibited VWF-A1A2A3 binding to purified LRP1 cluster II and cluster IV in a similar manner to pegylated BT200 in purified plate-binding studies (Figure 3E-F). Conversely, caplacizumab, which is a bivalent, humanized nanobody directed to a different part of the VWF-A1 domain29 (supplemental Figure 3A), did not significantly inhibit VWF-A1 binding to either LRP1 cluster II or cluster IV (supplemental Figure 3B).
BT200 concentration-dependently inhibits VWF interaction with LRP1 cluster II and cluster IV. (A-D) BT200 significantly inhibits binding of human pdVWF (A); commercial rVWF (B); VWF-A1A2A3 truncation (C); and isolated VWF-A1 domain (D) to purified LRP1 cluster II (blue) and cluster IV (red) in a concentration-dependent manner. (E-F) BT100 (blue) and BT200 (red) significantly inhibit the binding of VWF-A1A2A3 to LRP1 cluster II (E) and LRP1 cluster IV (F). Plate binding (%) is calculated from absorbance at 450 nm in which the value for 0-nM BT200 or BT100 was taken as 100%. Dots represent the mean plate binding (%) at corresponding aptamer concentrations. Error bars depict the standard deviations. P values are outcomes of extra-sum-of-squares F test and compare the binding capacity of pdVWF vs rVWF or VWF constructs with LRP1 cluster II and cluster IV. Abs, absorbance; pdVWF, plasma-derived VWF; rVWF, recombinant VWF.
BT200 concentration-dependently inhibits VWF interaction with LRP1 cluster II and cluster IV. (A-D) BT200 significantly inhibits binding of human pdVWF (A); commercial rVWF (B); VWF-A1A2A3 truncation (C); and isolated VWF-A1 domain (D) to purified LRP1 cluster II (blue) and cluster IV (red) in a concentration-dependent manner. (E-F) BT100 (blue) and BT200 (red) significantly inhibit the binding of VWF-A1A2A3 to LRP1 cluster II (E) and LRP1 cluster IV (F). Plate binding (%) is calculated from absorbance at 450 nm in which the value for 0-nM BT200 or BT100 was taken as 100%. Dots represent the mean plate binding (%) at corresponding aptamer concentrations. Error bars depict the standard deviations. P values are outcomes of extra-sum-of-squares F test and compare the binding capacity of pdVWF vs rVWF or VWF constructs with LRP1 cluster II and cluster IV. Abs, absorbance; pdVWF, plasma-derived VWF; rVWF, recombinant VWF.
VWF can interact with several macrophage cell surface receptors.12-14,30,31 To confirm that BT200 specifically inhibits VWF interaction with cell surface–exposed LRP1, full-length human LRP1 was stably expressed on HEK293T cells (HEK-LRP1). In keeping with previous studies that demonstrated that VWF interaction with LRP1 is enhanced in the presence of shear,10-12 we observed that recombinant full-length VWF binding to HEK-LRP1 cells was markedly enhanced in the presence of ristocetin (Figure 4A). Similar to macrophage binding, VWF interaction with HEK-LRP1 cells was again inhibited in a concentration-dependent manner by BT200 (Figure 4B-C). Finally, we investigated whether BT200 inhibited VWF interaction with LRP1 through competitive, allosteric, or mixed mechanisms. Importantly, we observed a linear relationship between Kd values for human VWF binding to LRP1 cluster IV over a range of different BT200 concentrations, suggesting a competitive rather than allosteric mechanism for BT200-mediated inhibition of VWF-LRP1 interaction (Figure 4D-E). Cumulatively, these results support the hypothesis that BT200 prolongs the half-life of the VWF-FVIII complex by interacting with a defined region of the VWF-A1 domain and thereby specifically inhibiting macrophage LRP1–mediated VWF clearance.
BT200 inhibits VWF interaction with HEK293 cells expressing LRP1. (A) Binding of full-length recombinant human VWF to HEK293 cells stably transfected with LRP1 (HEK-LRP1) was assessed by flow cytometry in the presence (blue) or absence (red) of ristocetin (1.5 mg/mL). The y-axis represents the binding capacity normalized to the number of cells. FITC-A on the x-axis represents the fluorescence intensity absorbance at the wavelength of 490 nm, in which higher values indicate more VWF binding. (B) Binding of rVWF to HEK-LRP1 in the presence of ristocetin was assessed by flow cytometry in the presence of increasing concentrations of BT200. Blue is VWF binding in the absence of BT200; gray is background in the absence of VWF; and green to red signals for increasing BT200 concentrations. The y-axis represents the binding capacity normalized to the number of cells. BL1-A::BL1-A on the x-axis represents the fluorescence intensity absorbance at the wavelength of 488 nm, in which higher values indicate more VWF binding. (C) rVWF binding (%) to HEK-LRP1 cells by flow cytometry is concentration-dependently inhibited by BT200. HEK-LRP1 binding (%) on the y-axis is calculated from mean fluorescent intensities at different BT200 concentrations presented on the x-axis. Error bars depict the standard deviations of the fluorescent intensities. P values are outcomes of extra-sum-of-squares F test and represent the significance of the binding of rVWF to HEK-LRP1. (D-E) To investigate whether BT200 inhibits VWF interaction with LRP1 through competitive, allosteric, or mixed mechanisms, Kd values for human rVWF binding to LRP1 cluster IV were determined over a range of different BT200 concentrations. (E) A linear relationship was observed, suggesting a competitive rather than allosteric mechanism for BT200-mediated inhibition of VWF-LRP1 interaction.
BT200 inhibits VWF interaction with HEK293 cells expressing LRP1. (A) Binding of full-length recombinant human VWF to HEK293 cells stably transfected with LRP1 (HEK-LRP1) was assessed by flow cytometry in the presence (blue) or absence (red) of ristocetin (1.5 mg/mL). The y-axis represents the binding capacity normalized to the number of cells. FITC-A on the x-axis represents the fluorescence intensity absorbance at the wavelength of 490 nm, in which higher values indicate more VWF binding. (B) Binding of rVWF to HEK-LRP1 in the presence of ristocetin was assessed by flow cytometry in the presence of increasing concentrations of BT200. Blue is VWF binding in the absence of BT200; gray is background in the absence of VWF; and green to red signals for increasing BT200 concentrations. The y-axis represents the binding capacity normalized to the number of cells. BL1-A::BL1-A on the x-axis represents the fluorescence intensity absorbance at the wavelength of 488 nm, in which higher values indicate more VWF binding. (C) rVWF binding (%) to HEK-LRP1 cells by flow cytometry is concentration-dependently inhibited by BT200. HEK-LRP1 binding (%) on the y-axis is calculated from mean fluorescent intensities at different BT200 concentrations presented on the x-axis. Error bars depict the standard deviations of the fluorescent intensities. P values are outcomes of extra-sum-of-squares F test and represent the significance of the binding of rVWF to HEK-LRP1. (D-E) To investigate whether BT200 inhibits VWF interaction with LRP1 through competitive, allosteric, or mixed mechanisms, Kd values for human rVWF binding to LRP1 cluster IV were determined over a range of different BT200 concentrations. (E) A linear relationship was observed, suggesting a competitive rather than allosteric mechanism for BT200-mediated inhibition of VWF-LRP1 interaction.
Lysine residues 1405-1408 in the VWF-A1 domain constitute a critical LRP1 binding site
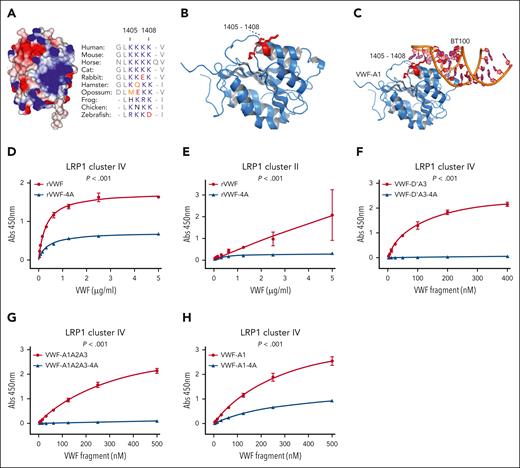
Lysine residues on other ligands have been reported to play a critical role in regulating LRP1-mediated clearance.24 Interestingly, a cluster of 4 conserved contiguous lysine residues (K1405, K1406, K1407, and K1408) are located on the surface of the VWF-A1 domain (Figure 5A-B). Previous cocrystallization studies have shown that the BT100 aptamer interacts with the VWF-A1 domain in proximity to the electronegative K1405-K1408 cluster (Figure 5B-C).19 To determine whether this lysine cluster is involved in VWF interaction with LRP1, we performed site-directed alanine mutagenesis studies. Interestingly, replacement of the entire K1405-K1408 lysine cluster with alanine residues (VWF-A1-4A) significantly (P < .001) attenuated binding of full-length recombinant human VWF to both LRP1 clusters II and IV binding (Figure 5D-E). We next assessed the effect of the K1405-K1408 cluster on interaction of VWF truncations with LRP1. Importantly, markedly attenuated binding to LRP1 cluster IV (P < .001) was observed for VWF-D’A3-4A and VWF-A1A2A3-4A compared with wild-type controls (Figure 5F-G). Similarly, removal of the K1405-K1408 lysine cluster (VWF-A1-4A) significantly (P < .001) reduced the ability of the isolated VWF-A1 domain to bind to LRP1 cluster IV (Figure 5H). In contrast, alanine mutagenesis of other lysine residues on the surface of the VWF-A1 domain, including K1362A and K1371A, had no significant effect on VWF binding to LRP1 (data not shown). Cumulatively, these data define K1405-K1408 in the VWF-A1 domain as a novel key region involved in regulating VWF interaction with LRP1.
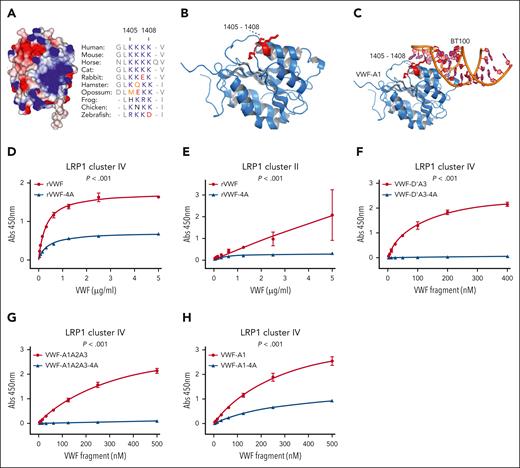
Lysine 1405-1408 residues in the VWF-A1 domain constitute a critical LRP1 binding site. (A) A cluster of 4 contiguous lysine residues (K1405, K1406, K1407, and K1408) are located on the surface of the VWF-A1 domain (electropositive residues in blue and negative residues in red). These Lys residues are conserved throughout VWF species sequences. (B-C) VWF-A1 domain cocrystallized with aptamer BT100 (pdb 7f49; Zhu et al19) showing the aptamer (orange) interacts in proximity to K1405-K1408 (red) within VWF-A1 (blue). To investigate the importance of the K1405-K1408 cluster in the VWF-A1 domain in regulating LRP1 binding, site-directed mutagenesis was used to replace the 4 lysine residues with alanine in full-length rVWF (rVWF-4A). Subsequently, the binding of wild-type rVWF and rVWF-4A to LRP1 cluster IV (D) and cluster II (E) were assessed in plate-binding studies. Similarly, the effect of alanine mutagenesis of K1405-K1408 on LRP1 cluster IV binding was also assessed in rVWF truncations including VWF-D’A3-4A (F), VWF-A1A2A3-4A (G) and VWF-A1-4A (H). Dots represent the mean absorbance at 450 nm at the corresponding VWF concentrations. Error bars depict the standard deviations. P values are outcomes of extra-sum-of-squares F test and compare the binding capacity of VWF constructs with or without –4A to LRP1 cluster IV or cluster II. Abs, absorbance.
Lysine 1405-1408 residues in the VWF-A1 domain constitute a critical LRP1 binding site. (A) A cluster of 4 contiguous lysine residues (K1405, K1406, K1407, and K1408) are located on the surface of the VWF-A1 domain (electropositive residues in blue and negative residues in red). These Lys residues are conserved throughout VWF species sequences. (B-C) VWF-A1 domain cocrystallized with aptamer BT100 (pdb 7f49; Zhu et al19) showing the aptamer (orange) interacts in proximity to K1405-K1408 (red) within VWF-A1 (blue). To investigate the importance of the K1405-K1408 cluster in the VWF-A1 domain in regulating LRP1 binding, site-directed mutagenesis was used to replace the 4 lysine residues with alanine in full-length rVWF (rVWF-4A). Subsequently, the binding of wild-type rVWF and rVWF-4A to LRP1 cluster IV (D) and cluster II (E) were assessed in plate-binding studies. Similarly, the effect of alanine mutagenesis of K1405-K1408 on LRP1 cluster IV binding was also assessed in rVWF truncations including VWF-D’A3-4A (F), VWF-A1A2A3-4A (G) and VWF-A1-4A (H). Dots represent the mean absorbance at 450 nm at the corresponding VWF concentrations. Error bars depict the standard deviations. P values are outcomes of extra-sum-of-squares F test and compare the binding capacity of VWF constructs with or without –4A to LRP1 cluster IV or cluster II. Abs, absorbance.
BT200 inhibits VWF interaction with LRP1 by blocking K1405-K1408 within the VWF-A1 domain
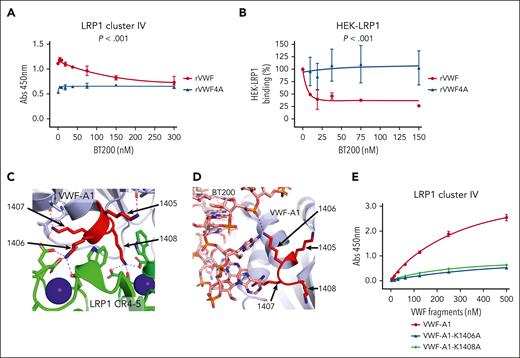
BT200 interacts with the VWF-A1 domain in proximity to K1405-K1408.19 Consequently, we next investigated whether the inhibitory effect of BT200 in blocking VWF interaction with LRP1 was mediated through this lysine cluster. Interestingly, although BT200 concentration-dependently inhibited the binding of full-length VWF to LRP1 cluster IV, it had no significant effect on the binding of full-length VWF-4A (Figure 6A). Similarly, BT200 did not attenuate the binding of VWF-4A to HEK-LRP1 cells (Figure 6B).
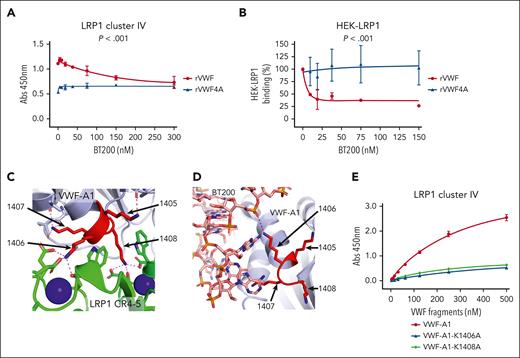
BT200 inhibits VWF interaction with LPR1 by blocking lysine 1405-1408 cluster in VWF-A1 domain. (A) Binding of full-length rVWF (red) and rVWF-4A (blue) to purified LRP1 cluster IV in an immunosorbent plate-binding assay in the presence or absence of increasing concentrations of BT200. Dots represent the mean absorbance at 450 nm at the corresponding VWF concentrations. Error bars depict the standard deviations. (B) Binding of full-length rVWF (red) and rVWF-4A (blue) to HEK-LRP1 cells assessed by flow cytometry in the presence or absence of increasing concentrations of BT200. HEK-LRP1 binding (%) on the y-axis is calculated from mean fluorescent intensities at different BT200 concentrations presented on the x-axis. Error bars depict the standard deviations of the fluorescent intensities. P values are outcomes of extra-sum-of-squares F test and compare the binding capacity of rVWF and rVWF-4A with LRP1 cluster IV (A) or HEK-LRP1 (B). (C) AlphaFold 3 model of the interaction between LRP1 cluster IV and VWF-A1 shown as a cartoon diagram. VWF-A1 lysine cluster amino acids 1405-1408 shown in red. LRP1 domains CR4,5 (green) engage the lysine cluster of VWF-A1, and calcium ions bound to LRP1 are shown as spheres (blue). Zoom-in view of contacts between LRP1 CR4-5 lysine binding sites with the VWF-A1 1406 and 1408 sidechains. Electrostatic interactions are shown as dashed lines. (D) Crystal structure of BT200 (sticks) bound to VWF-A1 shown as a cartoon diagram showing the proximity to the lysine cluster (red) and direct electrostatic interaction with K1406 shown as a dashed line. (E) Site-directed alanine mutagenesis was used to generate the VWF-A1 variants VWF-A1-K1406A and VWF-A1-K1408A. Binding of VWF-A1-K1406A (blue), VWF-A1-K1408A (green), and wild-type VWF-A1 domain (red) to LRP1 cluster IV was then assessed in a plate-binding assay. Dots represent mean absorbance at 450 nm values at corresponding VWF concentrations. Error bars depict standard deviations.
BT200 inhibits VWF interaction with LPR1 by blocking lysine 1405-1408 cluster in VWF-A1 domain. (A) Binding of full-length rVWF (red) and rVWF-4A (blue) to purified LRP1 cluster IV in an immunosorbent plate-binding assay in the presence or absence of increasing concentrations of BT200. Dots represent the mean absorbance at 450 nm at the corresponding VWF concentrations. Error bars depict the standard deviations. (B) Binding of full-length rVWF (red) and rVWF-4A (blue) to HEK-LRP1 cells assessed by flow cytometry in the presence or absence of increasing concentrations of BT200. HEK-LRP1 binding (%) on the y-axis is calculated from mean fluorescent intensities at different BT200 concentrations presented on the x-axis. Error bars depict the standard deviations of the fluorescent intensities. P values are outcomes of extra-sum-of-squares F test and compare the binding capacity of rVWF and rVWF-4A with LRP1 cluster IV (A) or HEK-LRP1 (B). (C) AlphaFold 3 model of the interaction between LRP1 cluster IV and VWF-A1 shown as a cartoon diagram. VWF-A1 lysine cluster amino acids 1405-1408 shown in red. LRP1 domains CR4,5 (green) engage the lysine cluster of VWF-A1, and calcium ions bound to LRP1 are shown as spheres (blue). Zoom-in view of contacts between LRP1 CR4-5 lysine binding sites with the VWF-A1 1406 and 1408 sidechains. Electrostatic interactions are shown as dashed lines. (D) Crystal structure of BT200 (sticks) bound to VWF-A1 shown as a cartoon diagram showing the proximity to the lysine cluster (red) and direct electrostatic interaction with K1406 shown as a dashed line. (E) Site-directed alanine mutagenesis was used to generate the VWF-A1 variants VWF-A1-K1406A and VWF-A1-K1408A. Binding of VWF-A1-K1406A (blue), VWF-A1-K1408A (green), and wild-type VWF-A1 domain (red) to LRP1 cluster IV was then assessed in a plate-binding assay. Dots represent mean absorbance at 450 nm values at corresponding VWF concentrations. Error bars depict standard deviations.
Structure predictions with AlphaFold 332 were performed to further investigate the roles of the K1405-K1408 cluster in regulating interaction with LRP1 and BT200. Across many simulations, we consistently observed that K1408 was the residue most effectively engaged by the lysine binding sites of LRP1 cluster IV due to its solvent accessibility and disposition with respect to the VWFA1 domain shape (Figure 6C). In addition, K1406 was also solvent accessible and could be engaged by cluster IV (Figure 6C). In contrast, both K1405 and K1407 demonstrated reduced solvent accessibility and were predicted to make contacts with other residues within the VWF-A1 domain. Furthermore, K1406 formed direct contacts to BT200 via a salt bridge to the phosphate backbone (Figure 6D). To investigate these structural prediction data, we performed site-directed mutagenesis to generate VWF-A1 variants including VWF-A1-K1406A and VWF-A1-K1408A. In LRP1 cluster IV plate-binding experiments, binding of VWF-A1-K1406A and VWF-A1- K1408A were both significantly (P < .001) reduced than that of wild-type VWF-A1 domain (Figure 6E). Cumulatively, these data are consistent with the hypothesis that BT200 inhibits VWF interaction with LRP1 by specifically blocking the interaction of the K1405-K1408 cluster region in the VWF-A1 domain with LRP1 cluster IV.
BT200 attenuates VWF interaction with additional macrophage receptors
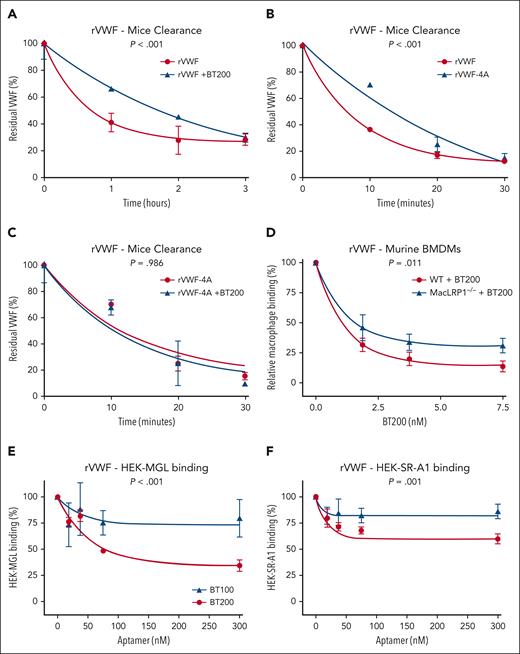
In vivo clearance experiments via tail vein injection in VWF–/– mice confirmed that the half-life of recombinant human VWF was significantly (P < .001) extended in the presence of BT200 (Figure 7A). Second, we observed that the half-life of recombinant human VWF-4A was significantly (P < .001) prolonged in VWF–/– mice than recombinant wild-type human VWF (Figure 7B). Finally, in contrast to wild-type VWF, we found that coinfusion of BT200 had no significant effect on the clearance of the VWF-4A variant (Figure 7C). Together, these data support the following hypotheses: (1) BT200 directly attenuates VWF clearance in vivo; (2) K1405-K1408 plays a role in regulating VWF clearance in vivo; and (3) the effect of BT200 in prolonging VWF half-life in vivo is mediated at least in part through interaction with the K1405-K1408 cluster.
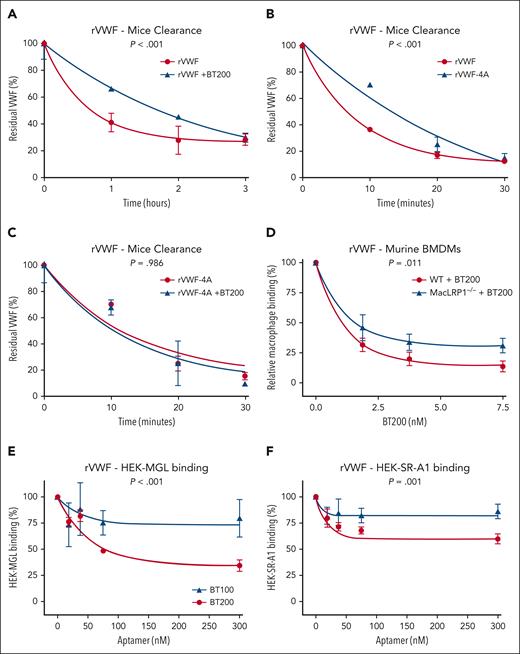
The effect of BT200 on VWF clearance in VWF–/– mice and ability to attenuate VWF interaction with macrophage MGL and SR-AI receptors. (A-C) In vivo clearance experiments were performed in VWF−/− mice weighing 20 to 25 g on a C57BL/6J background. Mice were infused with 7.5 μg rVWF or rVWF-4A with or without 0.9 μM BT200 via tail-vein injection. Blood was collected via submandibular bleeding or cardiac puncture into lithium-heparin–coated microtainers. Three to 6 mice per time point were used. Residual plasma VWF:Ag levels were determined at specific time points by VWF:Ag enzyme-linked immunosorbent assay, using polyclonal rabbit anti-human VWF (Dako). (D) Binding of full-length rVWF to WT BMDMs (red) or MX1cre+LRPflox/flox BMDMs (MacLRP1–/– blue) was assessed using flow cytometry in the presence of increasing concentrations of BT200. Relative macrophage binding (%) on the y-axis is calculated from mean fluorescent intensities at different BT200 concentrations presented on the x-axis. Error bars depict the standard error of the mean of the fluorescent intensities. P values are outcomes of extra-sum-of-squares F test. (E-F) Binding of full-length rVWF to HEK-MGL (E) and HEK-SR-AI cells (F) was assessed by flow cytometry in the presence or absence of increasing concentrations of BT100 (blue) and BT200 (red). (E-F) HEK-MGL binding (%) (E) and HEK-SR-AI binding (%) (F) on the y-axis are calculated from mean fluorescent intensities at different BT100 and BT200 concentrations presented on the x-axis. Error bars depict the standard deviations of the fluorescent intensities. P values are outcomes of extra-sum-of-squares F test and compare the binding capacity of BT100 and BT200 with HEK-MGL (E) or HEK-SR-AI (F).
The effect of BT200 on VWF clearance in VWF–/– mice and ability to attenuate VWF interaction with macrophage MGL and SR-AI receptors. (A-C) In vivo clearance experiments were performed in VWF−/− mice weighing 20 to 25 g on a C57BL/6J background. Mice were infused with 7.5 μg rVWF or rVWF-4A with or without 0.9 μM BT200 via tail-vein injection. Blood was collected via submandibular bleeding or cardiac puncture into lithium-heparin–coated microtainers. Three to 6 mice per time point were used. Residual plasma VWF:Ag levels were determined at specific time points by VWF:Ag enzyme-linked immunosorbent assay, using polyclonal rabbit anti-human VWF (Dako). (D) Binding of full-length rVWF to WT BMDMs (red) or MX1cre+LRPflox/flox BMDMs (MacLRP1–/– blue) was assessed using flow cytometry in the presence of increasing concentrations of BT200. Relative macrophage binding (%) on the y-axis is calculated from mean fluorescent intensities at different BT200 concentrations presented on the x-axis. Error bars depict the standard error of the mean of the fluorescent intensities. P values are outcomes of extra-sum-of-squares F test. (E-F) Binding of full-length rVWF to HEK-MGL (E) and HEK-SR-AI cells (F) was assessed by flow cytometry in the presence or absence of increasing concentrations of BT100 (blue) and BT200 (red). (E-F) HEK-MGL binding (%) (E) and HEK-SR-AI binding (%) (F) on the y-axis are calculated from mean fluorescent intensities at different BT100 and BT200 concentrations presented on the x-axis. Error bars depict the standard deviations of the fluorescent intensities. P values are outcomes of extra-sum-of-squares F test and compare the binding capacity of BT100 and BT200 with HEK-MGL (E) or HEK-SR-AI (F).
To investigate whether BT200 may block VWF clearance through additional receptors besides LRP1, we next isolated BMDM macrophages from cre/loxP-mediated conditional LRP-deficient mice (MX1cre+LRPflox/flox). Significantly elevated plasma VWF levels have been reported in MX1cre+LRPflox/flox mice after the inactivation of the LRP1 gene.33 Consistently, we observed that VWF binding to macrophages from MX1cre+LRPflox/flox mice was significantly (P = .048) reduced upon LRP1 inactivation. Critically, however, even in the absence of LRP1, BT200 was still able to significantly (P = .011) attenuate VWF binding to MX1cre+LRPflox/flox-derived macrophages (Figure 7D). These findings highlight that BT200 not only inhibits VWF binding to LRP1, but also attenuates the interaction of VWF with other macrophage receptors.
The VWF-A1 domain has been implicated in regulating VWF interaction with the MGL and the SR-AI scavenger receptors.13,14,34 Consequently, we next examined whether BT100 and/or BT200 influenced VWF binding to these macrophage clearance receptors. We observed that BT200 inhibited VWF binding to MGL (Figure 7E). In contrast, BT100 was significantly (P < .001) less efficient in attenuating MGL-VWF interaction. A mild inhibitory effect of BT200 on VWF binding to SR-AI was also seen (Figure 7F). Again, BT100 was significantly (P = .001) less efficient in attenuating the SRAI-VWF interaction. Cumulatively, these data demonstrate that the BT200 aptamer not only blocks VWF binding to LRP1 but also attenuates the interaction of VWF with additional macrophage receptors including MGL and SR-AI. Moreover, our findings suggest a role for the 40-kDa PEG moiety of BT200 in blocking VWF interaction with MGL and SR-AI.
Discussion
Although rondaptivon pegol (BT200) was originally developed as a novel antithrombotic therapy designed to target the VWF-A1 domain,19,35,36 clinical studies in healthy human volunteers demonstrated that BT200 significantly increased plasma VWF and FVIII levels.20 These observations led to a repurposing of BT200 as a potential novel therapy to increase plasma VWF-FVIII levels in patients with VWD or hemophilia A. Herein, we demonstrate, to our knowledge, for the first time that binding of BT200 to the VWF-A1 domain significantly attenuates VWF clearance by macrophages.
LRP1 is a large endocytic receptor that is highly expressed on macrophage cell surfaces.24,37 The extracellular domain of LRP1 is composed of 4 clusters (clusters I-IV) of low-density lipoprotein receptor type A repeats.24,38 To define how specific VWF domains contribute to LRP1-mediated clearance, we used a series of recombinant VWF truncations and assessed specific interactions with LRP1 clusters II and IV. Although full-length and truncated VWF fragments were able to interact with LRP1 cluster II, our data suggest that cluster IV plays a more important role in modulating VWF binding. Importantly, we observed that the isolated VWF-A1 domain bound in a concentration-dependent manner to both LRP1 clusters II and IV. Conversely, neither VWF-A2 nor VWF-A3 domain demonstrated significant binding to either cluster. In summary, therefore, our data confirm that multiple VWF regions (including notably the VWF-A1 domain) contribute to LRP1 binding, predominantly via the extracellular cluster IV domain of the receptor.
Previous evidence suggests that macrophage LRP1–mediated clearance plays a role in regulating physiological VWF clearance in vivo.12,39,40 Our findings demonstrate that BT200 binding to the VWF-A1 domain specifically attenuates the ability of full-length plasma-derived VWF and recombinant human VWF to bind to both cluster II and cluster IV of LRP1 in plate-binding assays. Moreover, BT200 also concentration-dependently inhibited VWF binding to full-length recombinant LRP1 expressed on HEK293.
Structural studies have provided insights into the mechanisms through which LRP1 can interact with >40 different target ligands.24 The canonical mode of ligand binding is characterized by the formation of salt bridges between amino groups of specific lysine residues in the ligand protein with carboxylate groups of aspartate residues in the clusters of LRP1.38,41,42 Although multiple VWF domains may have the potential to interact with LRP1, the marked effects of shear stress and ristocetin in promoting the binding of full-length VWF suggest a key role for VWF-A1 domain.10-12,27 This concept is further supported by the observation that VWD type 2B mutations in the VWF-A1 domain promote pathologically enhanced LRP1-dependent clearance.27 Our data demonstrate that a cluster of 4 lysine residues (K1405, K1406, K1407, and K1408) in the VWF-A1 domain constitutes a novel critical binding site for macrophage LRP1. Thus, site-directed alanine mutagenesis to remove this conserved cluster of lysine residues (K1405A-K1408A) ablated the ability of full-length VWF or truncated VWF-A1 domain containing VWF fragments to bind to LRP1 cluster II and cluster IV. Furthermore, the half-life of recombinant human VWF-4A was significantly (P < .001) prolonged in VWF–/– mice than recombinant wild-type human VWF.
Overall, our data, therefore, suggest that different VWF-A1 domains in a multimeric VWF chain will potentially interact with multiple LRP1 receptors (via cluster II or cluster IV) on the macrophage cell surface. Based upon our modeling studies, it seems unlikely that a single VWF-A1 domain will have the capacity to interact with both extracellular cluster II and cluster IV on the same LRP1 receptor. Additional studies will be required to determine the precise roles of the K1405-K1408 cluster in regulating physiological and pathological clearance of VWF. Notably however, many of the different VWF sequence variants associated with enhanced VWF clearance have also been mapped to the VWF-A1 domain.1,2 Importantly, the K1405-K1408 lysine cluster in the VWF-A1 domain is located close to the binding site of the BT200 aptamer.19 Moreover, the ability of BT200 to inhibit VWF binding to LRP1 or prolong the in vivo half-life of VWF infused into VWF–/– mice was no longer observed after mutagenesis of the K1405-K1408 cluster.
The VWF-A1 domain has also been implicated in regulating VWF clearance through additional macrophage receptors including MGL and SR-AI.13,14 We observed that VWF binding to BMDM from MX1cre+LRPflox/flox mice after inactivation of the LRP1 gene was still significantly attenuated (P < .001) in the presence of BT200. These findings support the hypothesis that BT200 not only inhibits VWF binding to LRP1 but also attenuates interaction with other macrophage receptors. Consistently, we found that VWF binding to MGL and, to a lesser extent, SR-AI were both inhibited by BT200. Interestingly, the inhibitory effect on MGL and SR-AI binding was significantly reduced for unpegylated BT100 than BT200. Thus, our data suggest that the 40-kDa PEG moiety of BT200 not only serves to prolong the plasma half-life of the aptamer but also potentiates its ability to inhibit macrophage-mediated clearance of VWF when bound to the VWF-A1 domain. This hypothesis is supported by recent studies in which we demonstrated that site-directed pegylation targeted to specific surface residues in the VWF-A1 domain could be used to extend the half-life of VWF-A1A2A3 in VWF-deficient mice.23 Further studies will be required to determine whether BT200 may also inhibit VWF clearance mediated through other cell types, including liver sinusoidal ECs and/or hepatocytes.2 In addition, it is possible that the PEG moiety of BT200 may also affect the interaction of other VWF domains (notably A2 and/or A3) with specific clearance receptors. Nonetheless, from a translational perspective, these data regarding the BT200 aptamer provide exciting proof of concept that targeted inhibition of VWF clearance pathways represents a novel therapeutic modality for the treatment of VWD and hemophilia A.
Acknowledgments
J.S.O. is supported by a Science Foundation Ireland Frontiers for the Future (FFP) Award (20/FFP-A/8952) and the National Institutes of Health, National Heart, Lung, and Blood Institute for the Zimmerman Program (grant HL081588). F.A. is supported by a Rubicon grant (452022310) from the Netherlands Organisation for Health Research and Development (ZonMw).
The visual abstract was created with BioRender.com.
Authorship
Contribution: A.C., F.A., D.D., C.B., S.A., J.F., P.L., E.K., A.A., T.A.J.M., J.E., and J.S.O. performed experiments, designed the research, and wrote the article; F.A. performed statistical analysis; A.C., F.A., D.D., R.J.S.P., R.I.B., T.A.J.M., S.Z., J.C.G., B.J., and J.S.O. designed the research and contributed to literature review, data interpretation, final draft writing, and critical revision; and all the authors have participated sufficiently in this work, take public responsibility for the content, and gave consent to the final version of the article.
Conflict-of-interest disclosure: J.S.O. has served on the speaker’s bureau for Baxter, Bayer, Novo Nordisk, Sobi, Boehringer Ingelheim, Leo Pharma, Takeda, and Octapharma; served on the advisory boards of Baxter, Sobi, Bayer, Octapharma, CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, Takeda, and Pfizer; and received research grant funding awards from 3M, Baxter, Bayer, Pfizer, Shire, Takeda, and Novo Nordisk. F.A. received research support from CSL Behring, Takeda, Octapharma, and Sobi. D.D. has received honoraria from Takeda; and educational support sponsorship from Novo Nordisk and Amgen. R.I.B.’s institution has received research support/clinical trial funding from Bayer, Takeda, Pfizer, Daiichi Sankyo, CSL Behring, Roche, Amgen, AstraZeneca, AbbVie, Sanofi, Acerta Pharma, Janssen-Cilag, Bristol Myers Squibb, Boehringer Ingelheim, Werfen, and Technoclone, unrelated to this study. B.J. is a consultant to Band Therapeutics LLC, a Guardian Therapeutics company, and has received reimbursement for travel and related to scientific advice from Sanofi. The remaining authors declare no competing financial interests.
Correspondence: James S. O’Donnell, Irish Centre for Vascular Biology, Royal College of Surgeons in Ireland, Ardilaun House, 111 St Stephen’s Green, Dublin 2, Ireland; email: jamesodonnell@rcsi.ie.
References
Author notes
Presented orally in abstract form at the 29th International Society on Thrombosis and Haemostasis Congress, Melbourne, Australia, 9 July 2019, and at the 31st International Society on Thrombosis and Haemostasis Congress, Montreal, Canada, 24 June 2023.
Data are available on request from the corresponding author, James S. O’Donnell (jamesodonnell@rcsi.ie).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.